Abstract
The concept of lower is better when considering the goal for glycemic control in patients with diabetes mellitus has recently been challenged due to recent studies, such as ACCORD, ADVANCE, and VADT, which have observed increased morbidity and mortality from intensive control, especially in older adults, and in those with long duration of diabetes disease and chronic complications. Although evidence in younger patients suggest that blood glucose levels should not be above 180 mg/dl (10.0 mmol/l), there are many unanswered questions and controversies regarding the benefits and risks, methods to achieve and maintain these levels while avoiding hypoglycemia (<70 mg% (3.9 mmol/l)) in the older population. Since the population is aging with a greater life expectancy, it is crucial that these questions be answered. Although several studies of inpatient non-ICU diabetes management have been published, few include older patients. This review will examine available recommendations and explore those controversies regarding non-ICU hospital management in this vulnerable patient population. Additional conditions that impact upon achieving glycemic control will also be discussed. Finally, the older individual has many special needs which may be more important to consider than in young or middle-aged individuals, when transitioning care from in-hospital to home in a patient-centered approach, as recommended by the American Diabetes Association (ADA) and European Society for the Study of Diabetes (EASD).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes Mellitus, a common medical disorder, which affects the older population, can result in chronic complications of retinopathy, nephropathy, neuropathy, peripheral vascular disease, and cardiovascular disorders [1]. This condition and its comorbidities have significant economic impacts on the individual, as well as society. Since, the population is aging and life expectancies are longer, elderly diabetic care is becoming more important to understand than previously. Despite the increasing number of older individuals with diabetes mellitus, few studies have focused upon management principles and requirements in the non-ICU hospital setting. One of the major reasons is the marked heterogeneity of functional status among these individuals. Physiological changes which occur with aging make it difficult to assume that the data obtained from studies of younger patients equally apply to the older population. Thus, there are many unanswered questions regarding recommendations for diabetes treatment of older adults in acute non-ICU hospital settings. Further, the principle of patient-centered diabetes care becomes most important in this age group. This review will highlight important considerations in the management of older adults with diabetes in noncritical care hospital settings.
Incidence
Currently, in the USA, >25 % of the population 65 years and older have diabetes mellitus versus 11.3 % of people aged 20 years or older [2, 3]. Although type 2 diabetes is due to increasing rates of overweight and obesity, the prevalence is expected to double in the next 20 years due to the aging of the population, even if diabetes incidence rates level off [4]. Further, the number of cases of diagnosed diabetes in those aged 65 years or more are expected to increase 4.5 times (compared to three-fold in the total population) from 2005 to 2050 [5]. The incidence of type 1 diabetes is also increasing by 2–5 % per year worldwide [6, 7, 8••]. About 25 % of type 1 diabetics are diagnosed as adults, and due to increasing longevity, patients may even be newly diagnosed in their nineties [9]. In addition, due to improved diabetes management, patients with type 1 diabetes are living into the later decades of life and earlier onset can be associated with a longer duration of disease and more diabetes-related complications. It is estimated that approximately 1/3 of older adults are undiagnosed, since postprandial hyperglycemia is more common in older adults with type 2 diabetes mellitus, it may be missed by standard tests for glycosylated hemoglobin or fasting plasma glucose [3, 10–12].
Demographic and clinical characteristics also differ by ethnicity and genetic heterogeneities in the population in the USA as well as the rest of the world due to increasing mobility. Thus, the one size-fits-all approach is no longer acceptable.
Characteristics of Diabetes Mellitus in the Older Population
In addition to having the highest prevalence of diabetes, this age group also has more comorbidities, acute microvascular and cardiovascular complications, and is associated with higher mortality, reduced functional status, and increased risk for institutionalization in hospital and long-term care settings [13]. Retinopathy is more common in older adults with middle-age onset diabetes than in those with older-age onset, but the prevalence of cardiovascular disease (CVD) and peripheral neuropathy do not differ by age of onset [14]. This age group also has the highest rates of major lower-extremity amputation, myocardial infarction (MI), visual impairment, and end-stage renal disease than in any other age group [15]. Further, diabetic individuals >75 years have higher rates of most complications than those 65–74 years. Deaths from hyperglycemia are also significantly higher in older adults. Studies in type 1 diabetics have shown that the incidence of diabetic ketoacidosis (DKA) was lower with increasing age and was not associated with duration of diabetes [16].
Economic Burden
The economic impact of this disease in older adults is enormous. Data from an analysis of 2010 show that more than 14 million hospital stays among adults ages 65 years and older account for more than one-third of all US community hospital stays [17]. In 2012, estimates (from various data sources, such as national surveys, medical standard analytical files, and claims databases for the commercially insured population in USA) suggested that the total cost of diabetes in the USA was approximately $245 billion, with $176 billion for direct medical costs and $69 billion in reduced productivity and mortality [18]. Furthermore, approximately 59 % of all health care expenditure attributed to diabetes is for health resources used by the older population, most of which is paid by Medicare programs. A recent study observed higher utilization of health resources for hospital inpatient days, nursing home and residential facility days, as well as for prescription medication use among this group. This excess expenditure has been estimated to be from $23,900–$40,900, depending upon sex and age at diagnosis [19]. Although recent estimates and trends suggest that newly diagnosed diabetics can be expected to add $4.6 billion to future medial spending, this population has often been excluded from randomized controlled trials (RCT) regarding interventions as well as treatment targets [19].
Pathophysiology of Aging
Progressive glucose intolerance due to age-related changes occurs from the following: (1) decreased insulin secretion due to decreases in pancreatic islet function and islet proliferative capacity, (2) impaired hepatic glucose metabolism, and (3) increases in insulin resistance [20–23]. In addition, insulin signaling mechanisms limit mobilization of glucose transporters needed for insulin-mediated glucose uptake and impaired metabolism in muscle and fat [24]. Aging is also associated with low-grade inflammation. There are higher levels of tumor necrosis factor, TNFα, and IL-6 from uncontrolled diabetes and increased morbidity [25]. This inflammatory environment contributes to the higher rates of diabetes in the elderly [26]. In addition, hyperglycemia leads to further generation of reactive oxygen species (ROS), lipid peroxidation, elevated cardiovascular inflammatory markers, and also increases proinflammatory cytokine such as tumor necrosis factor-α (TNFα), interleukin (IL)-6, and IL-1, which ultimately alter the immune system [27, 28]. TNFα mediates insulin resistance. Increased glycosylation from high glucose levels, as well as from aging, produce cross-linked products to form advanced glycosylation end products, which are partly responsible for abnormal cellular function [29]. In addition, insulin action may be further impaired due to obesity, sarcopenia, and reduced physical activity [24, 26]. Abdominal obesity is also associated with increased levels of free fatty acids, inflammatory cytokines, and multifunctional chemoattractant proteins, leptin, and osteopontin [25]. Further contributors to the abnormal glucose tolerance may occur from iatrogenic causes, such as (1) medications that have adverse effects on carbohydrate metabolism (such as: diuretics, olanzapine, sympathomimetics, glucocorticoids, and niacin); (2) increased risks for infections, myocardial infarctions, or strokes; and (3) decreases in physiological reserves, interacting with diabetes-related end-organ damage which can cause increased vulnerability to physiological stressors [24, 30].
