Summary
Total neoadjuvant therapy is a novel approach for locally advanced rectal cancer. The optimal treatment sequence is, however, a matter of debate and until now, no overall survival benefit has been reported. This review is a critical, objective summary viewed from different perspectives of the available literature of previous milestones that have been achieved in the treatment of locally advanced rectal cancer, of current recommendations, and of future perspectives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction with a short look back
Until the 1990s, the 5‑year overall survival (OS) in patients with rectal cancer was 30–70% after a curative resection and the local recurrence rate was 15–40% [1,2,3]. To improve the outcome in locally advanced rectal cancer, two pathways were taken:
The establishment of a special surgical technique—total mesorectal excision (TME)—and the involvement of radiotherapy has reduced the 5‑year local recurrence rate to 5–8%.
Two landmark studies, the German CAO/ARO-AIO-94 study [6] and the Dutch trial [7], examined the influence of neoadjuvant therapy on OS, disease-free survival (DFS), local relapse rate, complete response, and R0 resection. Both studies could decrease the local recurrence rate down to about 6% but had no impact on OS. The question of whether radiotherapy had a positive impact on local recurrence rate also in patients in whom a TME surgery was performed could be positively answered by the Dutch trial [7].
Further insights from these studies were
-
accuracy of staging (18% of patients were stage I in the postoperative arm, who shouldn’t be enrolled),
-
distant metastases rate was still about 30%,
-
uninfluenced OS.
From then on, new major questions were of interest (from about 2005 to 2016):
-
What should be the standard diagnostic procedure for local staging of rectal cancer?
-
Accurate preoperative staging of rectal cancer is crucial in planning treatment and is the strongest predictor for recurrence. Local staging incorporates the assessment of mural wall invasion, circumferential resection margin (CRM) involvement, and nodal status. For local radiological staging of rectal cancer, a combination of modalities involving magnetic resonance imaging (MRI) and/or endorectal ultrasonography (EUS) is the standard diagnostic procedure [8,9,10,11].
-
-
Could/should the pathologic procedure be optimized and standardized?
-
Pathologists play a key role in the modern multidisciplinary management of patients with rectal cancer. Pathological assessment of the resected specimen provides key prognostic information, e.g., primary staging of the tumor and identification of high-risk features, and allows evaluation of the quality of the surgery, accuracy of radiology, and the response to neoadjuvant therapy. The pathologic procedure was standardized and optimized over the years [12,13,14,15].
-
-
Should upper-third rectal tumors should be treated by neoadjuvant radiotherapy?
-
Despite recommendations [16], the Dutch trial showed that the main parameter is the positive circumferential margin until the distance of 15 cm [17].
-
Are there indications for adjuvant chemotherapy after neoadjuvant long-course radiotherapy?
-
Which is the optimal radiotherapy treatment: short- or long-course?
-
Neoadjuvant radiotherapy is the standard of care for locally advanced rectal cancer (LARC), which can be delivered with short-course fractionation (25 Gy in 5 fractions) or in a conventionally fractionated, long-course schedule over 25–30 fractions with concurrent chemotherapy. Head-to-head randomized trials have shown little or no significant difference between short- and long-course radiotherapy in terms of local control or OS. Long-course radiotherapy should be offered if downstaging is a goal or if the CRM is involved, because the Dutch trial showed less downstaging with short-course radiotherapy followed by surgery after 1 week and that there was no benefit if circumference is involved [23]. Both treatment schedules remain viable options depending on the clinical scenario.
-
-
Which group has the most advantage from neoadjuvant radiotherapy?
-
What should be the endpoint in studies about neoadjuvant treatment with curative intent and what should be the minimum follow-up time to record valid local recurrence rates?
-
Complete remission rate of long-course radiotherapy has often been discussed to be a sufficient argument for neoadjuvant treatment. Maas et al. examined long-term outcome in patients with pathologic complete remission (pCR) after chemoradiotherapy (CRT). 5‑year DFS, 5‑year risk for local relapse, 5‑year distant metastasis-free survival, and 5‑year OS were 83.3%, 2.8%, 88.8%, 87.6% for patients with pCR and 65.6%, 9.7%, 74.9%, 76.4% for those without pCR, respectively (p < 0.001). The multivariate analyses couldn’t show an additional favorable effect on DFS of administration of adjuvant chemotherapy, although it was associated with significantly improved OS [29]. A more recent meta-analysis of 22 randomized trials didn’t support the use of pCR and 3‑year DFS as appropriate surrogate endpoints for 5‑year OS [30]; also in colon cancer overall association between 2‑/3-year DFS and 5‑/6-year OS HRs were modest to poor. Only in stage III patients was the association increased, and a correlation could be found [31]. Only 5‑year local recurrence rates allow treatment quality to be definitely judged, especially when multimodal treatment was applied [32].
