Abstract
Purpose of Review
Gastroparesis is an important complication of diabetes that may have a major impact on the quality of life as a result of upper gastrointestinal symptoms and impaired glycaemic control. Current management strategies include optimising blood glucose control, dietary modifications and supportive nutrition. Pharmacologic approaches with drugs that have prokinetic and/or antiemetic effects are also used widely; however, current available treatments have major limitations. There is increasing recognition that the rate of gastric emptying (GE) is a key determinant of the glycaemic response to a meal.
Recent Findings
There is ongoing uncertainty regarding the impact of longstanding hyperglycaemia on GE, which requires clarification. New diagnostic techniques have been developed to better characterise the mechanisms underlying gastroparesis in individual patients, and these have the potential to lead to more personalised therapy.
Summary
Management of gastroparesis is complex and suboptimal; novel approaches are desirable. This review summarises recent advances in the understanding of diabetic gastroparesis, with an emphasis on the current therapies that influence GE, and the bidirectional relationship between glycaemic control and GE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroparesis is a chronic disorder usually associated with longstanding, complicated diabetes and characterised by delayed gastric emptying (GE) in the absence of mechanical obstruction. Gastroparesis can have a substantial impact on the quality of life and the management of diabetes [1, 2]. Associated symptoms include early satiety, belching, bloating, nausea, vomiting and abdominal discomfort. There are many challenges in the management of this condition and a need for an improved understanding of the underlying complex pathophysiology in order to overcome current limitations, and allow more personalised management. The focus of this review relates to the “bidirectional” relationship of GE with glycaemic control, and the effects of long- and short-acting glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) on GE.
Methods
A literature search using the PubMed database was performed using the search terms “gastric emptying” or “gastroparesis” with “glycaemic control” and “diabetes mellitus”. Full text articles and abstracts were included. Reference lists from articles were used to identify further relevant papers. Only English language articles pertaining to adult medicine were included.
Prevalence of Diabetic Gastroparesis
The Gastroparesis Clinical Research Consortium (GpCRC) has estimated that up to 5 million individuals in the US are affected by this condition [3]. A cross-sectional study analysing a US medical database reported that the cost of inpatient treatment for gastroparesis was $568 million in 2013 which was predicted to increase [4]. A recent US cross-sectional, population-based study reported the prevalence of gastroparesis to be 4.3% in type 1 diabetes (T1D), 1.3% in type 2 diabetes (T2D) and 0.16% in the general population [5]. However, only a minority of patients with gastroparesis are likely to have had their GE measured, therefore, many remain undiagnosed [6]. As assessed with a 13C-spirulina breath test to quantify GE, 47% of participants at year 20 of the Epidemiology of Diabetes Interventions and Complications (EDIC) study had abnormally delayed GE [7]. Although patients with T1D are 3.5 times more likely to have gastroparesis than T2D, the overall number of patients with gastroparesis is greater in T2D due to the substantially greater prevalence of the latter condition [5].
Pathophysiology of Diabetic Gastroparesis
The pathophysiology of diabetic gastroparesis is complex and multifactorial. From studies in animal models and humans, there has been some definition of the underlying processes, but there are still substantial gaps in knowledge. The pathogenetic mechanisms are heterogeneous and include dysfunction of vagal innervation, damage to interstitial cells of Cajal (ICCs) and reduction in neuronal nitric oxide synthase (nNOS).
Vagal innervation of the stomach is a key mechanism that modulates antral contractions, which are responsible for grinding solid food into small particles and pumping it across the pylorus. Diabetic autonomic neuropathy is associated with antral hypomotility [8], reduced fasting proximal gastric tone and decreased postprandial accommodation of the gastric fundus [9]; the latter may contribute to the sensation of bloating in patients with gastroparesis. Pyloric contractility may be abnormally increased in some patients with diabetes and would contribute to delayed GE [10].
ICCs generate action potentials and act as the “pacemaker” of the stomach [11, 12]. Animal studies suggest that impaired insulin and IGF-1 signalling may result in damage to myenteric cholinergic neurones and ICCs [13]. Furthermore, ICC numbers in full thickness antral biopsies are reduced in many patients with diabetic gastroparesis [14,15,16,17], associated with abnormalities of gastric slow waves, which potentially leads to both disordered motor function and symptoms [14]. A reduction or loss of nNOS has similarly been demonstrated [11, 15] and is likely to contribute to disordered coordination of gastric motility.
Haem oxygenase 1 (HO1) is an enzyme expressed by CD206+ M2 macrophages, a macrophage subset that is depleted in both mouse models of diabetic gastroparesis and patients with this condition, while the number of HO1-negative M1 macrophages is increased. HO1 is upregulated in diabetic mice in response to oxidative stress [18, 19] and may be protective against the development of gastroparesis. It has been suggested that upregulating expression of HO1 represents a potential target for the management of gastroparesis [18, 19]. This concept is supported by a study comparing CD206+ cell expression in diabetic patients with and without gastroparesis, where a positive relationship between CD206+ and number of ICCs was evident [20].
In health, enteroendocrine cells in the gastrointestinal mucosa respond to luminal glucose and other macronutrients by generating signals that stimulate the release of peptides. Small intestinal K cells release glucose-dependent insulinotropic polypeptide (GIP), while L cells in the small and large bowel release GLP-1 and GLP-2 [21]. In addition to stimulating insulin and suppressing glucagon in a glucose-dependent manner, GLP-1 slows GE, which is a major mechanism in the regulation of postprandial glycaemic excursions [22]. Recent studies suggest that in T2D, this feedback mechanism may be impaired due to dysregulation of small intestinal sweet taste receptors contributing to either abnormally delayed or rapid GE [23]. Multiple other hormones, including cholecystokinin, ghrelin, leptin, amylin, peptide tyrosine-tyrosine and oxyntomodulin, also modulate GE [24].
Specialised mucosal endocrine cells in the duodenal and gastric antral epithelium secrete the hormone, motilin [25]. The binding of motilin to its receptors present in the muscular layer of the gastrointestinal tract facilitates acetylcholine release from cholinergic motor neurons to increase contractility of upper GI smooth muscles, thereby accelerating GE [26]. In diabetic gastroparesis, while one study reported increased motilin levels in diabetes [27], thought to be due to cholinergic deficiency in the setting of autonomic neuropathy, in a subsequent study, levels of motilin in health and diabetes were comparable [28], and the biological activity of motilin to accelerate GE appeared to be preserved [28]. Ghrelin is released from the fundus of the stomach and, in pharmacological doses, accelerates GE [29].