Diagnostic Dilemma
Symptoms of hyperglycemia in the elderly are often atypical, not easily recognized. They may include: weight loss, fatigue, infections, impaired wound healing, neuropathic pain, nocturia, failure to thrive, falls, or even nonketotic hyperosmolar coma which can delay appropriate diagnosis and treatment [1]. Dehydration due to impaired thirst thresholds in older adult may occur. In addition, the renal threshold for glucose increases with age and glycosuria may not be present [31••]. Factors such as cognitive dysfunction, visual changes, depression, physical disabilities, polypharmacy, social and financial issues, and chronic pain, may further contribute to the reason for metabolic decompensation [31••, 32•].
Glycemic Control Trials
After the Van Den Berghe study of 2001 of intensive inpatient glycemic control for critically ill patients in the surgical intensive care unit, which showed a reduction in mortality from 8 to 4.6 % for blood glucose levels blood levels at or below 110 mg/dl (6.1 mmol/l), it was believed that inpatient blood sugars needed to be very tightly controlled [33]. Further trials of critically ill, general medicine, and surgical patients confirmed a reduction in hospital complications [34–36]. However, recent ICU trials and meta-analyses such as VISEP, NICE Sugar, and GLUCONTROL of 21 centers observed that intensified insulin therapy increased the risk of severe hypoglycemia, which is also associated with increased morbidity and mortality [34–37]. These studies did not focus upon elderly patients in a non-ICU hospital setting.
Long-term trials, such as DPP and UKPDS, showed the benefits of glycemic control for younger patients [38–41]. Although the importance of glycemic control was proven for younger individuals, these studies did not enroll a significant number of older patients, and were not designed for this group with poor health status. However, it was assumed that the conclusions could be equally applicable to older individuals.
Three major prospective randomized controlled long-term outpatient trials focusing upon middle-aged patients (the Action to Control Cardiovascular Risk in Diabetes [ACCORD] trial, the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation [ADVANCE] trial, and the Veterans Affairs Diabetes Trial [VADT]) failed to demonstrate improvement in cardiovascular (CV) outcomes in patients with type 2 diabetes [42, 43, 44••, 45–47]. The results suggested that there are possible adverse effects from tight glycemic control. Further subgroup analyses concluded that older patients treated with intensive control and multiple comorbidities, longer duration of diabetes, and those with preexisting macro and/or microvascular disease were more likely to be adversely affected. Other long-term studies of middle-aged patients, such as The UK General Practice Research Database showed that intensification was associated with a U-shaped relationship for all-cause mortality and CV events [48]. A longitudinal, observational study of Italian patients reported that an A1C of 6.5 or <7 % at baseline was associated with lower 5-year CV events in the low-to-moderate comorbidity subgroup, but not in the high comorbidity subgroup [49]. The results of these trials add to the controversy regarding the benefits and risks of more intensive treatment of hyperglycemia for older adults. Although these studies focused upon ambulatory older patients, it is not clear whether these same results can be translated to an inpatient setting for the elderly population. Furthermore, these studies also raised concerns about potential risks to intensive glycemic control which may outweigh benefits in elderly individuals who often have longer duration of diabetes, high risks for hypoglycemia, advanced complications, shorter life expectancy due to aging and malignancies, cognitive as well as other physical disabilities, and frailties.
Hypoglycemia
The exact relationship between hypoglycemia and mortality is unclear. Two recent studies reported that hypoglycemia (blood glucose <70 mg/dl (3.9 mmol/l)) was associated with an increased mortality risk in critically ill and ICU patients, independent of their diabetes status [50]. In contrast, a recent meta-analysis evaluating clinical studies in hospitalized patients, observed a small reduction in mortality in hospitalized patients, but there was no effect on ICU mortality [51].
Mechanisms Contributing to Morbidity and Mortality Associated with Hypoglycemia
Insulin-induced hypoglycemia has been associated with increases in C-reactive protein and proinflammatory cytokines (TNFα, IL-1β, IL-6, IL-8, endothelin-1), markers of lipid peroxidation, ROS, and leukocytosis [52]. Increases in these cytokines can lead to endothelial injury, as well as abnormalities in coagulation, both of which can result in an increased risk for cardiovascular events [53, 54]. In addition, cytokine IL increases the severity of hypoglycemia, leading to a positive feedback cycle [54]. Increases in epinephrine can cause increased platelet activation, leukocyte mobilization, and blood coagulability [55]. Acute hypoglycemia also causes this prothrombotic environment, due to increased levels of vasoconstrictors, platelet aggregation, and endothelial dysfunction. Endogenous insulin secretion is decreased with an increase in glucagon during an episode of hypoglycemia [56]. In addition, the increased sympathoadrenal response also results in increased levels of epinephrine and norepinephrine, as well as increased levels of ACTH and cortisol [56]. The increase in catecholamines causes increases of heart rate, abnormal cardiac repolarization, and increases in myocardial contractility, myocardial workload, and cardiac output, which can lead to angina and myocardial ischemia [57, 58]. These abnormalities can also contribute to the increased risk of CV events with severe hypoglycemia, especially when they occur in patients with preexisting coronary artery disease, longer duration of diabetes, or severe autonomic neuropathy [57–60]. These effects, in turn can contribute to increased cardiovascular morbidity and mortality [58].
Hypoglycemia can also cause changes in EKGs, leading to a higher risk of arrhythmias. This increase in catecholamines with lower levels of serum potassium during hypoglycemia can enhance the arrhythmogenic effects. Further, hypoglycemia due to hyperinsulinemia has been associated with abnormal atrioventricular conduction, ventricular depolarization, and repolarization, as well as prolongation of QT intervals [57–60].
Further, age impairs counter-regulatory responses with symptoms of cognitive dysfunction, and abnormal psychomotor coordination in response to hypoglycemia [61, 62]. The presenting symptoms most often are those of neuroglycopenia, such as dizziness, confusion, weakness, vertigo, and TIA’s, rather than adrenergic (tremors, sweating, palpitations) [61, 62]. Brain damage may also occur. Thus, the older patient may experience increased falls and resulting fractures, confusion, dizziness, weakness, poor coordination and balance, or cerebrovascular events.
