Abstract
Based on the World Health Organization (WHO) criteria, serrated lesions were classified as sessile serrated adenoma/polyp (SSA/P), traditional serrated adenoma (TSA), and hyperplastic polyp (HP). Large serrated lesions are found to be associated with advanced colonic adenoma in the colon. Serrated lesions of the colorectum are believed to account for 15–20 % of all colorectal cancers via the “serrated neoplastic pathway” with SSA/P being the main precursor lesion. Serrated lesions are also thought to account for around 30 % of cancers that develop after a negative colonoscopy or the interval cancers. While serrated lesions are often flat or sessile and inconspicuous on conventional white light colonoscopy, missed lesions are not uncommon. Increased detection of serrated lesions may potentially reduce the incidence and mortality of colorectal cancers, especially the risk of interval cancers. Further research shall be directed to improve detection of serrated lesions by colonoscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is a major cause of cancer-related morbidity and mortality worldwide. In the USA, it is the third most common diagnosed cancer and third leading cause of cancer death [1]. Although CRC is generally considered to be a western disease, the incidence of CRC is also rising at an alarming rate in many Asian countries. CRC has emerged as the top cancer in Korea with the highest age-standardized incidence rate in the world (45 per 100,000 population) [2].
Conventionally, CRC was believed to develop through the adenoma-carcinoma sequence with the continuous accumulation of genetic alterations [3]. Recently, evidence showed that around 10–20 % of CRCs developed through an alternative pathway called the serrated neoplastic pathway that arise from serrated lesions [4]. Certain subtypes of serrated lesions, such as sessile serrated adenoma/polyp (SSA/P), are considered to have higher potential for malignant transformation.
Serrated neoplastic pathway is also considered to have an important role in patients who developed CRCs after colonoscopy, often called postcolonoscopy/interval cancers [5–10]. Thus, identification and complete removal of these lesions appears to be instrumental in reducing CRC development. This review will discuss the pathology of serrated lesions, the association of serrated lesions with advanced neoplasia, and suggest ways to improve detection of these lesions.
Clinicopathologic Presentation of Serrated Lesions
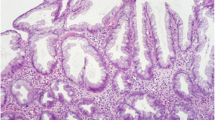
Serrated polyps are a group of heterogeneous lesions which are characterized by the serrated (“saw-tooth”) architecture of epithelium that lines the colonic crypts. There was much confusion in the nomenclature in the past. In 2010, the World Health Organization published a statement to standardize the terminology and diagnostic criteria of sporadic serrated lesions [11]. Serrated lesions are now classified into hyperplastic polyp (HP), sessile serrated adenoma or polyp (SSA/P), and traditional serrated adenoma (TSA).
Hyperplastic polyp is the most prevalent subtype of serrated lesions. Around one-quarter of average-risk individuals have at least one hyperplastic polyp in the colon. They are characterized by elongation of intestinal crypts, with serration of the upper half of the crypt. Cytological dysplasia does not occur in HPs. Microvesicular hyperplastic polyps (MVHPs) and goblet cell hyperplastic polyps (GCHPs) are the two most common subtypes of HPs. Both are more often located in the distal colon [12, 13].
Sessile serrated adenoma/polyps are the second commonest subtype of serrated lesions. It is usually flat or sessile and located in the proximal colon [13], which accounted for around 20–35 % of all serrated lesions. In SSA/P, the abnormal architecture characteristics are secondary to abnormal proliferation. Crypt proliferation leads to increase in crypts being asymmetric of T-shape or inverted L-shape. The other histological features are hyperserration throughout the base or in the crypts and muscle pseudoinvasion [4, 14]. SSA/Ps can be further subdivided into SSA/Ps without dysplasia and SSA/Ps with dysplasia, which is likely to indicate a more aggressive behavior [11]. Dysplastic component is present in around 15–30 % of SSA/Ps [12, 15, 16].