Glycaemic Control and Gastroparesis
An important concept is that the presence of upper gastrointestinal symptoms typically associated with gastroparesis correlates poorly with the rate of GE [30], such that in some cases, patients with markedly abnormally slow GE may be asymptomatic. Even in the absence of symptoms, delayed GE can pose management challenges, particularly for patients on insulin therapy. For example, gastroparesis may predispose to postprandial hypoglycaemia as a result of a mismatch between nutrient absorption and the action of exogenous insulin [31, 32].
Type 1 Diabetes
Glycaemia and GE
Multiple studies have demonstrated slowing of GE in the setting of acute hyperglycaemia, although the magnitude of this effect remains contentious [33,34,35,36,37]. There are limited prospective studies assessing the effect of long-term glycaemia on GE. One study [7] followed up participants in the Diabetes Control and Complications Trial and the follow-up Epidemiology of Diabetes Interventions and Complications (DCCT-EDIC) study. While intensive glycaemic control was not associated with a reduced risk of gastroparesis, assessed by 13C-spirulina breath testing, patients with a longer duration of diabetes and higher HbA1c before DCCT had a modestly increased risk of delayed GE. As GE was not assessed at initial enrolment, it was not possible to determine whether intensive glycaemic control had an early effect on GE.
In a retrospective review of a cohort of 299 patients (both T1D and T2D), those with HbA1c < 7% had more rapid GE measured by scintigraphy compared with patients with higher HbA1c levels [38]. In contrast, both another retrospective review of a cohort of 250 patients [39] and a cross-sectional study [40] failed to observe any correlation between GE and HbA1c [39]. Further studies are required.
In contrast to the effect of acute hyperglycaemia, insulin-induced hypoglycaemia accelerates GE markedly in patients with uncomplicated [41] and complicated [42] T1D and probably serves as an important counter-regulatory mechanism by increasing the rate of carbohydrate absorption.
Glycaemia and Symptoms of Gastroparesis
A case series [43] of 26 patients diagnosed with gastroparesis evaluated the effects of a continuous insulin infusion via a pump on GE and glycaemic control. All patients were initially on a multiple-dose insulin regimen and changed to an insulin pump for 12 months. HbA1c was improved on insulin pump treatment (8.0%) compared with the multiple-dose insulin regimen (9.8%), and the rate of hospital admissions decreased. While the authors suggested that insulin pump therapy was an effective treatment in patients with diabetic gastroparesis, the study was uncontrolled and the specific impact of improved glycaemic control on GE was not assessed.
An open-label pilot study of 45 patients with poorly controlled diabetes using continuous glucose monitoring (CGM) who were treated with subcutaneous insulin infusion over 6 months reported that improved glycaemic control (reduction in HbA1c from 9.4 to 8.3%) was associated with a reduction in gastrointestinal symptoms as assessed via the Gastroparesis Cardinal Symptom Index (GCSI) [44]. However, incidence of gastroparesis exacerbations (defined as severe nausea or vomiting) was similar before and after treatment.
These observations suggest that both acute and chronic hyperglycaemia may slow GE, although the magnitude of the effect is uncertain, and that improved glycaemic control may reduce symptoms of gastroparesis. Increased glycaemic variability (GV) has been associated with an increased risk of microvascular complications [45]. Given the potential that increased GV may result in autonomic neuropathy, information on day-to-day GV should ideally be incorporated in future trials addressing the relationship between glycaemic control and gastroparesis.
Type 2 Diabetes
Glycaemia and GE
Using gastric scintigraphy, the relationship between GE and glycaemic control was analysed in 20 patients with T2D and 20 matched controls [46]. Patients with T2D had slower gastric and oesophageal emptying compared with the controls, and there was a positive correlation between the magnitude of the delay in GE with the plasma glucose concentration.
A study involving 160 older patients (age ≥ 65 years) with T2D found that those with poor glycaemic control (defined by fasting BGL ≥ 7.8 mmol/L and/or 2 h postprandial BGL ≥ 11.1 mmol/L) had slower GE at baseline [47]. In a prospective trial of 30 participants with T2D, GE was measured with a 13C-Spirulina breath test at baseline and after overnight administration of insulin to achieve euglycaemia or saline control, with subsequent follow-up after 6 months of intensive therapy at the discretion of their endocrinologist using a combination of lifestyle modification, oral therapy or insulin. GE was not affected by an acute reduction in glycaemia with the overnight insulin infusion, nor after 6 months of treatment intensification, although the latter achieved only a modest reduction in HbA1c (from 10.6 to 9.0%) [48]. It is, accordingly, possible that the magnitude of improvement in glycaemic control was insufficient to impact GE.
In another small study [49], 10 patients with T2D underwent GE measurement by scintigraphy before and after a week of intensive glucose control using either additional oral agents or insulin. At baseline, as a group, the participants had delayed GE when compared with healthy controls, but GE did not change after the intervention. Limitations of this study, however, include its uncontrolled design, small sample size and short duration of euglycaemia.
In contrast, in 30 female patients with T2D, the prevalence of delayed GE (assessed by scintigraphy and defined by > 10% gastric retention of a solid meal at 4 h) was substantially lower 2–3 months after treatment intensification to achieve euglycaemia (fasting blood glucose of 5–6 mmol/L and HbA1c < 7%) [50]. It is, accordingly, possible that improved glycaemic control can correct abnormally slow GE in T2D, but that a specific “threshold” of good control may be required. Again, additional studies are required.
Glycaemia and Symptoms of Gastroparesis
The effect of glycaemia on symptoms of gastroparesis has been evaluated in two cross-sectional studies. In the first, population-based survey of 8657 subjects (almost 95% had T2D), poor glycaemic control was associated with an increased prevalence of upper and lower gastrointestinal symptoms [51]. A similar conclusion was reached in the second cross-sectional questionnaire study of 1101 subjects with T2D [52].
Effect of GE on Glycaemic Control
As described, there is evidence, albeit inconclusive, that improved glycaemic control may reduce both symptoms of diabetic gastroparesis and the degree of delayed GE. However, the second component of the “bidirectional relationship” is the effect of GE on glycaemic control.