Incidence of Hypoglycemia
Hypoglycemia in the inpatient setting is a common occurrence with potentially harmful consequences. In both inpatient and outpatient settings, large trials have found a correlation between hypoglycemia with morbidity and mortality. Although the incidence of inpatient hypoglycemia has been reported to range from 0.5 to 32.8 %, this is misleading, since the true incidence is difficult to assess due to lack of standardized definitions (blood glucose level of <40 mg/dl (2.2 mmol/l) versus the ADA definition of hypoglycemia which is any blood glucose level of <70 mg/dl (3.9 mmol/l)), which correlates with the threshold for release of counter-regulatory hormones [36, 37, 44••, 45, 63, 64••, 65, 66, 67••, 68]. There are varying methods data collection and reporting among hospital settings. Risk factors that predispose to hypoglycemia involve changes in clinical status, diet, and medication regimens, as well as breakdown of hospital processes.
Risk Factors for Inpatient Hypoglycemia
Although the cause of hypoglycemia in the hospital is generally due to treatment of hyperglycemia, some conditions predispose patients to hypoglycemia that are unrelated to the treatment of hyperglycemia. As shown by studies of Kosiborod, and that of Boucai, inpatient hypoglycemia may also be a marker of illness severity, rather than a cause of an adverse event or insulin treatment [69, 70]. Hypoglycemic episodes also increase costs due to increased length of stays, increased number of venipunctures, increased number of point-of-care glucose values, increased number of intravenous dextrose solutions, and increased nursing time. Risk factors for inpatient hypoglycemia in patients with diabetes also include: (1) patient factors; (2) nutrition factors, and (3) system issues.
Patient Factors
Underlying illnesses often lead to changes in insulin sensitivity, as in acute renal failure, sepsis, and change in medications such as beta-adrenergic agonists, quinolone, antibiotics, vasopressors, and changing doses of corticosteroids [71, 72]. In hospital use of long-acting sulfonylureas, such as glyburide, can cause hypoglycemia, especially in elderly patients with renal or hepatic insufficiency [72]. In addition, patients with an increased risk of hypoglycemia include those with a long duration of diabetes, low BMI, malnutrition, hemoglobin A1c <7 %, previous cardiovascular events, insulin treatment at baseline, advanced age, and a higher albumin-to-creatinine ratio [71]. It should be noted that in general, there is less risk of hypoglycemia in type 2 diabetes, as compared to those with type 1 [73].
Nutrition Factors
Hypoglycemia often occurs when a fixed dose of mealtime (prandial) insulin is administered despite erratic oral intake, missed meals, or lack of coordination between meal trays and blood glucose monitoring, interruption of enteral feedings or parenteral nutrition with lack of glucose replacement, inadequate adjustment of insulin when feedings are held prior to procedures, for elevated gastric residuals, or loss of intravenous or enteral access [67••, 71–73, 74••].
System Issues
Systems need to be designed considering issues of patient safety, quality control, multidisciplinary communication, and transitions of care. In recent years, there have been a large number of studies that illustrate the usefulness of system-wide approaches and protocols to prevent hypoglycemia, as well as root cause analyses [74••]. In addition, hypoglycemia unawareness, inadequate frequency of glucose monitoring in the inpatient setting, can lead to underestimation of the true rates of hypoglycemia. There are controversies regarding glucometrics, such as measurement and accuracies of bedside POCT versus plasma glucose. Further problems include: (1) failures to evaluate trends in blood glucose values, (2) lack of recognition of individual insulin sensitivities, (3) failure and delay to make appropriate changes in glycemic management, (4) use of sliding scale insulin as sole treatment for hyperglycemia, (5) insufficient glucose with insulin for the acute treatment of hyperkalemia, (6) hypoglycemia due to enteral tube feeds or parenteral nutrition, when different teams give nutrition orders that are not coordinated or communicated with those for insulin administration, (7) avoiding hypo or hyperglycemia when patients are receiving corticosteroids in varying doses, when adjustments of insulin are not adequately anticipated, and (8) others, such as nursing errors, communication errors, and lack of hospital policies [67••, 71–73, 74••]. Other obstacles to glycemic control and contributions to the occurrence of hypoglycemia also include differing levels of knowledge and training of staff, poor communication between various teams with conflicting glycemic orders, lack of standardized protocols or order sets, and during the transitions of care from inpatient to outpatient [74••].
Principles of Non-ICU Acute Hospital Management
Approximately, 38–46 % of non-ICU hospitalized patients have diabetes mellitus, either with or without a prior diagnosis. Hyperglycemia is associated with an increased risk of complications, increased lengths of stays, and with each 18 mg/dl increase in the admitting fasting plasma glucose, there is an associated increase in mortality by 33 % [75–77]. Furthermore, hyperglycemia is also associated with complications, regardless of whether the glucose level upon admission is elevated or the mean glucose level is elevated during the hospital stay. With new-onset or stress-induced hyperglycemia, there is a higher risk of complications than in those patients with a known diagnosis of diabetes.
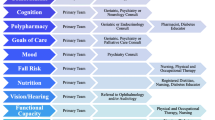
Few studies have focused upon the elderly population in the inpatient setting. Additional management requirements when treating this group include unique nutritional, behavioral, pharmacologic, and other interventions, as well as monitoring for increased risks of hypo- and hyperglycemia. The ADA recommends that upon admission, all patients be assessed for a history of diabetes, and, if previously known, the type of diabetes [78]. This should be documented in the medical record. Evaluation should also include screening and interventions for acute and chronic diabetic complications, and all in patients with hyperglycemia or diabetes should have a hemoglobin A1c measured, if not performed within the last 2–3 months [78]. For most patients, oral hypoglycemic agents should be discontinued at the time of admission [78]. Patients previously taking insulin at home need to have their dose re-evaluated for adequacy during hospitalization, as well as for discharge planning. It is also important to avoid the use of sliding scale insulin therapy as a substitute for proper management [79]. Supplemental insulin protocols should be used with extra caution at night, in order to avoid the increased incidence of nocturnal hypoglycemia. Special considerations need to be given to diabetes assessment and management of the older patient, since acute changes in cognitive function may occur during and after hospitalization, and may require more supervision to avoid errors in dosing after discharge. Team management is most important for coordinating proper diabetes management, and consists of physicians, diabetes nurse educators, advanced nurse practitioners, physician assistants, pharmacists, dietitians, social workers, and others involved in their care. In addition, communication between physicians, nursing staff, and dietary services is essential. Information about orders for nutrition, point-of-care glucose testing, and insulin all need to be communicated regularly to the patient care team [78]. Considerations should also be given to adequate control of blood pressure, lipids, and the use of aspirin, especially for those patients at high risk for CV disease (Table 1).