Traditional serrated adenoma is a rare subtype of serrated lesions. The term “serrated adenoma” was first introduced by Longacre et al. in 1990 [17]. It accounted for <1 % of serrated lesions and are most often located in the distal colon. Unlike SSA/Ps, TSA can be sessile or pedunculated. TSA has complex and distorted tubulovillous and villous configuration. Prominent serration and ectopic crypt foci are the other histological characteristics of TSA. Dysplasia is present by definition [18•, 19]. These features could allow distinction of TSA from other serrated lesions.
In contrast, hyperplastic polyposis syndrome (HPS), or serrated polyposis syndrome (SPS), is a rare form of polyposis syndrome, which was associated with even higher risk of CRCs. Torlakovic et al. showed that the polyps in patient with HPS are more similar to serrated adenomas rather than conventional HPs, further suggesting the link between serrated adenomas and cancer [20].
Serrated Neoplastic Pathway
CRCs develop through accumulation of genetic alteration and molecular changes. There are at least three proposed molecular mechanisms of colorectal tumorigenesis: chromosomal instability, defective mismatch repair gene leading to microsatellite instability (MSI) and epigenetic DNA promoter hypermethylation leading to CpG island methylator phenotype (CIMP). The serrated neoplastic pathway is often referred to as CIMP pathway or the sporadic MSI pathway [18•]. Hypermethylation of CpG islands in the promotor regions of the tumor suppressor genes and mutation of BRAF proto-oncogene are the most important molecular alterations in this pathway [13]. Hypermethylation of CpG island within the promotor region leads to reduced expression of a gene [21], and in the case of tumor suppressor genes, promotes carcinogenesis. To determine the CIMP status, a panel of five genes will be assessed and hypermethylation of at least three genes is considered to be CIMP-high [22]. BRAF functions as a molecular switch in the MAPK/ERK pathway, which regulates cell proliferation, differentiation, and survival. Mutation in BRAF will result in uncontrolled cell proliferation leading to neoplastic process [23, 24]. The mechanism leading to CIMP-high/positive status is not fully understood, but BRAF mutation may have a role [13]. Evidence showed that BRAF mutations are strongly associated with CIMP status in CRC [24, 25].
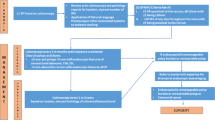
CIMP-high, BRAF mutant CRC was hypothesized to arise and progress through this sequence: colonic mucosa to MVHP, to SSA/P (or colonic mucosa directly to SSA/P), to SSA/P with dysplasia, then to CRC. This is based on the relative prevalence of BRAF mutation and CIMP-high phenotype in various serrated lesions [13, 26–28]. However, direct evidence to confirm the hypothesis does not exist.
TSA is less common than SSA/P and there is few data on its molecular profile. TSA is group of more heterogeneous lesion in terms of morphology and molecular characteristics. The frequency of KRAS and BRAF mutations varied among different subtypes of TSA. The mechanism of progression of TSA to carcinoma and the association with serrated neoplastic pathway is largely unclear [29, 30].
Detection of Serrated Lesions
Polyp Characteristics
Hyperplastic polyps are usually diminutive in size and most frequently found in the rectosigmoid colon, which are usually sessile or flat with a pale color. Small (<5 mm) hyperplastic polyps in the rectosigmoid colon are common and are generally considered to be benign [13]. On the other hand, SSA/Ps and TSA deserve more attention because of their malignant potential.
Pereya et al. showed that a vast majority (94 %) of SSA/Ps was located in the right-sided colon. Around half of the SSA/Ps is flat. Similar findings were reported in other studies. SSA/Ps are typically larger than HPs. The average size of SSA/Ps is 10.3 mm and 14 % of lesions are greater than or equal to 20 mm [31•, 32, 33].