-
-
Status of escalation/optimization chemotherapy in the neoadjuvant treatment part. Is there a benefit of adding a second chemotherapy drug such as oxaliplatin?
-
To the best of our knowledge there is no trial which could clearly show an advantage of adding a further chemotherapy drug combined with fluoropyrimidine-based CRT in the neoadjuvant part. Several randomized trials have been conducted to evaluate the efficacy of adding oxaliplatin to fluoropyrimidine-based CRT. Because of the inconsistent results of these trials, several meta-analyses have been carried out, but they have not been conclusive. The pCR rate and DFS were increased in a few studies but did not translate to improved OS and toxicity was increased [20, 33,34,35,36,37,38].
-
-
Most studies included patients with “LARC,” but what is the definition of LARC?
To date, there is no international consensus on a definition of LARC. In general, the term is used when no R0 resection and an increased risk of local relapse is expected. Other common descriptions are cT3/4, mesorectal facia (MRF) involvement (< 1 mm), nodal positivity (N+), extramural venous invasion (EMVI), cN2, extramesorectal N+. Because of different inclusion criteria in rectal cancer studies, a comparison between studies on the treatment of LARC is not easy.
-
What is the main aim of neoadjuvant therapy: local relapse, DFS, OS, watch and wait (W&W), sphincter saving, downstaging, downsizing, distant metastasis, etc.?
-
To date, there is no agreement about the main aim. Therefore, due to different endpoints and different inclusion criteria, it is impossible to draw a clear conclusion.
-
During the past few years, the above-listed points have been internationally discussed and examined from different points of view. Main goals were to decrease the rate of distant metastases with the hope of increasing OS, to increase the rate of pCR either with the intention to increase DFS, OS, complete response, or the rate of sphincter-saving surgery, and last but not least, to decrease the rate of local recurrences. All these discussion points were internationally discussed and described by the tasked force “workflow rectal cancer” and others [39,40,41].
Total neoadjuvant therapy
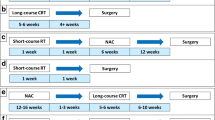
Total neoadjuvant therapy (TNT)—a novel therapeutic approach in LARC—has been intensively investigated in recent years. The results of five prospective, randomized trials were recently published and reactivated these discussion points. All studies used MRI for local rectal staging. The five trials are briefly summarized:
PRODIGE-23 trial
In the PRODIGE-23 trial [42], six cycles of modified FOFLIRINOX (mFOLFIRINOX) followed by CRT and surgery and 3 months of adjuvant mFOLFOX6 or capecitabine (experimental arm A; n = 231) were compared with standard CRT followed by surgery and 6 months of adjuvant mFOLFOX or capecitabine (standard arm B; n = 230). The primary endpoint was 3‑year DFS and was 75.7% in the experimental arm versus 68.5% in the control arm (p = 0.034). Distant metastasis occurred in 17% versus 25% of patients (p < 0.05) and pCR was 27.8% versus 12.1% (p < 0.001). No difference was found in terms of OS, R0 resection rate, and local relapse rate.
RAPIDO trial
In the RAPIDO trial [43], short-course radiotherapy followed by consolidation chemotherapy with six cycles of CAPOX or nine cycles of FOLFOX4 (experimental arm A; n = 462) were compared with standard CRT followed by surgery and adjuvant chemotherapy consisting of eight cycles of CAPOX or 12 cycles of FOLFOX4 (standard arm B; n = 450). The primary endpoint of this trial changed during the study from DFS to disease-related treatment failure (DRTF). 3‑year DRTF in arm A was 23.7% versus 30.4% (p = 0.019). Distant metastasis occurred in 20% versus 26.8% of patients (p = 0.0048) and pCR was 27.8% versus 12.1% (p < 0.001). No difference was found in OS, R0 resection rate, and local recurrence rate.
OPRA trial
In the OPRA trial [44], induction chemotherapy followed by CRT (arm A; n = 152) or CRT followed by consolidation chemotherapy (arm B; N = 166) and either TME or W&W on the basis of tumor response were assessed. Patients in both groups received 4 months of chemotherapy (eight cycles FOLFOX or five cycles CAPEOX). The primary endpoint was 3‑year DFS and was 76% in both arms. 3‑year DFS did not differ when compared to historical controls treated with CRT (75%). 3‑year TME-free survival was 41% in the induction group and 53% in the consolidation group. No differences were found between groups in local recurrence-free survival, distant metastasis-free survival, and OS.