Nutrition
The elderly often have unique nutritional needs. There is a physiologic decrease in taste and olfactory sensations, and changes in thirst thresholds with aging. Further, the elderly may present in a malnourished rather than obese state. They often skip meals and are anorexic due to cognitive disorders, swallowing difficulties, gastrointestinal disorders, loneliness, and depression [65]. Relaxation of restriction of calories, salt, or sugar content may be required in order to increase palatability. Consideration of a soft or pureed diet, and assistance with feeding may be required in cases of cognitive dysfunction, cerebrovascular accidents, or dental disorders. Other factors, such as inadequate nutrient content and disruptions in timing of meals, can predispose patients to hypoglycemia.
Therefore, it is important to provide a well-balanced and palatable diet, where meal delivery is matched to prandial insulin. Carbohydrate amount may be used to calculate prandial insulin doses. However, we often find that it is easier for nursing staff to estimate the prandial insulin based upon the amount of the meal that is consumed; e.g., less insulin when less of the meal is eaten, such as 50 % of the calculated insulin dose, if half of the meal is consumed, 25 % if less, or no prandial insulin, if the patient does not eat or is NPO or a procedure is planned, rather than the complex carbohydrate counting system. Often, this is best determined after the tray is inspected and the prandial insulin is administered immediately after the meal. In patients receiving enteral or parenteral nutrition, glucose monitoring should be done every 4–6 h and individualized for controlling hyperglycemia during feedings while avoiding hypoglycemia, if there are interrupted feedings [80].
Standard Protocols
Structured insulin order sets and management algorithms improve not only rates of hyperglycemia, as well as decrease the rates of hypoglycemia [68, 82]. Protocols should be used for (1) continuous intravenous insulin infusions, (2) subcutaneous insulin (involving basal insulin, prandial insulin, and corrective insulin), (3) transitioning from intravenous to subcutaneous insulin, and (4) nurse-driven hypoglycemia treatment [71, 72]. Although Quality Improvement Programs are required for tracking the frequency and severity of hypoglycemia, there are no standardized protocols [74••].
Glucose monitoring should match the patient’s medication regimen and nutritional intake. Hypoglycemia can suggest clinical deterioration [69, 70, 77]. In the majority of patients who are eating, blood glucose monitoring needs to be performed before meals and at bedtime. Patients that are NPO should have their glucose monitored every 4–6 h [71, 72]. Any glucose value that does not correlate with the patient’s clinical status should be confirmed with a laboratory plasma glucose measurement. Blood glucose monitored every 30 min to 2 h, needs to be performed in patients receiving intravenous insulin infusions. IV insulin requires that it be administered as part of a standardized protocol [51, 67••, 74••, 78].
Insulin Administration
Insulin administration for treating hyperglycemia has many beneficial effects [67••, 78, 81, 82, 83••, 84]. Recent studies have shown that insulin exerts anti-inflammatory, antithrombotic, and anti-atherogenic effects as well as inhibition of lipolysis and platelet aggregation, and can also prevent many of the counter-regulatory hormones and proinflammatory transcription factors, formation of reactive oxidation species associated with hyperglycemia, including free radical formation, oxidative stress, and apoptotic cell death [81, 82, 85, 86]. In addition, there are decreases in hepatic glucose production, improvements in insulin resistance, improvements in energy delivery to peripheral tissues, and improvements in endothelial dependent vasodilation. The anti-inflammatory effects are mediated by inhibition of TNFα, reactive oxygen species, inhibition of NF-Kß, intracellular adhesion molecule-1 in macrophages and leukocytes, inhibition of c-Jun N-terminal kinase, which mediates inflammatory processes downstream of TNFα, and TNF α is suppressed through PI3 kinase-AKT mediated activation of endothelial nitric oxide synthase. The inhibition of mitogen-activated protein kinase (MAPK) suppresses the IL-6 signaling. Additionally, insulin has been shown to increase cardiac contractility and coronary vasodilation and preserve endothelial function by the potent vasodilator nitric oxide [85, 86].
Therefore, insulin therapy is the current standard of care for achieving glycemic control in hospitalized patients [67••, 78, 83••, 84, 87, 88]. However, a study between 2006 and 2008 demonstrated that insulin is the drug with the greatest number of medication errors in hospitals [89]. Insulin treatment in hospital settings requires careful planning and monitoring. In general, glucose levels should be maintained <200 mg/dl (11.2 mmol/l). This decreases acute hyperglycemic symptoms, as well as minimizes fluid and electrolyte abnormalities, and risks for infection due to altered immune function. Although clinical guidelines recommend targeting a glucose level of <140 mg/dl (7.8 mmol/l) before meals and a random glucose level of <180 mg/dl (10.0 mmol/l) for most non-ICU hospitalized patients (provided that these targets can be safely achieved without hypoglycemia), data is lacking regarding glycemic goals and specific therapeutic recommendations for the elderly in hospitalized settings. However, it is generally recommended that less stringent glycemic targets may be appropriate for some older patients who have multiple comorbidities, high risks for hypoglycemia, and reduced life expectancy.
Multiple strategies have been proposed to calculate the total daily insulin dose requirement. In patients who are already receiving intravenous insulin, 80 % of the daily requirement can be used as the total daily dose. In patients with newly diagnosed hyperglycemia or patients on inadequate home regimens, a new insulin regimen needs to be designed. If the patient is eating, 50 % of this should be administered as basal insulin analogs (glargine or detemir), or intermediate acting insulin (NPH) given once or twice daily, with the remaining 50 % administered as bolus or prandial insulin, using a rapid-acting insulin analog (lispro, aspart or glulisine) divided into three and given prior to meals [67••, 78, 83••, 84, 87, 88]. One choice has been to start a total daily dose, based on the patient’s weight in kilograms. Although insulin doses vary widely for younger diabetic patients (from 0.3 to 1.5 units/kg/day), it is recommended that 0.25–0.3 units/kg/day be used as initial therapy for the elderly and for patients with renal failure (eGFR < 60 ml/min). Although the total amount of daily insulin is generally divided into 50 % basal and 50 % bolus, these balances may be readjusted to 70 % long-acting and 30 % short-acting insulin preparations for patients with increased sensitivity, or 60 % long-acting basal with 40 % rapid-acting insulin for patients with greater insulin resistance. Patients with inadequate oral intake or who will be kept NPO should receive a daily dose of basal insulin (0.15–0.25 units/kg/day) and rapid-acting insulin analogs as correctional insulin coverage for glucose values >140 (7.8 mmol/l) to 180 mg/dl (10.0 mmol/l). Another approach to insulin therapy recently reported is the basal plus regimen, administering basal glargine with corrections by glulisine. This regimen was found to be effective in patients requiring low-dose insulin or in those with poor oral intake [84].