One of the distinctive features of SSA/P is the presence of a mucus cap, which comprised of a layer of mucus adherent to the surface of the lesion, giving the lesion a yellow or rust color on white light endoscopy. This feature was considered the most prevalent distinctive feature of SSA/Ps and it is present in around 40–60 % of SSA/Ps [18•, 31•, 32, 34, 35]. Apart from the presence of mucus cap, peripheral rim of debris or bubbles and lesions obscuring underlying submucosal blood vessel and nodular surface are also distinctive features of SSA/Ps [32]. Other endoscopic features that are independently associated with SSA/Ps are lesions in the proximal colon, flat morphology, and red-colored surface [31•]. [15]. Unlike SSA/Ps, TSAs are usually located in the distal colon. They are often >5 mm and can be pedunculated or sessile [13].
Ways to Improve Detection of Serrated Lesions
Apart from conventional white light colonoscopy, the use of image enhanced colonoscopy such as narrow band imaging (NBI) can enhance the visibility of SSA/Ps (Fig. 1). Hazewinkel et al. showed that cloud-like surface, indistinct border, irregular shape, and dark spots inside crypts are independent predictors of SSA/Ps on NBI examination. Combining these endoscopic features on NBI examination, the accuracy of differentiating SSA/Ps from HPs is 93 % [36•]. A Dutch group developed the “Workgroup serrAted polypS and Polyposis (WASP)” classification for optical diagnosis of different polyps including adenoma, SSA/Ps, and HPs. It combined the NBI International Colorectal Endoscopic classification and criteria for differentiation of SSA/Ps described by Hazewinkel et al. [36•] in a stepwise approach. The use of NBI and the WASP classification may improve the diagnostic accuracy of serrated lesions by colonoscopy. It is especially useful in determining whether a diminutive lesion can be left in situ safely as the negative predictive value for diminutive neoplastic lesions (adenoma and SSA/Ps) was 91 % [37].
Type II Kudo pit pattern is frequently described in SSA/Ps [32, 33]. A Japanese group proposed a new Type II open-shape pit pattern (Type II-O). This is similar to the conventional Type II pattern, but the pits are wider and more roundish in shape. This pit pattern is highly predictive of SSA/Ps with specificity of 97.3 % [38]. Recent evidence showed that combination of NBI with optical magnification can allow for more detailed examination of polyps and are potentially useful to discriminate SSA/Ps from other lesions [39, 40].
These lesions, such as SSA/Ps, are easily masked by feces, debris, or non-transparent fluid in the colon [33], which illustrates the importance of satisfactory bowel preparation. A recent retrospective study showed that cap-assisted colonoscopy detected more significant serrated polyps (defined as SSA/Ps, TSA, proximal HPs and HPs ≥ 1 cm) than colonoscopy performed without a cap [41].
In real-life clinical practices, the detection rates of serrated lesions can be highly variable and operator dependent. A study showed that the detection rates for proximal serrated lesions ranged from 0–9.8 % in different centers. Another study from the Netherlands showed that the detection rate of proximal serrated polyps differed significantly among endoscopists, ranging from 6 to 22 % [42, 43]. In order to increase the detection rate of serrated lesions, endoscopists should have training on the features of these lesions. As there is a strong correlation between adenoma detection rates and detection rates of serrated lesions [44, 45], endoscopist could measure their adenoma detection rates as a surrogate marker for detection of serrated lesions. One recent study showed that longer withdrawal time was also associated with higher detection rate of proximal serrated lesions [46].
Association of Serrated Lesions With Advanced Neoplasia
Multiple studies have demonstrated that serrated lesions, especially the large or proximal serrated lesions, are associated with advanced colonic neoplasm [12, 47–53, 54•]. In a large cohort of asymptomatic average-risk subjects undergoing screening colonoscopy, the presence of large (≥1 cm) serrated polyp was found to be an independent predictor of advanced colorectal neoplasia with an odds ratio (OR) of 3.24. Both right and left-sided serrated polyps were associated with advanced colorectal neoplasia [47]. Another study involving 5059 average-risk patients undergoing screening colonoscopy showed that 6.5 % patients had proximal serrated polyps while 1.8 % patients had large (≥1 cm) serrated polyps. Large serrated polyps were associated with advanced colorectal neoplasia (OR 2.49), regardless of their proximal (OR 4.15) or distal (OR 2.61) locations [51]. There were similar results with studies involving Asian patients. A study involving 1282 Chinese patients undergoing screening colonoscopies showed that the presence of advanced neoplasia was associated with the presence of large or proximal serrated polyps (OR 1.8) [52]. Another study involving average-risk Chinese undergoing colonoscopy showed that the independent predictors of synchronous advanced colorectal neoplasia were the presence of SSA (OR 4.52), proximal serrated polyps (OR 2.23), hyperplastic polyps (OR 1.66), and the presence of three or more serrated polyps (OR 4.86) [53].