CAO/ARO/AIO-12 trial
In the CAO/ARO/AIO-12 trial [45], a phase II study, three cycles of FOLFOX followed by CRT and TME (experimental arm A; n = 156) were compared to CRT followed by three cycles FOLFOX and TME (control arm B). Adjuvant chemotherapy after TME was not recommended. The primary endpoint was DFS. The 3‑year DFS was 73% in both arms. Up-front CRT followed by consolidation chemotherapy resulted in higher rates of pCR (27% vs 38%). Long-term oncologic outcomes did not differ between the groups.
STELLAR
In the STELLAR trial [46], short-course radiotherapy followed by four cycles of CAPOX and surgery followed by two cycles of adjuvant CAPOX (experimental arm A; n = 302) were compared with RCT followed by surgery and six cycles of CAPOX (control arm B; n = 297). The primary endpoint was DFS. 3‑year DFS in arm A was 64.5% versus 62.2% in the control arm (n. s.). The pCR was 21.8% versus 12.3% (p = 0.002) and OS was 86.5% versus 75.1% (p = 0.033). No difference was found in the occurrence of distant metastases, R0 resection rate, and local recurrence rate.
Discussion
In 2018, the National Comprehensive Cancer Network (NCCN) incorporated TNT as the preferred treatment for LARC [47]. This incorporation was based on high complete response rates of about 30% and a favored DFS rate, but with no influence on OS [48,49,50,51]. The guidelines were not accepted in general because of a missing influence on survival, and most of the studies were not prospective and randomized.
Recently, a big step forward was achieved with the publication of five prospective randomized trials: the Prodige23 trial [42], Rapido trial [43], OPRA trial [44], Stellar trial [46], and CAO/ARO/AIO-12 trial [45] trials.
Two trials (Prodige-23 trial, Rapido trial) showed an almost identical benefit of 7% in DFS, of 6% in distant metastases, and 10% in pCR. The Stellar trial, on the other hand, had different results, with no benefit in DFS and in distant metastases, but benefit in OS (11%) and in pCR (10%). Differences in study protocols are the inclusion criteria—cT3/4 and/or N+ versus cT3/4 Nx versus rectal cancer with risk factors (Table 1)—and the used treatment strategy—induction chemotherapy/CRT versus short-course radiotherapy/consolidation chemotherapy versus short-course radiotherapy/consolidation chemotherapy/adjuvant chemotherapy. The difference of about 10% in DFS (> 70% versus 65%) is difficult to explain.
Although in none of the studies except for the Stellar trial could a benefit in OS be shown, a systemic review and meta-analysis demonstrated a significant increase in OS with an HR 0.81 (0.67, 0.99; p = 0.04) [52]. On the other hand, in a recently published systemic review and meta-analysis including the at ASCO 2022 published data of the Prodige-23 trial and Rapido trial trials, no statistically significant differences in OS were observed in the TNT treatment compared to the standard treatment, with HR 0.88 (0.74–1.05; p = 0.15) [53].
This is all the more remarkable since the inclusion criteria were different. Rapido trial included the homogenous group of high-risk rectal cancers: cT4a/b, cN2, MRF+, lateral lymph node, and EMVI, and this group had in DFS, distant metastases, and pCR a significant benefit. In the Prodige-23 trial, about 40% were stage cT3a/b nodal status unknown, and in this group neoadjuvant treatment is in discussion [27, 28, 54, 55].
Grade 3–4 acute toxicities were more common in the TNT treatment group (9–41%) than in the standard treatment group (2–29%) [53], although the meta-analysis by Riesco-Martinez et al. [52] did not show statistically significant differences in grade 3–4 adverse events (OR 1.43, 95% CI 0.75–2.70; Tables 1 and 2).
Factors to consider
In 2016, Bujko et al. [56] published the results of a phase II trial in patients with primary or locally recurrent rectal cancer involving or abutting adjacent organs or structures (cT4) or a palpably fixed cT3 lesion. They compared either preoperative short-course irradiation over 5 days with consolidation chemotherapy consisting of three cycles of FOLFOX4 (group A) or preoperative long-course chemoradiation concomitantly with oxaliplatin and boluses of 5‑fluorouracil and leucovorin (group B). After a median follow-up of 35 months (IQR 21–50), 3‑year DFS rate was 53% versus 52% (n. s.) and the OS rate 73% versus 65% (p = 0.046). And as Bruce Minsky said at ASCO 2010, “Good news comes early, bad news comes later, we need a follow-up in rectal cancer of at least 5 years,” the long-term results put the primarily promising results into perspective [57]. After a median follow-up of 7.0 years (IQR 5.8–8.3), OS at 8 years was 48% in both groups and DFS 43% versus 41%.