Supplemental insulin should also be given prior to meals. Insulin doses should be readjusted based upon the previous day’s blood glucose responses [67••, 78, 83••, 90]. Insulin doses should be reduced by approximately 20 % for unexplained blood sugars of <70 md/dl (3.9 mmol/l). Insulin doses should also be reduced if blood glucose levels are between 70 (3.9 mmol/l) and 100 mg/dl (5.6 mmol/l), since these values indicate a potential risk for subsequent hypoglycemia [67••, 78, 83••]. Older patients on insulin may need to increase or decrease their dose as they recuperate from their acute illness and their diet improves. Further, studies such as RABBIT 2 (Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes Undergoing General Surgery), the DEAN TRIAL (Insulin Detemir versus NPH Insulin in Hospitalized Patients with Diabetes), and the Basal trial have confirmed the benefits and feasibility of the use of insulin in a basal and meal-related bolus fashion [84, 87, 88]. The use of sliding scale insulin (SSI) is nonphysiologic and should not be used since it causes significant hypoglycemia, as well as hyperglycemia [67••, 78, 79, 83••, 91]. Premixed insulins, such as 70/30, are often the cause of hypoglycemia in the hospital setting. Therefore, these insulin preparations have been taken off many hospital formularies. We do not recommend 70/30 insulin for use in hospital settings due to the high rate of hypoglycemia, inability to use flexible dosing, and inadequate prandial coverage. It is also not used for transition off IV insulin (as the SQ option). However, the premix insulin may be considered for home use when a simplified insulin regimen is required after hospital discharge.
Special Situations for Insulin Management
Patients receiving enteral tube feeding or parenteral nutrition can be treated with long-acting insulin to attain goal blood glucose levels. Caution should be used if feedings are interrupted and insulin is not appropriately adjusted or the glucose content from feedings is not replaced. This most frequently happens when feedings are held prior to procedures, for elevated gastric residuals, or if intravenous or enteral access is lost. Alternatively, NPH insulin can be administered every 8 h for parenteral nutrition, twice daily for tube feedings, and pm NPH for pm tube feedings, with glucose monitoring and correction with short-acting insulin every 4 h. For enteral feedings, an alternative regimen is to use continuous a IV insulin infusion to establish the total insulin dose, then add basal insulin every 12 h and stop IV insulin 2 h after the second basal dose with correction doses every 4 h. In cases of interrupted tube feedings, D10 is required to avoid hypoglycemia [80]. Glucocorticoids, which cause much insulin resistance, are often used in high doses for chemotherapy/solid organ and bone marrow transplants [92]. Although this therapy is associated with very high glucose levels, there is no consensus about protocols for treatment [74••]. Options have included: insulin drips, NPH insulin, long-acting basal insulin daily to twice daily with meal time short-acting insulin analogs. The exact insulin regimen varies according to timing, frequency of steroids and various regimens, as well as the rapidity of tapering doses. For example, if am prednisone is administered, am NPH insulin can be used. There are no specific protocols for insulin administration for patients with renal failure and peritoneal dialysis. Insulin regimens that have been used include NPH insulin, basal insulin, and insulin drips.
Other Antihyperglycemic Pharmacologic Agents
Metformin is often considered the first-line therapy in type 2 diabetes due to its low risk of hypoglycemia. The risk for lactic acidosis is minimal, unless the GFR is <30 mL/min, but increases during cases of changing renal function and procedures. Its low cost may be considered when transitioning to outpatient management or when there is a need to simplify complex regimens. Sulfonylureas are also a low-cost option for home use, but there is an increased risk of hypoglycemia, especially with glyburide, due to its long duration of action and decreased clearance. Thiazolidinediones have associated risks of weight gain, edema, heart failure, bone fractures, and possibly bladder cancer. We recommend that this drug also be stopped. It should be noted that the effects may remain even after it is discontinued. Therefore, we do not recommend any of these antihyperglycemic agents be administered in the acute hospital setting.
There is recent increased interest and experience using incretin-based therapy, such as dipeptidyl peptidase-4 (DDP4 inhibitors) and glucagon-like peptide-1 agonists (GLP-1 agonists) [93]. They decrease postprandial hyperglycemia with less hypoglycemia in the acute hospital setting, especially when glucocorticoids are used. The results of a recent study showed that patients with T2DM, who were treated with diet, oral antidiabetic agents, and low-dose insulin (daily dose ≤0.4 unit/kg) can be safely given a daily dose of DPP-4 inhibitor plus correction (supplemental) doses of rapid-acting insulin before meals, or with the combination of DPP-4 inhibitor plus low-dose basal insulin therapy. There are marked decreases in hypoglycemia, insulin doses, and they are well tolerated [93]. They also have cardioprotective effects. For some agents, dose reduction is required in renal dysfunction. Patients on combination therapy of DPP-4 inhibitor plus basal group can be started on a total daily dose of basal insulin of 0.25 units/kg/day, except in elderly patients (age >70 years) and/or with a glomerular filtration rate <45 mL/min, when a lower starting insulin dose of 0.15 units/kg should be given [93]. Further randomized studies are needed to further evaluate the safety and efficacy of incretin therapy in the management of older patients in non-ICU settings.
Transitions of Care
Transitioning from the inpatient to outpatient setting can be a source of medical errors, leading to adverse outcomes [64••, 74••, 94]. The elderly present with many unique challenges which affect their ability to follow recommendations. Successful transitions require a multidisciplinary team of doctors, nurses, social workers, family members, diabetes educators, and social workers [78, 94, 95••, 96]. Close attention should be given to not only to age, but life expectancy and functional status. Addressing the unique issues associated with aging can significantly improve the quality of life of older adults and help avoid unnecessary health care costs from morbidities and hospital readmission. Medication reconciliation, patient and caregiver evaluation and education are required. There should be communication between inpatient and outpatient care teams to monitor patient safety and reduce readmission rates.