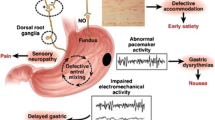
A randomised placebo-controlled trial of 30 patients with T1D utilised CGM in patients receiving either intravenous or oral erythromycin, or placebo [53]. Intravenous erythromycin predictably accelerated GE (as assessed by the 13C-spirulina breath test) and was associated with postprandial hyperglycaemia. Similar findings were observed in a case control study comparing GE measured by a 13C-octanoate breath test in patients with T1D and healthy subjects suggesting that accelerated GE results in hyperglycaemia [54]. Conversely, abnormally delayed gastric emptying may predispose to hypoglycaemia. In one study, 31 patients with unexplained hypoglycaemic events within 2 h of insulin injection were compared with 18 insulin-treated controls who had not experienced hypoglycaemic events. Using a stable isotope breath test, GE was shown to be abnormally delayed in the hypoglycaemia group [32], supporting this concept of “gastric” hypoglycaemia. The clinical implication is that GE should be measured in patients with diabetes using insulin therapy who have recurrent, unexplained episodes of hypoglycaemia particularly early in the postprandial period, and that changes in the insulin regimen or therapies to improve the predictability of GE (typically by accelerating it) should then be instituted. (Fig. 1).
Effects of GLP-1-Based Therapies and Pramlintide on GE
Medical therapy for diabetes may also affect GE. As discussed, incretin hormones are secreted in response to nutrient ingestion, and endogenous GLP-1 is one of several hormones that have physiological effects to slow GE [55, 56], thereby providing a negative feedback mechanism to diminish further entry of nutrients into the small intestine and attenuate the postprandial glycaemic excursion. Degradation-resistant GLP-1RAs and dipeptidyl peptidase-4 (DPP-4) inhibitors have been developed for the treatment of T2D. GLP-1RAs can be subclassified into “short-acting” (exenatide BD, lixisenatide) or “long-acting” (exenatide QW, liraglutide, dulaglutide, semaglutide). Continuous exposure to GLP-1 is associated with tachyphylaxis for its effects on GE so that after 24 h of continuous IV GLP-1 infusion in a pharmacological dose, the slowing of gastric emptying, while still significant, is less [57, 58]. A number of studies have assessed the impact of incretin-based therapies on GE, although in many, the value of the information provided is limited due to the use of suboptimal methodology (e.g. the kinetics of acetaminophen absorption) to quantify GE.
A randomised, double-blind, placebo-controlled trial demonstrated that exenatide (5 mcg twice daily) slowed GE compared with placebo at 30 days, as assessed by gastric scintigraphy. [59] In another randomised, double-blind, placebo-controlled trial involving 43 participants with T2D, lixisenatide was up-titrated over 28 days to 20 mcg daily, at which point GE—assessed by a 13C-octanoic acid breath test—was found to be substantially slower than with placebo [60]. The effect of a lower than clinically used dose of lixisenatide (10 mcg) on GE in well-controlled T2D and health was recently assessed using scintigraphy, and a profound slowing was evident in both groups in the absence of nausea [61]. Another multicentre, randomised, open-label trial compared the effects of lixisenatide (20 mcg daily) and liraglutide (1.2 mg and 1.8 mg daily) on GE, assessed via a 13C-octanoic acid breath test. At week 8, GE was slower than at baseline with both GLP-1RAs arms, but with much more pronounced slowing with lixisenatide [62]. In a further randomised, double-blind trial, liraglutide was up-titrated to 3.0 mg for 5 weeks and continued at this dose until week 16. GE—assessed by gastric scintigraphy—was slower than placebo at both 5 and 16 weeks, but the difference was less marked at 16 weeks, indicative of tachyphylaxis [63]. In a study reported in abstract form [64], the long-acting GLP-1RA exenatide QW was shown to moderately slow GE of solids and liquids measured by scintigraphy after 8 weeks administration. The magnitude of this slowing was shown to be predictive of the reduction in postprandial glucose. Finally, in a randomised, double-blind, placebo-controlled, crossover study of subcutaneous semaglutide up-titrated to 1.0 mg per week, GE was reported to be slower in the first hour with semaglutide than placebo, based on acetaminophen absorption from a yoghurt meal, but the difference was not statistically significant over the entire postprandial period [65].
DPP-4 inhibitors, in contrast to GLP1-RAs, appear not to have a substantial effect on GE. This was demonstrated in three randomised, double-blind, placebo-controlled studies using the “gold standard” of scintigraphy to assess GE, the first with sitagliptin in 15 healthy volunteers [66], the second with vildagliptin in 14 patients with T2D [67] and the third with sitagliptin in 14 patients with T2D [68]. Similar outcomes were reported in studies using stable isotope breath tests in health and T2D [69,70,71] One study, however, evaluating emptying of a 13C acetate-labelled glucose drink did report modestly delayed GE after sitagliptin, which was abolished by the GLP-1 receptor antagonist, exendin 9-39 [72].
Pramlintide is an analogue of islet amyloid polypeptide, which is co-secreted with insulin by beta cells. Pramlintide delays GE substantially in both healthy subjects and patients with insulin-treated diabetes [73,74,75,76].
It is important to recognise the effects of diabetes treatments on GE when determining the management of individual patients. For example, patients with T2D and relatively rapid GE may particularly benefit from a GLP-1RA that slows GE in order to reduce postprandial hyperglycaemic excursions. In contrast, patients with pre-existing delayed GE stand to gain much less from medications that slow GE [77].
Non-pharmacologic Management of Gastroparesis
Dietary modification is advocated as first line management for gastroparesis, although its efficacy has not been clearly established. In one trial, 56 patients with insulin-treated diabetes and symptomatic gastroparesis were randomised to a diet incorporating small particle size or a standard diet [78]. Patients who received the former diet experienced a greater reduction in the severity of nausea, vomiting, postprandial fullness and bloating, without any change in abdominal pain [78].
Based on the premise that exercise upregulates GLUT4 gene expression in skeletal muscle, which may improve glycaemic control and potentially GE, a trial was conducted assessing the role of rehabilitative exercises in the treatment of gastroparesis [46]. Eighty older patients (age ≥ 65) with T2D were randomly assigned into 4 groups: rehabilitation exercise and the prokinetic drug mosapride, rehabilitation exercise only, mosapride only and a control group. Rehabilitation exercises were associated with a modest improvement in GE, and this effect was greater when combined with prokinetic therapy. Given the low risk of harm and the additional benefits of exercise on glycaemic control, an exercise plan should arguably be a component of the management of diabetic gastroparesis.