A multicenter study in Japan showed that the presence of large (≥10 mm) serrated polyps was associated with both advanced neoplasia (OR 4.01) and CRC (OR 3.34) [48]. Schreiner et al. showed that the presence of proximal serrated polyp is associated with higher chance of interval neoplasia at surveillance colonoscopy (OR 3.14) [55]. Although there is considerable heterogeneity among these studies, a recent meta-analysis confirmed the association between proximal and large serrated polyps with advanced neoplasia [54•].
Association of Serrated Lesions With Cancer
The natural history of serrated polyp is uncertain. However, there were case reports describing the rather unexpected rapid development of CRCs from serrated lesions. Two Japanese case reports described progression of a 15-mm ascending colon SSA into cancer in 8 months [56] and a 20-mm SSA with severe dysplasia progressed to adenocarcinoma in 2 years [57]. On the other hand, some evidences showed that serrated lesions progressed slowly and some serrated lesions might not turn malignant even left in situ for a median of 11 years [58]. A study that evaluated 2416 SSAs showed that the median age of patient increases in the following order: SSA without dysplasia (61 years), SSA with low-grade dysplasia (66 years), SSA with high-grade dysplasia (72 years), and SSA with adenocarcinoma (76 years). Based on this, the authors indirectly concluded that SSA progressed to cancer in a stepwise manner in a period over 10–15 years [16]. A case series showed that serrated adenocarcinoma was often accompanied by synchronous residual serrated adenomas and remote serrated adenoma, suggesting a possible etiological link between serrated adenoma and serrated adenocarcinoma [59].
Although evidence showed that some serrated lesions may not progress to cancer, its presence was associated with a higher risk of CRC. A large population-based trial involving 12,955 patients screened with flexible sigmoidoscopy showed that those with large serrated polyps, as compared to those without polyps, have higher risk of developing CRC (hazard ratio of 2.5) over a median of 10.9 years [58]. Hiroaka et al. showed that the presence of large, serrated polyps was associated with synchronous CRC (OR 3.34) [48].
The risk of CRCs varies in patients harboring different types of serrated lesions. A study showed that the incidence of subsequent CRCs was significantly higher in patients with SSAs at baseline than those with HPs at baseline (12.5 vs 1.8 %). In this study, 15 % of patients with SSA at baseline developed subsequent CRCs or adenomatous polyps with high-grade dysplasia [60].
Interval Cancers
There are overwhelming evidences that colonoscopy and polypectomy significantly reduce the incidence and mortality of CRC. However, recent studies showed that the protection may be more prominent for left side than right side colonic cancer. In fact, some studies showed that there may be no significant protection from right-side cancer by colonoscopy [61–65].
SSA/P, an important precursor lesion in serrated pathway, is more often diagnosed in the proximal colon. SSA/P is often flat and inconspicuous on endoscopy [49, 66] and can be easily missed. With increased recognition in the recent years, the reported prevalence of SSA/Ps has increased, indicating some of these lesions were missed in the past [44, 67, 68]. Pohl et al. showed that around one third of SSA/Ps and almost half of the serrated lesions of 10–20 mm were incompletely resected [69•]. An endoscopist may also fail to correctly size the entire polyp because of the indistinct border, resulting in incomplete removal. Snare polypectomy may also be inadequate in the larger, sessile/flat lesions, leaving residual tissue. More specialized technique such as endoscopic mucosal resection (EMR) may be required for complete removal of these lesions. Even with endoscopic mucosal resection, residual polyp was identified in 8.7 % of patients with SSA/Ps [70]. As the growth rate of serrated lesions is variable, some of these residual lesions may potentially grow into malignant tumor before the usual surveillance interval.