Special care should be given when prescribing medications, such as insulin or oral antihyperglycemic agents, since a recent hospital discharge is a predictor of serious outpatient hypoglycemia in older patients [8••, 13, 65]. As in Table 2, it is recommended that upon discharge, consideration should be given to switching from insulin to oral antihyperglycemic therapy, if possible, due to ease of administration, generally lower costs, and less risk for hypoglycemia. However, it is important to remember that insulin therapy is still required for type 1 diabetes in order to avoid serious hyperglycemia and DKA [10, 16]. With the availability of various types of insulin, with different time-action curves, it is now possible to target postprandial hyperglycemia in elderly while avoiding fasting hypoglycemia. However, when requiring insulin treatment, regimens and delivery approaches must be individualized. The multiple daily injection (MDI) insulin regimen uses basal insulin (glargine or detemir) with rapid-acting insulin (aspart, lispro, or glulisine) which is administered before, with, or after meals, and takes into account anticipated carbohydrate intake or meal amounts and activity. If food intake is uncertain, rapid-acting prandial insulin can be given immediately after the meal, so the dose can be adjusted based upon actual intake. In our experience, using meal amounts for determining short-acting insulin doses tends to be easier for older patients to comprehend, rather than a complex carbohydrate counting system. Further, insulin pens may be easier to use or patients with visual problems, arthritis, or peripheral neuropathy, and who lack abilities for fine hand coordination and manipulation. Some older patients can use NPH insulin twice daily with regular insulin before breakfast and dinner (and at lunch if necessary). The cost of these insulin formulations is less than that of insulin analogs. However, there is an increased risk for hypoglycemia with NPH insulin. Snacks may be required between meals and at bedtime to avoid hypoglycemia. Bedtime NPH insulin, instead of before dinner, can also be given to avoid nighttime hypoglycemia. Premixed insulins are rarely used in type 1 diabetes because of their nonphysiological profiles. However, in specific circumstances for both type 1 and type 2 patients, twice daily dosing (before breakfast and dinner) can be considered. Snacks may be needed between meals and at bedtime to avoid hypoglycemia. Continuous subcutaneous insulin infusion (CSII) or insulin pump therapy is another sophisticated option for insulin delivery in older adults. CSII may become difficult to use for many older patients without a caretaker who can assume much responsibility. In addition, few studies have evaluated its use for this population.
Long-Term Care Facilities
Few studies have been published regarding long-term or nursing home diabetic management in the elderly population, even though these individuals are more likely to be discharged to long-term care, and approximately, 33 % of patients living in these facilities have diabetes. Further, due to longer life expectancies, it is anticipated that more and more elderly diabetic patients will require long-term care or nursing home placements. In addition, these patients require more complex management and often have other comorbidities, more severe diabetic complications (such as retinopathy, neuropathy, foot, and other ulcerations), cardiovascular and cerebrovascular disease, renal failure, increased risk for infections, cognitive disorders, falls/fractures, and other physical frailties. There are increased risks for hypo and hyperglycemia, polypharmacy, emergency room visits with increased number of hospital admissions and readmission, and longer length of stays. The staff of long-term care or nursing homes are required to perform the typical self-care measures (diet planning, preparation, and administration, glucose monitoring, medication administration, and monitoring for hypo and hyperglycemia) for the elderly patient. However, insulin management might be safer in this observed environment than in community dwelling individuals due to better supervision. In addition, their care requires a more holistic approach compared to that of individual chronic disease management for the independent (community-living) patient [24].
A Shared Decision-Making Process
Shared decision making has been advocated as an approach to improving the quality of preferences to medical decisions and can begin while in the hospital [94, 95••, 96]. This fosters an ongoing partnership between the patient and provider for information exchanges, medical decision making, and acting upon these decisions and choices. Diabetes self-management education/training (DSME/T) team members can assist in this regard, since special attention can be given to unique medical, cultural, and social needs, including transportation and logistical barriers. Adaptations can be developed for patients with visual, hearing, and cognitive impairments, as well as impairments in functional/physical status. It is also important to recognize that recommendations may need to be simplified and or adjusted due to problems with mobility, dexterity, depression, and chronic pain, polypharmacy, which can interfere with their ability to follow complex diabetic treatment plans. In addition, they may also develop geriatric syndromes of unpredictable eating, and frailty and further adjustments must be made to these regimens. Comorbidities (such as increased rates of arthritis, cancer, congestive heart failure, depression, emphysema, falls, hypertension, incontinence, renal insufficiency, cardiovascular and cerebrovascular disease) must be balanced against the risks for hyperglycemia, which can lead to increased risks of hypoglycemia resulting in traumatic falls, further decreases in cognition, as well as increased morbidity and mortality [8••, 13, 65]. Diabetes care team and other social service interventions started during hospitalization help reduce diabetes-related distress at home. Care partners—family, friends, or other caregivers should be involved in DSME/T.
Discharge orders should include written instructions regarding diet, recommended doses of medications, frequency of self-monitoring of blood glucose, avoidance and treatment of acute hypoglycemia and hyperglycemia, follow-up provider appointments, as well as methods of emergency contacts. Outpatient diabetes education and home health services should be arranged prior to discharge [78, 95••, 96]. These orders need to be discussed at least 1 day before discharge.
Conclusions
The anticipated growth in the US population between 2002 and 2020 population is estimated to be approximately 17 %, with an expected increase in patients with diabetes mellitus of about 44 %; This increase is largely due to increase in numbers in the elderly population [97]. Further contributing to the rise in numbers is that adults are living longer with diabetes and that multiple comorbidities will continue to increase. Several unanswered questions remain regarding treatment for the non-ICU hospitalized older patient. Due to the lack of evidence and controversies regarding best practices, the Planning Research in Inpatient Diabetes (PRIDE) Research Group has recommended that clinical trials be designed to evaluate high-quality and cost-effective inpatient management strategies to address these issues [74••]. Furthermore, we need a better understanding of the goals for diabetes treatment in these settings, better guidelines on how to achieve these goals, as well as strategies for achieving healthy-lifestyles and disease prevention. The exclusion of older and especially frail from most traditional randomized controlled trials of diabetes interventions has left large gaps in our knowledge about how to address diabetes in this age group with the highest prevalence. Answers to these gaps will need to be balanced against societal needs as a whole. Future research should focus upon the complexity and heterogeneity of older adults. Randomized controlled trials are needed that include patients with multiple comorbidities, in both dependent and independent living situations, as well as those with geriatric syndromes, in order to advance our knowledge about best care practices in this population. There is also a need for more research into the disparities of diabetes, and evaluation of the full impact of quality improvement programs and culturally tailored interventions in this vulnerable age group.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Association AMD. Diabetes management in the long-term care setting clinical practice guideline. In: Columbia, MD: AMDA; 2008; revised 2010.
Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta Georgia: U.S. Department of Health and Human Services; 2014.
National Diabetes Fact Sheet. General information and national estimates on diabetes in United States. In: U.S. Department of Health and Human Services CDC (ed.) Atlanta, GA; 2011. This data sheet has the current prevalence of diabetes in various age groups and shows the burden of the disease and its complications in the U.S.
Boyle JP, Thompson TJ, Gregg EW, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metrics. 2010;8:29.