In cases where nutrition is compromised, support such as enteral or parenteral nutrition may be indicated, but a discussion is beyond the scope of this review.
Pharmacologic Management of Gastroparesis
Multiple drug classes have been evaluated for the treatment of gastroparesis. Many of these are associated with significant adverse effects and only modest efficacy in relieving symptoms or accelerating GE.
Dopamine Agonists
Metoclopramide is a D2 receptor antagonist and serotonin 5HT4 receptor agonist that has been evaluated in two randomised, double-blind placebo-controlled trials involving a total of 53 participants [79, 80]. It was shown to reduce symptoms of nausea, vomiting, fullness and early satiety, and accelerate GE as assessed by scintigraphy. Metoclopramide carries a risk of tardive dyskinesia with a long-term use reported to be 1–10% [81] However, a lower risk of 0.1% per 1000 patient years was reported in a recent review [82]. A nasal spray formulation has also been evaluated in two trials with mixed results. In an open-label study [83], there was a reduction in symptoms of gastroparesis with intranasal metoclopramide compared with oral administration. In a follow-up double-blind study, symptomatic improvement only occurred in females. No serious adverse events were reported. Metoclopramide can also be self-administered subcutaneously to potentially manage episodes of severe nausea/vomiting [84].
Domperidone is another D2 receptor antagonist and was evaluated in an open-label prospective study [85] involving 34 participants (5 had diabetic gastroparesis and 29 idiopathic). Symptom severity was reduced significantly from baseline to the final week of treatment. Adverse effects of domperidone include QTc prolongation and cardiac arrhythmia, while increased prolactin levels may potentially result in galactorrhea and gynecomastia.
Motilin Receptor Agonists
Both erythromycin and azithromycin were assessed in a retrospective case-control analysis (n = 120) [86]. Both agents modestly accelerated GE, but symptoms were not evaluated. Erythromycin has the potential for drug interactions related to cytochrome P450 C3A4 inhibition, unlike azithromycin. However, both may cause QTc prolongation and cardiac arrhythmia. Tachyphylaxis has been observed in patients who receive ongoing treatment with erythromycin [87].
Mitemcinal has been evaluated in two randomised, double-blind, placebo-controlled trials. In the smaller trial of 106 participants with idiopathic or diabetic gastroparesis [88], there was no benefit over placebo for symptom improvement, while a subsequent trial of 392 participants with insulin-treated diabetes [89] reported symptomatic improvement in a subgroup of patients with BMI < 25 kg/m2 and HbA1c < 10%. Mild liver enzyme elevation has been observed [88], and this drug has hitherto not received regulatory approval.
Another motilin agonist, camicinal was evaluated by a small double-blind, placebo-controlled crossover study [90] of 10 participants and showed acceleration of GE for solids in T1D. The drug was well tolerated.
5-HT4 Receptor Agonists
Revexepride was assessed in a randomised, double-blind, placebo-controlled trial of (n = 80) patients with symptoms suggestive of gastroparesis [91] but showed no benefit over placebo for symptom improvement or GE, however, a higher incidence of diarrhoea and nausea was reported compared with placebo. It is not currently available.
The non-selective 5-HT4 receptor agonist tegaserod has been used to treat gastroparesis. However, it was withdrawn from the market in 2007 following concerns regarding cardiovascular safety [92]. Cisapride, once used widely, remains available, but is not used frequently due to similar concerns [92].
Prucalopride was evaluated in 34 participants (28 had idiopathic gastroparesis) in a randomised, double-blind, placebo-controlled, crossover trial [93]. Prucalopride improved both the GCSI score and GE t½ as assessed by a C-octanoic acid breath test after 4 weeks of treatment. Small bowel volvulus occurred in one patient in the prucalopide group. It has been approved for treatment of chronic constipation.
Velusetrag was recently evaluated in 232 patients (51% had diabetes) with gastroparesis symptoms [94•]. Treatment with 5 mg velusetrag improved the GCSI score as well as accelerated GE as assessed by scintigraphy at 4 weeks. At 12 weeks, however, there was no significant improvement in GCSI compared with placebo. Interestingly, even though higher doses of velusetrag 15 mg and 30 mg accelerated GE to a greater extent, the GCSI scores were not improved. Velusetrag was well tolerated. It is awaiting further phase 3 trials [94•].
Ghrelin Agonists
Ulimorelin was evaluated in a randomised, double-blind, placebo-controlled trial [95] of participants with T1D and T2D (n = 23) with symptomatic gastroparesis and was found to reduce the frequency and severity of nausea and vomiting, accompanied by a reduction in symptom scores, but there were only 4 participants in the placebo arm and the development of this drug has not progressed.
TZP-102 was assessed in two randomised, double-blind, placebo-controlled trials. In the smaller trial [96] (n = 92), GE was not accelerated, but symptoms improved—but this finding was not reproduced in a larger study [97] (n = 201) and the development of this drug has not progressed.
Relamorelin was evaluated in two randomised, placebo-controlled trials of participants with T1D or T2D with symptomatic gastroparesis and delayed GE assessed by 13C-Spirulina breath test. One trial [98] (n = 204) was double-blind and associated with accelerated GE and a reduction in symptoms in a subgroup with frequent vomiting. The other trial [99•] (n = 393) was a single-blind study that showed acceleration of GE as assessed by 13C-Spirulina breath test, and a reduction in vomiting frequency. The latter, however, did not differ from the large placebo response. Relamorelin is well tolerated and a phase 3 trial is proceeding.
Interventional Treatment
Invasive treatment options such as use of botulinum toxin, transpyloric stenting, gastric electrical stimulation and surgical interventions have been reported. They have typically been used when symptoms of gastroparesis are refractory to other treatments and are described in another review [100]. A significant limitation of these therapies is the lack of well-controlled trials to establish benefit over sham procedures.
Conclusion
Significant advances have been made in our understanding of the pathophysiology of diabetic gastroparesis which have stimulated the development of novel approaches to management. Highly effective, safe pharmacologic options for diabetic gastroparesis still remain elusive, but there is potential in the newer generation motilin and ghrelin agonists. Given the complex and varied factors involved in the pathogenesis of diabetic gastroparesis, it is likely that specific drugs may be more effective in some individuals than others. Recognition of the effects of the bidirectional relationship between glycaemic control and GE is also important in tailoring individual treatment. Ideally, with a better understanding of gastroparesis and the further development of techniques to assess the underlying abnormality, the approach to treatment will become much more personalised.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yu D, Ramsey FV, Norton WF, Norton N, Schneck S, Gaetano T, et al. The burdens, concerns, and quality of life of patients with gastroparesis. Dig Dis Sci. 2017;62(4):879–93. https://doi.org/10.1007/s10620-017-4456-7.