Interval cancers shared similar biology with serrated lesions such as CIMP status and MSI [71, 72]. Around 30 % of interval cancers are thought to originate from serrated lesions. A study showed that CIMP positive status was present in 57 % of interval cancers, as compared to 33 % of non-interval cancers (p = 0.004) [72]. In another study, MSI was found in 30.4 % of interval cancers compared to 10.3 % of non-interval cancers (p = 0.03). After adjusting for age, interval cancers were 3.7 times more likely to have MSI than are non-interval cancers [71]. The similarity of biology in serrated lesions and interval cancers suggested that serrated neoplastic pathway may have important role in the development of these cancers.
The nomenclature and diagnostic criteria of serrated lesions are evolving over the years, which lead to inconsistency on reporting of different serrated lesions. Payne et al. showed that the reporting rate of SSA/Ps is highly variable among different centers, ranging from 0–9.8 %. Some pathologists never reported proximal serrated lesions such as SSA/Ps, even in high-volume centers [42]. Another study showed that around one third of the HPs (>5 mm) diagnosed between 2003 and 2008 could be reclassified as SSA/Ps after a review in 2011 [73]. Underreporting of important precursor lesions, such as SSA/Ps, could potentially lead to inappropriate surveillance interval, thus contributing to postcolonoscopy/interval cancers.
Current Management Strategy
Owing to the malignant potential, it is recommended that all serrated lesions proximal to the sigmoid colon should be fully resected. In addition, all serrated lesions of greater than 5 mm in size in the rectosigmoid region should also be resected [18•].
As the natural history of different types of serrated lesions is largely unclear, surveillance recommendations are mainly based on expert opinion [18•, 74, 75]. The current recommended surveillance intervals for various serrated lesions by the U.S. Multi-Society Task Force Guideline (2012) and the expert consensus opinion by Rex et al. were summarized in Table 1, which are largely dependent on the size, multiplicity, and the histology of the serrated lesions..
Conclusion
Serrated neoplastic pathway is now generally considered to be another important pathway of colorectal carcinogenesis. Certain subtypes of serrated lesions, large SSA/Ps and TSAs, are of higher malignant potential. While there are increasing evidences that these lesions are associated with advanced neoplasia and CRCs, high-quality longitudinal studies on the natural history of serrated lesions are lacking. Some of the sessile lesions, such as SSA/Ps, are often difficult to detect because of its unique endoscopic features. Increased recognition and awareness among endoscopist is necessary to improve the detection rate. Newer techniques such as NBI and magnification endoscopy can help better characterize the lesion and improve detection. Accurate pathological diagnosis is of equal importance, and appropriate surveillance interval can then be recommended. As serrated lesions contribute to a significant proportion of CRCs, especially proximal CRCs and interval cancer, increased detection and complete removal of these precursor lesions will be the key to further enhance the efficacy of colonoscopy in preventing CRCs.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.1, cancer incidence and mortality worldwide: IARC CancerBase No.11 [Internet]. Lyon: International Agency for Research on Cancer; 2014.
Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32.
Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–100.
le Clercq CM, Sanduleanu S. Interval colorectal cancers: what and why. Curr Gastroenterol Rep. 2014;16:375.
Rabeneck L, Paszat L. Circumstances in which colonoscopy misses cancer. Frontline Gastroenterol. 2010;1:52–8.
le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63:957–63.
Samadder NJ, Curtin K, Tuohy TM, et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 2014;146:950–60.
Huang Y, Gong W, Su B, et al. Risk and cause of interval colorectal cancer after colonoscopic polypectomy. Digestion. 2012;86:148–54.
Singh H, Nugent Z, Demers AA, et al. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588–96.
Snover DC, Ahnen DJ, Burt RW, et al. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system, vol. 3 Ch.8. Berlin: Springer; 2010.