The findings of the RAPIDO trial, PRODIGE-23 trial, and OPRA trial have found their way into the NCCN guidelines for rectal cancer treatment [58]. The recommendations preferred for cT3, any N with clear circumferential resection margin (CRM), T1/2 N+ total neoadjuvant therapy (12–16 weeks of FOLFOX/CAPEOX followed by RCT or CRT followed by chemotherapy with 12–16 weeks of FOLFOX/CAPEOX). Furthermore, standard CRT is still an option for this patient group. For patients with T3 any N, involved or threatened CRM, T4, any N or medically inoperable CRT followed by chemotherapy (12–16 weeks of FOLFOX/CAPEOX or consider FOLFIRINOX [for T4, N+]) or chemotherapy (12–16 weeks of FOLFOX/CAPEOX or consider FOLFIRIONOX [for T4N+]) followed by CRT is recommended. This recommendation shows an interpretation of the published data, but only in the Prodige-23 trial cT3N0 without CRM involvement were included, therefore this group should be treated with 6 × mFOLFIRINOX. Further facts to consider are that both trials didn’t include cT1/2N1 without risk factors and there is no randomized trial which examined FOLFOX → CRT or CRT → FOLFOX versus CRT alone.
At the beginning, the hope for TNT was not only to increase DFS and OS, but also—based on a higher pCR—that it should have an impact on R0 resection rate, local recurrence rate, and sphincter-saving procedures. But at the end of the day, although there is a CR rate higher than 20% (nearly doubled), no influence on the type of surgery nor the percentage of colostomy could be detected. There was no impact of this intensification on the percentage of patients requiring temporary (78.4% in experimental arm versus 77.8% in control arm) or permanent (14.1% versus 14.6%) stoma, as demonstrated in PRODIGE-23 trial [42]. Therefore, stoma avoidance or “what patients really want” was not achieved [59].
As in breast cancer, there is an ongoing discussion on what is the best time for chemotherapy: before surgery, after surgery, doesn’t matter? Yes, there is a positive influence of complete remission on OS; on the other hand, an accurate answer regarding tumor stage could only be given after knowing the tumor stage and the best staging is after surgery, done by pathology. Independently, an argument for neoadjuvant treatment is that the patient will get this therapy anyway. In rectal cancer, proof is missing.
Still an unanswered question in rectal cancer is adjuvant therapy based on staging before or after neoadjuvant therapy? And at least in patients staged cT3 without risk factors, no data are available showing a benefit of chemotherapy additionally to CRT and operation [16, 20, 38, 60]. Moreover, a not entirely valid comparison to the CAO/ARO/AIO-12 trial [45] and the PETACC‑6 trial [20], which evaluated the benefit of oxaliplatin neoadjuvant and adjuvant, showed surprising but frequently ignored results. The clear differences in toxicity between the studies are striking (from about 13 to 36% in CRT); however, there is a significant increase in the TNT–chemotherapy arm, with about 50% grade > 3 toxicity (Table 2).
Watch and wait
The published OPRA trial [44] evaluated chemotherapy before or after CRT. 3‑year DFS in both arms was 76%. Overall, this prospective trial could show that organ preservation is achievable in half of the patients with rectal cancer treated with TNT, without an apparent detriment in survival compared with historical controls treated with CRT, TME, and postoperative chemotherapy.
To begin with, we should answer two basic questions:
-
a)
How to evaluate clinical complete remission (cCR) after CRT?
-
A lot of publications have discussed advantages and disadvantages of evaluation without a clear recommendation [61,62,63]. A systematic review [64] evaluated 17 studies, the methodology of tumor assessment was reported in 16 studies, of which 88% used a combination of DRE, endoluminal assessment, and radiological imaging to detect cCR; use of biopsy was not standardized in most of the studies. Habr-Gama A et al. was the first to enroll carefully selected patients with cCR into a W&W program [65]. Patients with cCR should harbor no more than whitening of the mucosa, telangiectasia with mucosal integrity, and these patients should be considered for a nonoperative approach [66]. ESMO guidelines defined cCR as the absence of any palpable tumor or irregularity at digital rectal examination (DRE), no visible lesion at rectoscopy except a flat scar, telangiectasia, or whitening of the mucosa. These minimal criteria can be complemented by the absence of any residual tumor in the primary site and draining lymph nodes on imaging with MRI or ERUS, and negative biopsies from the scar [67].