Jung HK, Choung RS, Locke GR 3rd, Schleck CD, Zinsmeister AR, Szarka LA, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225–33. https://doi.org/10.1053/j.gastro.2008.12.047.
NIDDK. Gastroparesis Clinical Research Consortium (GpCRC). 2019. https://repository.niddk.nih.gov/studies/gpcrc/. Accessed 3 Aug 2019 2019.
Wadhwa V, Mehta D, Jobanputra Y, Lopez R, Thota PN, Sanaka MR. Healthcare utilization and costs associated with gastroparesis. World J Gastroenterol. 2017;23(24):4428–36. https://doi.org/10.3748/wjg.v23.i24.4428.
Syed AR, Wolfe MM, Calles-Escandon J. Epidemiology and diagnosis of gastroparesis in the United States: a population-based study. J Clin Gastroenterol. 2019. https://doi.org/10.1097/MCG.0000000000001231.
Rey E, Choung RS, Schleck CD, Zinsmeister AR, Talley NJ, Locke GR 3rd. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18(1):34–42. https://doi.org/10.5056/jnm.2012.18.1.34.
Bharucha AE, Batey-Schaefer B, Cleary PA, Murray JA, Cowie C, Lorenzi G, et al. Delayed gastric emptying is associated with early and long-term hyperglycemia in type 1 diabetes mellitus. Gastroenterology. 2015;149(2):330–9. https://doi.org/10.1053/j.gastro.2015.05.007.
Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9(1):5–12; quiz e7. doi:https://doi.org/10.1016/j.cgh.2010.09.022.
Samsom M, Roelofs JM, Akkermans LM, van Berge Henegouwen GP, Smout AJ. Proximal gastric motor activity in response to a liquid meal in type I diabetes mellitus with autonomic neuropathy. Dig Dis Sci. 1998;43(3):491–6. https://doi.org/10.1023/a:1018894520557.
Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90(6):1919–25. https://doi.org/10.1016/0016-5085(86)90262-3.
He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121(2):427–34.
Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94(3):859–907. https://doi.org/10.1152/physrev.00037.2013.
Yang S, Wu B, Sun H, Sun T, Han K, Li D et al. Impaired insulin/IGF-1 is responsible for diabetic gastroparesis by damaging myenteric cholinergic neurones and interstitial cells of Cajal. Biosci Rep. 2017;37(5). doi:https://doi.org/10.1042/BSR20170776.
Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9(1):102–8. https://doi.org/10.1016/j.gassur.2004.10.001.
Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41(11):1076–87. https://doi.org/10.1007/s00535-006-1909-8.
Grover M, Bernard CE, Pasricha PJ, Lurken MS, Faussone-Pellegrini MS, Smyrk TC, et al. Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24(6):531–9, e249. https://doi.org/10.1111/j.1365-2982.2012.01894.x.
Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140(5):1575–85 e8. https://doi.org/10.1053/j.gastro.2011.01.046.
Choi KM, Kashyap PC, Dutta N, Stoltz GJ, Ordog T, Shea Donohue T et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138(7):2399–409, 409 e1. doi:https://doi.org/10.1053/j.gastro.2010.02.014.
Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135(6):2055–64, 64 e1–2. doi:https://doi.org/10.1053/j.gastro.2008.09.003.
Bernard CE, Gibbons SJ, Mann IS, Froschauer L, Parkman HP, Harbison S, et al. Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol Motil. 2014;26(9):1275–84. https://doi.org/10.1111/nmo.12389.
Kreuch D, Keating DJ, Wu T, Horowitz M, Rayner CK, Young RL. Gut mechanisms linking intestinal sweet sensing to glycemic control. Front Endocrinol (Lausanne). 2018;9:741. doi:https://doi.org/10.3389/fendo.2018.00741.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. https://doi.org/10.1152/physrev.00034.2006.
Young RL, Chia B, Isaacs NJ, Ma J, Khoo J, Wu T, et al. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes. 2013;62(10):3532–41. https://doi.org/10.2337/db13-0581.
Camilleri M. Gastrointestinal hormones and regulation of gastric emptying. Curr Opin Endocrinol Diabetes Obes. 2019;26(1):3–10. https://doi.org/10.1097/MED.0000000000000448.
Christofides ND. Importance of the jejunal hormone motilin. J Clin Pathol Suppl (Assoc Clin Pathol). 1978;8:51–7. https://doi.org/10.1136/jcp.s1-8.1.51.
Sanger GJ, Wang Y, Hobson A, Broad J. Motilin: towards a new understanding of the gastrointestinal neuropharmacology and therapeutic use of motilin receptor agonists. Br J Pharmacol. 2013;170(7):1323–32. https://doi.org/10.1111/bph.12075.
Achem-Karam SR, Funakoshi A, Vinik AI, Owyang C. Plasma motilin concentration and interdigestive migrating motor complex in diabetic gastroparesis: effect of metoclopramide. Gastroenterology. 1985;88(2):492–9. https://doi.org/10.1016/0016-5085(85)90512-8.
Peeters TL, Muls E, Janssens J, Urbain JL, Bex M, Van Cutsem E, et al. Effect of motilin on gastric emptying in patients with diabetic gastroparesis. Gastroenterology. 1992;102(1):97–101. https://doi.org/10.1016/0016-5085(92)91788-6.
Levin F, Edholm T, Schmidt PT, Gryback P, Jacobsson H, Degerblad M, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91(9):3296–302. https://doi.org/10.1210/jc.2005-2638.
Horowitz M, Maddox AF, Wishart JM, Harding PE, Chatterton BE, Shearman DJ. Relationships between oesophageal transit and solid and liquid gastric emptying in diabetes mellitus. Eur J Nucl Med. 1991;18(4):229–34. https://doi.org/10.1007/bf00186645.
Horowitz M, Jones KL, Rayner CK, Read NW. ‘Gastric’ hypoglycaemia--an important concept in diabetes management. Neurogastroenterol Motil. 2006;18(6):405–7. https://doi.org/10.1111/j.1365-2982.2006.00804.x.