Hazewinkel Y, de Wijkerslooth TR, Stoop EM, et al. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy. 2014;46:219–24.
IJspeert JE, Vermeulen L, Meijer GA, et al. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol. 2015;12:401–9.
Szylberg L, Janiczek M, Popiel A, et al. Serrated polyps and their alternative pathway to the colorectal cancer: a systematic review. Gastroenterol Res Pract. 2015;2015:573814.
Bouwens MW, van Herwaarden YJ, Winkens B, et al. Endoscopic characterization of sessile serrated adenomas/polyps with and without dysplasia. Endoscopy. 2014;46:225–35.
Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63:681–6.
Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524–37.
Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–29. Quiz 1314, 1330. Expert consensus recommendations on pathological diagnosis, endoscopic treatment, and surveillance strategy of serrated lesions.
Aust DE, Baretton GB. Serrated polyps of the colon and rectum (hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, and mixed polyps)-proposal for diagnostic criteria. Virchows Arch. 2010;457:291–7.
Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748–55.
Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–13.
Toyota M, Ahuja N, Ohe-Toyota M, et al. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–6.
Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305.
Kambara T, Simms LA, Whitehall VL, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–44.
Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–93.
O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491–501.
Fernando WC, Miranda MS, Worthley DL, et al. The CIMP phenotype in BRAF mutant serrated polyps from a prospective colonoscopy patient cohort. Gastroenterol Res Pract. 2014;2014:374926.
Kim KM, Lee EJ, Ha S, et al. Molecular features of colorectal hyperplastic polyps and sessile serrated adenoma/polyps from Korea. Am J Surg Pathol. 2011;35:1274–86.
Tsai JH, Liau JY, Lin YL, et al. Traditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesis. Mod Pathol. 2014;27:1375–85.
Bettington ML, Walker NI, Rosty C, et al. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod Pathol. 2015;28:414–27.
Pereyra L, Gomez EJ, Gonzalez R, et al. Finding sessile serrated adenomas: is it possible to identify them during conventional colonoscopy? Dig Dis Sci. 2014;59:3021–6. Study evaluating the endoscopic features of SSAs.
Tadepalli US, Feihel D, Miller KM, et al. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video). Gastrointest Endosc. 2011;74:1360–8.
Jaramillo E, Tamura S, Mitomi H. Endoscopic appearance of serrated adenomas in the colon. Endoscopy. 2005;37:254–60.
Rex DK, Hewett DG, Snover DC. Editorial: detection targets for colonoscopy: from variable detection to validation. Am J Gastroenterol. 2010;105:2665–9.
Rosty C, Hewett DG, Brown IS, et al. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48:287–302.
Hazewinkel Y, Lopez-Ceron M, East JE, et al. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013;77:916–24. Study evaluating the endoscopic features of SSAs on white-light endoscopy and narrow-band imaging.
JE IJ, Bastiaansen BA, van Leerdam ME, et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut. 2015. doi:10.1136/gutjnl-2014-308411.
Kimura T, Yamamoto E, Yamano HO, et al. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol. 2012;107:460–9.
Yamashina T, Takeuchi Y, Uedo N, et al. Diagnostic features of sessile serrated adenoma/polyps on magnifying narrow band imaging: a prospective study of diagnostic accuracy. J Gastroenterol Hepatol. 2015;30:117–23.
Yamada M, Sakamoto T, Otake Y, et al. Investigating endoscopic features of sessile serrated adenomas/polyps by using narrow-band imaging with optical magnification. Gastrointest Endosc. 2015;82:108–17.
Rzouq F, Gupta N, Wani S, et al. Cap assisted colonoscopy for the detection of serrated polyps: a post-hoc analysis. BMC Gastroenterol. 2015;15:11.
Payne SR, Church TR, Wandell M, et al. Endoscopic detection of proximal serrated lesions and pathologic identification of sessile serrated adenomas/polyps vary on the basis of center. Clin Gastroenterol Hepatol. 2014;12:1119–26.
Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–6.