-
-
b)
What is the optimal interval between CRT and evaluation?
-
A review evaluated the outcome after an interval > 8 weeks (LI, n = 9070) compared to an interval < 8 weeks (SI, n = 14,207) between CRT and surgery. There was no significant difference in the R0 resection rate, anal preservation rate, morbidity rate, anastomotic leakage rate, operation time, local recurrence rate, distant metastasis rate, or OS rate between the two groups [68]. The analysis showed that the pCR rate in the LI group was higher than that in the SI group (P < 0.00001). Another meta-analysis showed the same results [69]. However, it is to be observed that minor or no tumor response (ypT stage of 2 to 3 or ypN positive) and a longer waiting time to surgery (> 8 weeks) compared with a shorter waiting time (< 8 weeks) was not only associated with worse OS rates 67.6% versus 80.3% at 5 years and 40.1% versus 57.8% at 10 years (p < 0.001), but also associated with worse DFS 59.6% versus 72.0% at 5 years and 36.2% versus 53.9% at 10 years (p < 0.001) [70].
-
What we have to keep in mind if the therapy goal is W&W, nearly all data using CRT in the neoadjuvant treatment arm. Therefore, combining TNT with short-course radiotherapy to achieve a better cCR for W&W is not evidence based.
Upcoming innovations
Circulating tumor DNA
In the discussion regarding cCR as an endpoint in TNT, several studies have shown the accuracy and ability of circulating tumor DNA (ctDNA) [71] to detect minimal residual disease in nonmetastatic rectal cancer (MRD) [72]. A prospective multicenter study investigated 159 LARC patients treated with neoadjuvant CRT and TME. Patients with detectable ctDNA after CRT or surgery had a significantly decreased relapse-free survival. Interestingly, the conversion of ctDNA status from positive at baseline to negative at 4–6 weeks after completing CRT was not associated with pCR. In another study in patients with LARC treated with TNT, patients with presurgery ctDNA positivity had an increased risk of recurrence compared to patients with negative ctDNA and a significantly decreased OS. However, again, no correlation was found between presurgery ctDNA and pCR [73]. At the ASCO GI 2022, preliminary results of the GALAXY study were presented [74]. These authors observed the association of ctDNA with clinical outcomes in the adjuvant setting for patients with colorectal cancer. A total of 1365 CRC patients were included in the analysis: 116 patients with stage I, 478 patients with stage II, 503 patients with stage III, and 268 oligometastatic resectable patients with stage IV disease. The presence of ctDNA at a 4-week interval from surgery correlated with inferior DFS. In addition, adjuvant chemotherapy cleared ctDNA and improved prognosis in two thirds of the patients. Those treated with adjuvant chemotherapy had 6‑ and 12-month DFS rates of 98.6% and 96.2% compared with 97.5% and 94.7% for those without chemotherapy, respectively (p = n. s.). This study highlighted the lack of benefit of adjuvant chemotherapy in postsurgery ctDNA negative patients.
As a little food for thought, in patients with cT1‑3 N0‑1 M0 disease with rectal cancer within 6 cm of the anal verge, with a radiation dose increase from 50.4 to 62 Gy, it was possible to reach a cCR of 86%, and 61% had no locoregional regrowth after 2 years of follow-up without adjuvant treatment [75]. A meta-analysis showed that pCR rates approaching 25% are achievable with moderate escalation (54–60 Gy) with modern inverse-planning techniques [76].