Lysy J, Israeli E, Strauss-Liviatan N, Goldin E. Relationships between hypoglycaemia and gastric emptying abnormalities in insulin-treated diabetic patients. Neurogastroenterol Motil. 2006;18(6):433–40. https://doi.org/10.1111/j.1365-2982.2006.00800.x.
Jones KL, Horowitz M, Wishart MJ, Maddox AF, Harding PE, Chatterton BE. Relationships between gastric emptying, intragastric meal distribution and blood glucose concentrations in diabetes mellitus. J Nucl Med. 1995;36(12):2220–8.
Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33(11):675–80.
MacGregor IL, Gueller R, Watts HD, Meyer JH. The effect of acute hyperglycemia on gastric emptying in man. Gastroenterology. 1976;70(2):190–6.
Samsom M, Akkermans LM, Jebbink RJ, van Isselt H, vanBerge-Henegouwen GP, Smout AJ. Gastrointestinal motor mechanisms in hyperglycaemia induced delayed gastric emptying in type I diabetes mellitus. Gut. 1997;40(5):641–646. doi:https://doi.org/10.1136/gut.40.5.641.
Schvarcz E, Palmer M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113(1):60–6. https://doi.org/10.1016/s0016-5085(97)70080-5.
Izzy M, Lee M, Johns-Keating K, Kargoli F, Beckoff S, Chun K, et al. Glycosylated hemoglobin level may predict the severity of gastroparesis in diabetic patients. Diabetes Res Clin Pract. 2018;135:45–9. https://doi.org/10.1016/j.diabres.2017.10.016.
Reddy S, Ramsubeik K, Vega KJ, Federico J, Palacio C. Do HbA1C levels correlate with delayed gastric emptying in diabetic patients? J Neurogastroenterol Motil. 2010;16(4):414–7. https://doi.org/10.5056/jnm.2010.16.4.414.
Merio R, Festa A, Bergmann H, Eder T, Eibl N, Stacher-Janotta G, et al. Slow gastric emptying in type I diabetes: relation to autonomic and peripheral neuropathy, blood glucose, and glycemic control. Diabetes Care. 1997;20(3):419–23. https://doi.org/10.2337/diacare.20.3.419.
Schvarcz E, Palmer M, Aman J, Lindkvist B, Beckman KW. Hypoglycaemia increases the gastric emptying rate in patients with type 1 diabetes mellitus. Diabet Med. 1993;10(7):660–3. https://doi.org/10.1111/j.1464-5491.1993.tb00141.x.
Russo A, Stevens JE, Chen R, Gentilcore D, Burnet R, Horowitz M, et al. Insulin-induced hypoglycemia accelerates gastric emptying of solids and liquids in long-standing type 1 diabetes. J Clin Endocrinol Metab. 2005;90(8):4489–95. https://doi.org/10.1210/jc.2005-0513.
Sharma D, Morrison G, Joseph F, Purewal TS, Weston PJ. The role of continuous subcutaneous insulin infusion therapy in patients with diabetic gastroparesis. Diabetologia. 2011;54(11):2768–70. https://doi.org/10.1007/s00125-011-2282-6.
Calles-Escandon J, Koch KL, Hasler WL, Van Natta ML, Pasricha PJ, Tonascia J, et al. Glucose sensor-augmented continuous subcutaneous insulin infusion in patients with diabetic gastroparesis: an open-label pilot prospective study. PLoS One. 2018;13(4):e0194759. https://doi.org/10.1371/journal.pone.0194759.
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603. https://doi.org/10.2337/dci19-0028.
Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LM, Chatterton BE, et al. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32(3):151–9.
Shao ZM, Yao JF, Chen J, Yu ZW, Yu XF, Zheng JJ, et al. Effects of rehabilitation management on gastric emptying function in older adults with diabetes. Genet Mol Res. 2014;13(4):9244–52. https://doi.org/10.4238/2014.January.24.5.
Bharucha AE, Kudva Y, Basu A, Camilleri M, Low PA, Vella A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol. 2015;13(3):466–76 e1. https://doi.org/10.1016/j.cgh.2014.06.034.
Holzapfel A, Festa A, Stacher-Janotta G, Bergmann H, Shnawa N, Brannath W, et al. Gastric emptying in type II (non-insulin-dependent) diabetes mellitus before and after therapy readjustment: no influence of actual blood glucose concentration. Diabetologia. 1999;42(12):1410–2. https://doi.org/10.1007/s001250051311.
Laway BA, Malik TS, Khan SH, Rather TA. Prevalence of abnormal gastric emptying in asymptomatic women with newly detected diabetes and its reversibility after glycemic control-a prospective case control study. J Diabetes Complicat. 2013;27(1):78–81. https://doi.org/10.1016/j.jdiacomp.2012.08.001.
Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161(16):1989–96.
Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97(3):604–11. https://doi.org/10.1111/j.1572-0241.2002.05537.x.
Parthasarathy G, Kudva YC, Low PA, Camilleri M, Basu A, Bharucha AE. Relationship between gastric emptying and diurnal glycemic control in type 1 diabetes mellitus: a randomized trial. J Clin Endocrinol Metab. 2017;102(2):398–406. https://doi.org/10.1210/jc.2016-2809.
Perano SJ, Rayner CK, Kritas S, Horowitz M, Donaghue K, Mpundu-Kaambwa C, et al. Gastric emptying is more rapid in adolescents with type 1 diabetes and impacts on postprandial glycemia. J Clin Endocrinol Metab. 2015;100(6):2248–53. https://doi.org/10.1210/jc.2015-1055.
Deane AM, Nguyen NQ, Stevens JE, Fraser RJ, Holloway RH, Besanko LK, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab. 2010;95(1):215–21. https://doi.org/10.1210/jc.2009-1503.
Little TJ, Pilichiewicz AN, Russo A, Phillips L, Jones KL, Nauck MA, et al. Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: relationships with postprandial glycemic and insulinemic responses. J Clin Endocrinol Metab. 2006;91(5):1916–23. https://doi.org/10.1210/jc.2005-2220.
Umapathysivam MM, Lee MY, Jones KL, Annink CE, Cousins CE, Trahair LG, et al. Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide 1 receptor on gastric emptying and glycemia. Diabetes. 2014;63(2):785–90. https://doi.org/10.2337/db13-0893.
Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60(5):1561–5. https://doi.org/10.2337/db10-0474.