Narayan KM, Boyle JP, Geiss LS, et al. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29:2114–6.
DIAMOND project group. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–66.
Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. EURODIAB study group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–33.
Dhaliwal R, Weinstock RS. Management of type 1 diabetes in older adults. Diabetes Spectrum. 2014;27(1):9–20. doi:10.2337/diaspect.27.1.9. This article describes specific management issues important for elderly Type 1 diabetes care.
Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, et al. Autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet. 1997;50:1288–93.
Schutt M, Fach EM, Seufert J, Kerner W, Lang W, Zeyfang A, et al. DPV Initiative and the German BMBF competence network diabetes network: multiple complications and frequent severe hypoglycaemia in ‘elderly’ and ‘old’ patients with type 1 diabetes. Diabet Med. 2012;29:e176–9.
Szoke E, Shrayyef MZ, Messing S, et al. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008;31:539–43.
Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7–E12.
Brown AF, Mangione CM, Saliba D, et al. California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(Suppl Guidelines):S265–80.
Selvin E, Coresh J, Brancati FL. The burden and treatment of diabetes in elderly individuals in the US. Diabetes Care. 2006;29:2415–9.
Li Y, Burrows NR, Gregg EW, et al. Declining rates of hospitalization for nontraumatic lower-extremity amputation in the diabetic population aged 40 years or older: U.S., 1988–2008. Diabetes Care. 2012;35:273–7.
Weinstock RS, Xing D, Maahs DM, Michels A, Rickels MR, Peters AL, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2013;98:3411–9.
Weir, LM, (Thomson Reuters) Pfuntner A (Thomson Reuters) and Steiner C (AHRQ). Hospital Utilization among Oldest Adults, 2008. HCUP Statistical Brief #103. December 2010, Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb103.pdf.
American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–46.
Zhuo X, Zhang P, Barker L, Albright A, Thompson TJ, Gregg E. The lifetime cost of diabetes and its implications for diabetes prevention. Diabetes Care. 2014;37:2557–64. doi:10.2337/dc13=2484.
Reers C, Erbel S, Esposito I, et al. Impaired islet turnover in human donor pancreata with aging. Eur J Endocrinol. 2009;160:185–91.
Maedler K, Schumann DM, Schulthess F, et al. Aging correlates with decreased beta-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes. 2006;55:2455–62.
Rankin MM, Kushner JA. Adaptive b-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–72.
Meneilly GS. The pathophysiology of diabetes in the elderly. In: Medha N, Munshi LAL, editors. Geriatric diabetes. New York: Informa Healthcare; 2007. p. 29–36.
Aaditya S, Segal AR, Munshi MN. Diabetes Long Term Care Facil Curr Diab Rep. 2014;14:464. doi:10.1007/s11892-013-0464-y.
Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance—a mini-review. Gerontology. 2009;55:379–86.
Amati F, Dube JJ, Coen PM, et al. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care. 2009;32:1547–9.
Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72.
Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079–86.
Vlassara H, Palace MR, Palace. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251(2):87–101. doi:10.1046/j.1365-2796.2002.00932.x.
Blaum C. Diabetes mellitus. In: Durso S, Sullivan GM, editors. Geriatric review syllabus: a core curriculum in geriatric medicine. 8th ed. New York: American Geriatric Society; 2013. p. 566–75.
Gates BJ, Walker KM. Physiological changes in older adults and their effect on diabetes treatment. Diabetes Spectrum. 2014;27:20–9. doi:10.2337/diaspect.27.1.20. This article describes the important issues specific to managing geriatric diabetic patients.
Schafer R, Letassy N, Randal D, Dorey C, Haas L, Barrier P. AADE position statement. Special considerations in the management and education of older persons with diabetes. 2009: 1–6. This article considers important diabetic care requirements for older persons with diabetes.
Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67.
Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007–21.
Brunkhorst FM, Engel C, Bloos F, German Competence Network Sepsis (SepNet), et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. New Engl J Med. 2008 Jan 10;358(2):125–39.
Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–7.
Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35:1738–48.
Knowler WC, Fowler SE, Hamman RF, et al. Diabetes prevention program research group. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. 2009;374:1677–86.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–65.
Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577.
Gerstein HC, Miller ME, Byington RP, et al. Action to control cardiovascular risk in diabetes study group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
Miller ME, Bonds DE, Gerstein HC, ACCORD Investigators, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
Skyler JS, Bergenstal R, Bonow RO, American Diabetes Association; American College of Cardiology Foundation; American Heart Association, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American diabetes association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–92. This position statement provides an analysis of recent studies regarding the question tight glucose control.
Patel A, MacMahon S, Chalmers J, ADVANCE Collaborative Group, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Duckworth W, Abraira C, Moritz T, VADT Investigators, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39.
Duckworth WC, Abraira C, Moritz TE, Investigators of the VADT, et al. The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA diabetes Trial. J Diabetes Complications. 2011;25:355–61.
Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–9.
Huang ES, Liu JY, Moffet HH, et al. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34:1329–36.
Sechterberger MK, Bosman RJ, Oudemans-van Straaten HM, et al. The effect of diabetes mellitus on the association between measures of glycaemic control and intensive care unit mortality: a retrospective cohort study. Crit Care. 2013;17:R52.
Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40:3251–76.
Eiland L, Goldner L, Drincic A, Desouza C. Inpatient hypoglycemia: a challenge that must be addressed. Curr Diab Rep. 2014;14:445. doi:10.1007/s11892-013-0445-1.
Razavi Nematollahi L, Kitabchi AE, Stentz FB, Wan JY, Larijani BA, Tehrani MM, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism. 2009;58:443–8. doi:10.1016/j.metabol.2008.10.018.
Del Rey A, Roggero E, Randolf A, Mahuad C, McCann S, Rettori V, et al. IL-1 resets glucose homeostasis at central levels. Proc Natl Acad Sci U S A. 2006;103:16039–44. doi:10.1073/pnas.0607076103.
Fisher BM, Hepburn DA, Smith JG, Frier BM. Responses of peripheral blood cells to acute insulin-induced hypoglycaemia in humans: effect of alpha-adrenergic blockade. Horm Metab Res Suppl. 1992;26:109–10.
Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Ciofetta M, Modarelli F, et al. Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia. 1994;37:797–807.
Robinson RT, Harris ND, Ireland RH, Lee S, Newman C, Heller SR. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes. 2003;52:1469–74.
Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389–94.
Laitinen T, Lyyra-Laitinen T, Huopio H, Vauhkonen I, Halonen T, Hartikainen J, et al. Electrocardiographic alterations during hyperinsulinemic hypoglycemia in healthy subjects. Ann Noninvasive Electrocardiol. 2008;13:97–105. doi:10.1111/j.1542-474X.2008.00208.x.