Dostarlimab
At ASCO 2022, an impressive late-breaking abstract (abstract LBA5) about dostarlimab, an anti-PD‑1 monoclonal antibody, as single-agent neoadjuvant therapy in patients with mismatch repair-deficient (dMMR) LARC was presented. Approximately 5–10% of rectal cancers are dMMR, and these tumors have been shown to respond poorly to neoadjuvant chemotherapy in LARC [77]. On the other hand, immune checkpoint inhibitors are highly effective as first-line treatment for patients with dMMR metastatic colorectal cancer as well as for patients with treatment-refractory disease, with an objective response rate of 33–55% and prolonged OS [78, 79]. Preliminary results of neoadjuvant immunotherapy were presented at ASCO 2022. In this study patients, with dMMR stage II or III rectal cancer receive dostarlimab 500 mg every 3 weeks for 6 months. Patients with a cCR after completion of dostarlimab therapy have nonoperative follow-up every 4 months. Patients without cCR after 6 months will receive standard radiation therapy (total dose of 50.4 Gy) with concurrent administration of capecitabine at standard doses followed by TME (in case of residual disease after CRT) or nonoperative follow-up (in case of cCR after CRT). 30 patients will be included in this ongoing study. Primary endpoints are the overall response to neoadjuvant dostarlimab therapy with or without CRT and the cCR rate or pCR rate 12 months after completion of dostarlimab. At the ASCO 2022 meeting, baseline characteristics of the 18 patients enrolled to date and the overall response of the first 14 patients, who have already finished therapy, were presented. An impressive 100% cCR were seen in the first 14 consecutive patients without grade 3 or 4 adverse events. None of these patients needed CRT or TME or CTx, and no recurrences were detected in the follow-up. The median follow-up is currently only 6.8 months, but 4 patients have been followed for nearly 2 years without recurrences. Most of the patients (78%) had T3 or T4 rectal cancer, with lymph node positivity in 95% of patients. The median age was 54 years. All patients had dMMR and BRAF V600E wildtype tumors. The mean tumor mutational burden was 67 mut/Mb. In 60% of cases, a germline mutation associated with a Lynch syndrome was detected [80]. Despite the fact that longer follow-up and completion of the study are required, this concept of treatment may be able to replace chemotherapy, radiation, and surgery in a subgroup of patients with dMMR rectal cancer and the final results are eagerly awaited.
Conclusion
New studies clearly showed a benefit regarding pCR, distant metastasis, and DFS with TNT compared to no TNT. But as in other studies with escalating chemotherapy, to date, no OS benefit has been demonstrated. Therefore, especially in a treatment with curative intent, for a general statement like, e.g., “new standard” it is too early. Or we have to check and prove that DFS is a clear surrogate marker for OS in rectal cancer.
Yes, the results are promising, but we need answers regarding the optimal radiotherapy strategy in TNT (short-course radiotherapy versus CRT); whether a triplet chemotherapy regimen (mFOLFIRINOX) is superior to a doublet chemotherapy regimen (FOLFOX); are 3, 6 or, 8 cycles FOLFOX required; and is adjuvant chemotherapy necessary? Furthermore, more subgroup selection is necessary for individualized therapy like immunotherapy in microsatellite instability-high (MSI‑h) rectal cancer which possibly will not need radiotherapy, chemotherapy, and surgery in the future.
The following points still need to be clarified in detail:
-
a)
better identify the group that has an advantage (LARC is far too imprecise),
-
b)
therapy intention should be determined before starting of therapy (W&W, DM, DFS),
-
c)
sequence and dose of the multidisciplinary therapy (e.g., chemotherapy dose, drug; radiotherapy dose, fractionation; sequence; time interval to surgery).
During the long period of developments in rectal cancer, we should not focus on one or two results, we should discuss the results in the context of multidisciplinary therapy development.
References
Enker WE, Laffer UT, Block GE. Enhanced survival of patients with colon and rectal cancer is based upon wide anatomic resection. Ann Surg. 1979;190(3):350–60.
Pollett WG, Nicholls RJ. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg. 1983;198(2):159–63.
Buyse M, Zeleniuch-Jacquotte A, Chalmers TC. Adjuvant therapy of colorectal cancer. Why we still don’t know. JAMA. 1988;259(24):3571–8.
Heald RJT. “Holy Plane” of rectal surgery. J R Soc Med. 1988;81(9):503–8.
Cedermark B, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336(14):980–7.
Sauer R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Kapiteijn E, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–46.
Brown G, et al. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91(1):23–9.
Brown G. Local radiological staging of rectal cancer. Clin Radiol. 2004;59(3):213–4.
Beets-Tan RG, Beets GL. Local staging of rectal cancer: a review of imaging. J Magn Reson Imaging. 2011;33(5):1012–9.
Beets-Tan RG. MRI in rectal cancer: the T stage and circumferential resection margin. Colorectal Dis. 2003;5(5):392–5.
Maughan NJ, Quirke P. Modern management of colorectal cancer—a pathologist’s view. Scand J Surg. 2003;92(1):11–9.
Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26(2):303–12.
Nagtegaal ID, et al. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23(36):9257–64.