Acosta A, Camilleri M, Burton D, O’Neill J, Eckert D, Carlson P et al. Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiol Rep. 2015;3(11). doi:https://doi.org/10.14814/phy2.12610.
Lorenz M, Pfeiffer C, Steinstrasser A, Becker RH, Rutten H, Ruus P, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes--relationship to postprandial glycemia. Regul Pept. 2013;185:1–8. https://doi.org/10.1016/j.regpep.2013.04.001.
Jones KL, Rigda RS, Buttfield MDM, Hatzinikolas S, Pham HT, Marathe CS, et al. Effects of lixisenatide on postprandial blood pressure, gastric emptying and glycaemia in healthy people and people with type 2 diabetes. Diabetes Obes Metab. 2019;21(5):1158–67. https://doi.org/10.1111/dom.13633.
Meier JJ, Rosenstock J, Hincelin-Mery A, Roy-Duval C, Delfolie A, Coester HV, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open-label trial. Diabetes Care. 2015;38(7):1263–73. https://doi.org/10.2337/dc14-1984.
Halawi H, Khemani D, Eckert D, O’Neill J, Kadouh H, Grothe K, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2(12):890–9. https://doi.org/10.1016/S2468-1253(17)30285-6.
Jones KL, Huynh LQ, Hatzinikolas S, Rigda RS, Phillips L, Pham HT, et al. Effect of exenatide once-weekly (QW) on gastric emptying in health: impact on glycaemia and glucose absorption [abstract]. Diabetologia. 2019;62(Suppl):S377.
Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, Blundell J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes Metab. 2018;20(3):610–9. https://doi.org/10.1111/dom.13120.
Stevens JE, Horowitz M, Deacon CF, Nauck M, Rayner CK, Jones KL. The effects of sitagliptin on gastric emptying in healthy humans-a randomised, controlled study. Aliment Pharmacol Ther. 2012;36(4):379–90. https://doi.org/10.1111/j.1365-2036.2012.05198.x.
Vella A, Bock G, Giesler PD, Burton DB, Serra DB, Saylan ML, et al. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes. 2007;56(5):1475–80. https://doi.org/10.2337/db07-0136.
Stevens JE, Buttfield M, Wu T, Hatzinikolas S, Pham H, Lange K, et al. Effects of sitagliptin on gastric emptying of, and the glycaemic and blood pressure responses to, a carbohydrate meal in type 2 diabetes. Diabetes Obes Metab. 2019. https://doi.org/10.1111/dom.13864.
Nonaka T, Sekino Y, Iida H, Yamada E, Ohkubo H, Sakai E, et al. Early effect of single-dose sitagliptin administration on gastric emptying: crossover study using the (13)C breath test. J Neurogastroenterol Motil. 2013;19(2):227–32. https://doi.org/10.5056/jnm.2013.19.2.227.
Nauck MA, Kind J, Kothe LD, Holst JJ, Deacon CF, Broschag M, et al. Quantification of the contribution of GLP-1 to mediating insulinotropic effects of DPP-4 inhibition with vildagliptin in healthy subjects and patients with type 2 diabetes using exendin [9-39] as a GLP-1 receptor antagonist. Diabetes. 2016;65(8):2440–7. https://doi.org/10.2337/db16-0107.
Wu T, Bound MJ, Zhao BR, Standfield SD, Bellon M, Jones KL, et al. Effects of a D-xylose preload with or without sitagliptin on gastric emptying, glucagon-like peptide-1, and postprandial glycemia in type 2 diabetes. Diabetes Care. 2013;36(7):1913–8. https://doi.org/10.2337/dc12-2294.
Aulinger BA, Bedorf A, Kutscherauer G, de Heer J, Holst JJ, Goke B, et al. Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes using DPP-4 inhibition and GLP-1 receptor blockade. Diabetes. 2014;63(3):1079–92. https://doi.org/10.2337/db13-1455.
Samsom M, Szarka LA, Camilleri M, Vella A, Zinsmeister AR, Rizza RA. Pramlintide, an amylin analog, selectively delays gastric emptying: potential role of vagal inhibition. Am J Physiol Gastrointest Liver Physiol. 2000;278(6):G946–51. https://doi.org/10.1152/ajpgi.2000.278.6.G946.
Kong MF, King P, Macdonald IA, Stubbs TA, Perkins AC, Blackshaw PE, et al. Infusion of pramlintide, a human amylin analogue, delays gastric emptying in men with IDDM. Diabetologia. 1997;40(1):82–8. https://doi.org/10.1007/s001250050646.
Kong MF, Stubbs TA, King P, Macdonald IA, Lambourne JE, Blackshaw PE, et al. The effect of single doses of pramlintide on gastric emptying of two meals in men with IDDM. Diabetologia. 1998;41(5):577–83. https://doi.org/10.1007/s001250050949.
Hinshaw L, Schiavon M, Dadlani V, Mallad A, Dalla Man C, Bharucha A, et al. Effect of Pramlintide on postprandial glucose fluxes in type 1 diabetes. J Clin Endocrinol Metab. 2016;101(5):1954–62. https://doi.org/10.1210/jc.2015-3952.
Linnebjerg H, Park S, Kothare PA, Trautmann ME, Mace K, Fineman M, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept. 2008;151(1–3):123–9. https://doi.org/10.1016/j.regpep.2008.07.003.
Olausson EA, Storsrud S, Grundin H, Isaksson M, Attvall S, Simren M. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol. 2014;109(3):375–85. https://doi.org/10.1038/ajg.2013.453.
McCallum RW, Ricci DA, Rakatansky H, Behar J, Rhodes JB, Salen G, et al. A multicenter placebo-controlled clinical trial of oral metoclopramide in diabetic gastroparesis. Diabetes Care. 1983;6(5):463–7. https://doi.org/10.2337/diacare.6.5.463.
Ricci DA, Saltzman MB, Meyer C, Callachan C, McCallum RW. Effect of metoclopramide in diabetic gastroparesis. J Clin Gastroenterol. 1985;7(1):25–32.
Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127(5):1592–622.
Al-Saffar A, Lennernas H, Hellstrom PM. Gastroparesis, metoclopramide, and tardive dyskinesia: risk revisited. Neurogastroenterol Motil. 2019:e13617. doi:https://doi.org/10.1111/nmo.13617.
Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray is effective in symptoms of gastroparesis in diabetics compared to conventional oral tablet. Neurogastroenterol Motil. 2014;26(4):521–8. https://doi.org/10.1111/nmo.12296.