Ross WA, Thirumurthi S, Lynch PM, et al. Detection rates of premalignant polyps during screening colonoscopy: time to revise quality standards? Gastrointest Endosc. 2015;81:567–74.
Kahi CJ, Li X, Eckert GJ, et al. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc. 2012;75:515–20.
de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc. 2013;77:617–23.
Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol. 2009;104:695–702.
Hiraoka S, Kato J, Fujiki S, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology. 2010;139:1503–10. 1510 e1501-1503.
Rondagh EJ, Masclee AA, Bouwens MW, et al. Endoscopic red flags for the detection of high-risk serrated polyps: an observational study. Endoscopy. 2011;43:1052–8.
Buda A, De Bona M, Dotti I, et al. Prevalence of different subtypes of serrated polyps and risk of synchronous advanced colorectal neoplasia in average-risk population undergoing first-time colonoscopy. Clin Transl Gastroenterol. 2012;3:e6.
Alvarez C, Andreu M, Castells A, et al. Relationship of colonoscopy-detected serrated polyps with synchronous advanced neoplasia in average-risk individuals. Gastrointest Endosc. 2013;78:333–41. e331.
Leung WK, Tang V, Lui PC. Detection rates of proximal or large serrated polyps in Chinese patients undergoing screening colonoscopy. J Dig Dis. 2012;13:466–71.
Ng SC, Ching JY, Chan VC, et al. Association between serrated polyps and the risk of synchronous advanced colorectal neoplasia in average-risk individuals. Aliment Pharmacol Ther. 2015;41:108–15.
Gao Q, Tsoi KK, Hirai HW, et al. Serrated polyps and the risk of synchronous colorectal advanced neoplasia: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:501–9. quiz 510. A meta-analysis evaluating the association of serrated polyps with colorectal advanced neoplasia.
Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010;139:1497–502.
Oono Y, Fu K, Nakamura H, et al. Progression of a sessile serrated adenoma to an early invasive cancer within 8 months. Dig Dis Sci. 2009;54:906–9.
Yamauchi T, Watanabe M, Hasegawa H, et al. Serrated adenoma developing into advanced colon cancer in 2 years. J Gastroenterol. 2002;37:467–70.
Holme O, Bretthauer M, Eide TJ, et al. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut. 2015;64:929–36.
Garcia-Solano J, Perez-Guillermo M, Conesa-Zamora P, et al. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: further insights into the full recognition of a new subset of colorectal carcinoma. Hum Pathol. 2010;41:1359–68.
Lu FI, van Niekerk de W, Owen D, et al. Longitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinoma. Am J Surg Pathol. 2010;34:927–34.
Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup N Engl J Med. 1993;329:1977–81.
Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96.
Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105.
Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8.
Lakoff J, Paszat LF, Saskin R, et al. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008;6:1117–21. quiz 1064.
Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656–64.
Abdeljawad K, Vemulapalli KC, Kahi CJ, et al. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest Endosc. 2015;81:517–24.
Burgess NG, Tutticci NJ, Pellise M, et al. Sessile serrated adenomas/polyps with cytologic dysplasia: a triple threat for interval cancer. Gastrointest Endosc. 2014;80:307–10.
Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–80 e71. A study evaluating the rate of incomplete removal of various colonic polyps during colonoscopy.
Rex KD, Vemulapalli KC, Rex DK. Recurrence rates after EMR of large sessile serrated polyps. Gastrointest Endosc. 2015;82:538–41.
Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700–5.
Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105:1189–95.
Tinmouth J, Henry P, Hsieh E, et al. Sessile serrated polyps at screening colonoscopy: have they been under diagnosed? Am J Gastroenterol. 2014;109:1698–704.
Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57.
Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yuk Fai Lam and Wai K. Leung declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Prevention and Early Detection
Rights and permissions
About this article
Cite this article
Lam, Y.F., Leung, W.K. The Importance of Increased Serrated Polyp Detection Rate. Curr Colorectal Cancer Rep 12, 81–87 (2016). https://doi.org/10.1007/s11888-016-0313-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-016-0313-x