Quirke P. Training and quality assurance for rectal cancer: 20 years of data is enough. Lancet Oncol. 2003;4(11):695–702.
van den Broek CB, et al. Differences in pre-operative treatment for rectal cancer between Norway, Sweden, Denmark, Belgium and the Netherlands. Eur J Surg Oncol. 2014;40(12):1789–96.
van Gijn W, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–82.
Breugom AJ, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16(2):200–7.
Bosset J‑F, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184–90.
Schmoll HJ, et al. Pre- and postoperative capecitabine without or with oxaliplatin in locally advanced rectal cancer: PETACC 6 trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J Clin Oncol. 2021;39(1):17–29.
Rödel C, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679–87.
Kim TW, et al. Randomized trial of postoperative adjuvant therapy in Stage II and III rectal cancer to define the optimal sequence of chemotherapy and radiotherapy: 10-year follow-up. Int J Radiat Oncol Biol Phys. 2011;81(4):1025–31.
Marijnen CA, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: report of a multicenter randomized trial. Int J Radiat Oncol Biol Phys. 2003;55(5):1311–20.
Strassburg J, et al. MRI-based indications for neoadjuvant radiochemotherapy in rectal carcinoma: interim results of a prospective multicenter observational study. Ann Surg Oncol. 2011;18(10):2790–9.
Junginger T, et al. Rectal carcinoma: is too much neoadjuvant therapy performed? Proposals for a more selective MRI based indication. Zentralbl Chir. 2006;131(4):275–84.
Kreis ME, et al. Use of preoperative magnetic resonance imaging to select patients with rectal cancer for neoadjuvant chemoradiation—interim analysis of the German OCUM trial (NCT01325649). J Gastrointest Surg. 2016;20(1):25–32. discussion 32–33.
Ruppert R, et al. Oncological outcome after MRI-based selection for neoadjuvant chemoradiotherapy in the OCUM rectal cancer trial. Br J Surg. 2018;105(11):1519–29.
Ruppert R, et al. Avoidance of overtreatment of rectal cancer by selective chemoradiotherapy: results of the optimized surgery and MRI-based multimodal therapy trial. J Am Coll Surg. 2020;231(4):413–425.e2.
Maas M, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–44.
Petrelli F, et al. Pathologic complete response and disease-free survival are not surrogate endpoints for 5‑year survival in rectal cancer: an analysis of 22 randomized trials. J Gastrointest Oncol. 2017;8(1):39–48.
Sargent D, et al. Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X‑ACT, PETACC‑3, C‑06, C‑07 and C89803. Eur J Cancer. 2011;47(7):990–6.
Merkel S, et al. Time to locoregional recurrence after curative resection of rectal carcinoma is prolonged after neoadjuvant treatment: a systematic review and meta-analysis. Colorectal Dis. 2011;13(2):123–31.
Allegra CJ, et al. Neoadjuvant 5‑FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(11):djv248. https://doi.org/10.1093/jnci/djv248.
Aschele C, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29(20):2773–80.
Deng HY, et al. Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur J Cardiothorac Surg. 2017;51(3):421–31.
Wiśniowska K, et al. Does the addition of oxaliplatin to preoperative chemoradiation benefit cT4 or fixed cT3 rectal cancer treatment? A subgroup analysis from a prospective study. Eur J Surg Oncol. 2016;42(12):1859–65.
Gerard JP, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28(10):1638–44.
Rödel C, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979–89.
Bittner R, et al. Report on the workshop “workflow rectal cancer II” in Burghausen. Zentralbl Chir. 2007;132(2):95–8.
Buhr HJ, et al. Clinical pathway (workflow) for diagnostic, therapy and follow-up in patients with rectal cancer. Zentralbl Chir. 2006;131(4):285–97.
Roxburgh CSD, et al. Changes in the multidisciplinary management of rectal cancer from 2009 to 2015 and associated improvements in short-term outcomes. Colorectal Dis. 2019;21(10):1140–50.
Conroy T, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(5):702–15.
Bahadoer RR, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42.
Garcia-Aguilar J, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. 2022;40(23):2546–56.
Fokas E, et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol. 2022;8(1):e215445.
Jin J, et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol. 2022; https://doi.org/10.1200/JCO.21.01667.
Benson AB, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(7):874–901.
Cercek A, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4(6):e180071.
Smith JJ, et al. Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3‑year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767.
Ludmir EB, et al. Total neoadjuvant therapy for rectal cancer: an emerging option. Cancer. 2017;123(9):1497–506.
Wang X, et al. Total neoadjuvant treatment (CAPOX plus radiotherapy) for patients with locally advanced rectal cancer with high risk factors: a phase 2 trial. Radiother Oncol. 2018;129(2):300–5.