McCallum RW, Valenzuela G, Polepalle S, Spyker D. Subcutaneous metoclopramide in the treatment of symptomatic gastroparesis: clinical efficacy and pharmacokinetics. J Pharmacol Exp Ther. 1991;258(1):136–42.
Heckert J, Parkman HP. Therapeutic response to domperidone in gastroparesis: a prospective study using the GCSI-daily diary. Neurogastroenterol Motil. 2018;30(1). doi:https://doi.org/10.1111/nmo.13246.
Larson JM, Tavakkoli A, Drane WE, Toskes PP, Moshiree B. Advantages of azithromycin over erythromycin in improving the gastric emptying half-time in adult patients with gastroparesis. J Neurogastroenterol Motil. 2010;16(4):407–13. https://doi.org/10.5056/jnm.2010.16.4.407.
Janssens J, Peeters TL, Vantrappen G, Tack J, Urbain JL, De Roo M, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies N Engl J Med. 1990;322(15):1028–31. https://doi.org/10.1056/NEJM199004123221502.
McCallum RW, Cynshi O. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis-a randomized, multicentre, placebo-controlled study. Aliment Pharmacol Ther. 2007;26(8):1121–30. https://doi.org/10.1111/j.1365-2036.2007.03461.x.
McCallum RW, Cynshi O. Efficacy of mitemcinal, a motilin agonist, on gastrointestinal symptoms in patients with symptoms suggesting diabetic gastropathy: a randomized, multi-center, placebo-controlled trial. Aliment Pharmacol Ther. 2007;26(1):107–16. https://doi.org/10.1111/j.1365-2036.2007.03346.x.
Hellstrom PM, Tack J, Johnson LV, Hacquoil K, Barton ME, Richards DB, et al. The pharmacodynamics, safety and pharmacokinetics of single doses of the motilin agonist, camicinal, in type 1 diabetes mellitus with slow gastric emptying. Br J Pharmacol. 2016;173(11):1768–77. https://doi.org/10.1111/bph.13475.
Tack J, Rotondo A, Meulemans A, Thielemans L, Cools M. Randomized clinical trial: a controlled pilot trial of the 5-HT4 receptor agonist revexepride in patients with symptoms suggestive of gastroparesis. Neurogastroenterol Motil. 2016;28(4):487–97. https://doi.org/10.1111/nmo.12736.
Giudicessi JR, Ackerman MJ, Camilleri M. Cardiovascular safety of prokinetic agents: a focus on drug-induced arrhythmias. Neurogastroenterol Motil. 2018;30(6):e13302. https://doi.org/10.1111/nmo.13302.
Carbone F, Rotondo A, Andrews CN, Holvoet L, Van Oudenhove L, Vanuytsel T, et al. A controlled cross-over trial shows benefit of prucalopride for symptom control and gastric emptying enhancement in idiopathic gastroparesis. Gastroenterology. 2016;150(4):S213–S4. https://doi.org/10.1016/s0016-5085(16)30792-2.
. Abell T, Kuo B, Esfandyari T, Canafax D, Camerini R, Grimaldi M et al. Velusetrag improves gastoparesis both in symptoms and gastric emptying in patients with diabetic or idiopathic gastroparesis in a 12-week global phase 2B study. Gastroenterology. 2019;156(6):S-164. Doi:https://doi.org/10.1016/s0016-5085(19)37201-4. The study reports the results of a newer pharmacologic agent for gastroparesis of which larger studies are in process.
Wo JM, Ejskjaer N, Hellstrom PM, Malik RA, Pezzullo JC, Shaughnessy L, et al. Randomised clinical trial: ghrelin agonist TZP-101 relieves gastroparesis associated with severe nausea and vomiting--randomised clinical study subset data. Aliment Pharmacol Ther. 2011;33(6):679–88. https://doi.org/10.1111/j.1365-2036.2010.04567.x.
Ejskjaer N, Wo JM, Esfandyari T, Mazen Jamal M, Dimcevski G, Tarnow L, et al. A phase 2a, randomized, double-blind 28-day study of TZP-102 a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013;25(2):e140–50. https://doi.org/10.1111/nmo.12064.
McCallum RW, Lembo A, Esfandyari T, Bhandari BR, Ejskjaer N, Cosentino C, et al. Phase 2b, randomized, double-blind 12-week studies of TZP-102, a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013;25(11):e705–17. https://doi.org/10.1111/nmo.12184.
Lembo A, Camilleri M, McCallum R, Sastre R, Breton C, Spence S, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology. 2016;151(1):87–96. https://doi.org/10.1053/j.gastro.2016.03.038.
. Camilleri M, McCallum RW, Tack J, Spence SC, Gottesdiener K, Fiedorek FT. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo-controlled study. Gastroenterology. 2017;153(5):1240-50 e2. Doi:https://doi.org/10.1053/j.gastro.2017.07.035. The study reports the results of a newer pharmacologic agent for diabetic gastroparesis of which larger studies are in process.
Krishnasamy S, Abell TL. Diabetic gastroparesis: principles and current trends in management. Diabetes Ther. 2018;9(Suppl 1):1–42. https://doi.org/10.1007/s13300-018-0454-9.
Author information
Authors and Affiliations
Contributions
RJ is the corresponding author for the manuscript. All authors contributed to the design and drafting the paper and reviewed and approved the manuscript for scholarly content.
Corresponding author
Ethics declarations
Conflict of Interest
Ryan Jalleh and Chinmay S. Marathe declare that they have no conflict of interest. Christopher K. Rayner has received research funding from Merck, Eli Lilly, Sanofi, AstraZeneca and Novartis. Karen L. Jones has received research funding from Sanofi-Aventis and AstraZeneca. Michael Horowitz has participated in advisory boards and/or symposia for Novo Nordisk, Sanofi-Aventis, Novartis, Eli Lilly, Merck Sharp & Dohme, Boehringer Ingelheim and AstraZeneca and received honoraria.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Other Forms of Diabetes and Its Complications
Rights and permissions
About this article
Cite this article
Jalleh, R., Marathe, C.S., Rayner, C.K. et al. Diabetic Gastroparesis and Glycaemic Control. Curr Diab Rep 19, 153 (2019). https://doi.org/10.1007/s11892-019-1281-8
Published:
DOI: https://doi.org/10.1007/s11892-019-1281-8