Koivikko ML, Karsikas M, Salmela PI, Tapanainen JS, Ruokonen A, Seppanen T, et al. Effects of controlled hypoglycaemia on cardiac repolarisation in patients with type 1 diabetes. Diabetologia. 2008;51:426–35. doi:10.1007/s00125-007-0902-y.
Bremer JP, Jauch-Chara K, Hallschmid M, et al. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32:1513–7.
Matyka K, Evans M, Lomas J, et al. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135–41.
Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32:1153–7. doi:10.2337/dc08-2127.
Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342–56. doi:10.1111/jgs.12035. This position paper evaluates evidence and recommendations for treatment decisions of older adults with diabetes.
Elliott MB, Schafers SJ, McGill JB, Tobin GS. Prediction and prevention of treatment-related inpatient hypoglycemia. J Diabetes Sci Technol. 2012;6:302–9.
Cook CB, Kongable GL, Potter DJ, Abad VJ, Leija DE, Anderson M. Inpatient glucose control: a glycemic survey of 126 U.S. hospitals. J Hosp Med. 2009;4:E7–E14. doi:10.1002/jhm.533.
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American association of clinical endocrinologists and American diabetes association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15:353–69. doi:10.4158/EP09102.RA. This paper provides recommendations for inpatient glycemic control.
Workgroup on Hypoglycemia, American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American diabetes association workgroup on hypoglycemia. Diabetes Care. 2005;28:1245–9. This is a useful guideline to what is defined as hypoglycemia and why and how it can be reported.
Kosiborod M, Inzucchi SE, Goyal A, Krumholz HM, Masoudi FA, Xiao L, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA. 2009;301:1556–64. doi:10.1001/jama.2009.496.
Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med. 2011;124:1028–35. doi:10.1016/j.amjmed.2011.07.011.
Farrokhi F, Klindukhova O, Chandra P, Peng L, Smiley D, Newton C, et al. Risk factors for inpatient hypoglycemia during subcutaneous insulin therapy in non-critically ill patients with type 2 diabetes. J Diabetes Sci Technol. 2012;6:1022–9.
Maynard G, Wesorick DH, O’Malley C, Inzucchi SE. Society of hospital medicine glycemic control task force. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3:29–41. doi:10.1002/jhm.354.
Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389–94. doi:10.2337/dc09-2082. Useful review of the CVD risk that might be associated with severe hypoglycemia and the mechanisms involved.
Draznin B, Gilden J, Golden SH, Inzucchi SE, for the PRIDE Investigators. Pathways to quality inpatient management: a call to action. Diabetes Care. 2013;36:1807–14. doi:10.2337/dc12-2508. This paper highlights the controversies and unanswered questions regarding inpatient management of diabetes and calls for studies designed to answer these challenges and gaps.
Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–82.
Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37:3001–9.
Kosiborod M. Blood glucose and its prognostic implications in patients hospitalised with acute myocardial infarction. Diab Vasc Dis Res. 2008;5:269–75.
American Diabetes Association. Standards of medical care in diabetes 2014. Diabetes Care. 2014;37 Suppl 1:S14–80.
Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin: myth or insanity? Am J Med. 2007;120:563–7.
Baldwin D, Kinnare K, Draznin B, et al. Insulin treatment of hyperglycemia in hospitalized patients receiving total parenteral nutrition (TPN). Diabetes. 2012;61 Suppl 1:A1070.
Dandona P, Mohanty P, Chaudhuri A, Garg R, Aljada A. Insulin infusion in acute illness. J Clin Invest. 2005;115:2069–72.
Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, et al. Insulin inhibits intranuclear factor kappa ß and stimulates Ikappaß in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect. J Clin Endocrinol Metab. 2001;86:3257–65.
Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:16–38. These guidelines provide clinical practice recommendations for management of patients with diabetes mellitus.
Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34:256–61.
Chaudhuri A, Dandona P, Fonseca V. Cardiovascular benefits of exogenous insulin. J Clin Endocrinol Metab. 2012;97:3079–91.
Ng KW, Allen ML, Desai A, Macrae D, Pathan N. Cardioprotective effects of insulin: how intensive insulin therapy may benefit cardiac surgery patients. Circulation. 2012;125:721–8.
Umpierrez GE, Smiley D, Hermayer K, Khan A, Olson DE, Newton C, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes. Basal Plus Trial Diabetes Care. 2013;32:2169–74.
Umpierrez GE, Hor T, Smiley D, Temponi A, Umpierrez D, Ceron M, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:564–9.
Rashidee A, Hart J, Chen J, Kumar S. High-alert medications: error prevalence and severity. Available at: http://www.psqh.com/julyaugust-2009/164-data-trends.html.
Maynard G, Lee J, Phillips G, Fink E, Renvall M. Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med. 2009;4:3–15. doi:10.1002/jhm.391.
Ib H. Sliding scale insulin: time to stop sliding. JAMA. 2009;301:213–4.
Oyer DS, Shah A, Bettenhausen S. How to manage steroid diabetes in the patient with cancer. J Support Oncol. 2006;4:479–83.
Umpierrez GE, Schwartz S. Use of Incretin-based therapy in hospitalized patients with hyperglycemia. Endocrine Practice 2014: (rapid electronic article in press) DOI 10.4158/EPI 13471.RA.
Hirschman KB, Bixby B. Transitions in care from the hospital to home for patients with diabetes. Diabetes Spectrum. 2014;27:192–5.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–79. These guidelines recommend that diabetes management should be individualized as a patient-centered approach.
Healy SJ, Dawn B, Cara H, Andrew L, Dungan KM. Inpatient diabetes education is associated with less frequent hospital readmission among patients with poor glycemic control. Diabetes Care. 2013;36(10):2960–7. dc13-0108v1 36/10/2960.
American Diabetes Association. Economic consequences of diabetes mellitus in the U.S. in 1997. Diabetes Care. 1998;21:296–309.
Acknowledgments
The contents of this article do not reflect the views of the JAL FHCC and are the opinion of the authors.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Janice L. Gilden declares that she has been an investigator in multi-center trials for Novo Nordisk and Boehringer Ingelheim, and on the Speaker’s Bureau for Novo Nordisk.
Aditi Gupta declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Hospital Management of Diabetes
Rights and permissions
About this article
Cite this article
Gilden, J.L., Gupta, A. Non-ICU Hospital Care of Diabetes Mellitus in the Elderly Population. Curr Diab Rep 15, 26 (2015). https://doi.org/10.1007/s11892-015-0596-3
Published:
DOI: https://doi.org/10.1007/s11892-015-0596-3