Riesco-Martinez MC, et al. Impact of total neoadjuvant therapy vs. standard chemoradiotherapy in locally advanced rectal cancer: a systematic review and meta-analysis of randomized trials. Cancers (Basel). 2020;12(12):3655.
Liu S, et al. Total neoadjuvant therapy (TNT) versus standard neoadjuvant chemoradiotherapy for locally advanced rectal cancer: a systematic review and meta-analysis. Oncologist. 2021;26(9):e1555–e66.
Valadão M, et al. Do we have to treat all T3 rectal cancer the same way? Clin Colorectal Cancer. 2020;19(4):231–5.
Lee JB, et al. Role of preoperative chemoradiotherapy in clinical stage II/III rectal cancer patients undergoing total mesorectal excision: a retrospective propensity score analysis. Front Oncol. 2020;10:609313.
Bujko K, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27(5):834–42.
Ciseł B, et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol. 2019;30(8):1298–303.
Benson AB, et al. NCCN guidelines insights: rectal cancer, version 6.2020. J Natl Compr Canc Netw. 2020;18(7):806–15.
Harrison JD, et al. Patient and physician preferences for surgical and adjuvant treatment options for rectal cancer. Arch Surg. 2008;143(4):389–94.
Breugom AJ, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26(4):696–701.
Goodman KA. Definitive chemoradiotherapy (“watch-and-wait” approach). Semin Radiat Oncol. 2016;26(3):205–10.
Lopez-Campos F, et al. Watch and wait approach in rectal cancer: current controversies and future directions. World J Gastroenterol. 2020;26(29):4218–39.
Xu Q, et al. MRI evaluation of complete response of locally advanced rectal cancer after neoadjuvant therapy: current status and future trends. Cancer Manag Res. 2021;13:4317–28.
Dattani M, et al. Oncological and survival outcomes in watch and wait patients with a clinical complete response after neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and pooled analysis. Ann Surg. 2018;268(6):955–67.
Habr-Gama A, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–7. discussion 717–8.
Habr-Gama A, et al. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(12):1692–8.
Glynne-Jones R, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22–iv40.
Yu M, et al. Does a long interval between neoadjuvant chemoradiotherapy and surgery benefit the clinical outcomes of locally advanced rectal cancer? A systematic review and meta analyses. Int J Colorectal Dis. 2022;37(4):855–68.
Petrelli F, et al. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studies. Ann Surg. 2016;263(3):458–64.
Deidda S, et al. Association of delayed surgery with oncologic long-term outcomes in patients with locally advanced rectal cancer not responding to preoperative Chemoradiation. JAMA Surg. 2021;156(12):1141–9.
Dizdarevic E, Hansen TF, Jakobsen A. The Prognostic Importance of ctDNA in Rectal Cancer: A Critical Reappraisal. Cancers (Basel). 2022;14(9):2252.
Murahashi S, et al. Serial circulating tumour DNA analysis for locally advanced rectal cancer treated with preoperative therapy: prediction of pathological response and postoperative recurrence. Br J Cancer. 2020;123(5):803–10.
Vidal J, et al. Clinical impact of presurgery circulating tumor DNA after total neoadjuvant treatment in locally advanced rectal cancer: a biomarker study from the GEMCAD 1402 trial. Clin Cancer Res. 2021;27(10):2890–8.
Kotaka M, et al. Association of circulating tumor DNA dynamics with clinical outcomes in the adjuvant setting for patients with colorectal cancer from an observational GALAXY study in CIRCULATE-Japan. J Clin Oncol. 2022;40(4_suppl):9.
Bach SP, et al. STAR-TREC phase II: Can we save the rectum by watchful waiting or transanal surgery following (chemo)radiotherapy versus total mesorectal excision for early rectal cancer? J Clin Oncol. 2022;40(16_suppl):3502–3502.
Hearn N, et al. Neoadjuvant radiotherapy dose escalation in locally advanced rectal cancer: a systematic review and meta-analysis of modern treatment approaches and outcomes. Clin Oncol. 2021;33(1):e1–e14.
Cercek A, et al. Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res. 2020;26:3271–9.
André T, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–18.
Overmann MJ, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–9.
Cercek A, et al. PD‑1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Eng J Med. 2022;386:2363–76.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Piringer and A. De Vries declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piringer, G., De Vries, A. Total neoadjuvant therapy in rectal cancer. memo 16, 21–30 (2023). https://doi.org/10.1007/s12254-022-00854-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-022-00854-1