Abstract
Purpose of Review
This review describes the presentation, diagnosis, and management of congenital coronary artery fistulas (CAFs) in adults.
Recent Findings
CAFs are classified as coronary-cameral or coronary arteriovenous fistulas. Fistulous connections at the distal coronary bed are more likely to be aneurysmal with higher risk of thrombosis and myocardial infarction (MI). Medium-to-large or symptomatic CAFs can manifest as ischemia, heart failure, and arrhythmias. CAF closure is recommended when there are attributable symptoms or evidence of adverse coronary remodeling. Closure is usually achievable using transcatheter techniques, though large fistulas may require surgical ligation with bypass. Given their anatomic complexity, cardiac CT with multiplanar 3-D reconstruction can enhance procedural planning of CAF closure. Antiplatelet and anticoagulation are essential therapies in CAF management.
Summary
CAFs are rare cardiac anomalies with variable presentations and complex anatomy. CAF management strategies include indefinite medical therapy, percutaneous or surgical CAF closure, and lifelong patient surveillance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery fistulas (CAFs) are abnormal communications between coronary arteries and other vascular structures. CAFs can be subdivided into [1] coronary-cameral fistulas that drain into cardiac chambers (e.g., atria, ventricles) and [2] coronary arteriovenous fistulas that drain into the pulmonary/systemic circulation (e.g., pulmonary artery, bronchial veins, vena cava, or coronary sinus) [1].
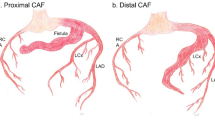
The absence of an outlet capillary bed allows for minimal vascular resistance to flow, which can lead to shunting of blood and progressive dilatation of the fistula and chamber over time. If a CAF originates within the proximal one-third of the artery, only the proximal feeding arteries are typically dilated, and the coronary artery diameter distal to the CAF is normal. Distal CAFs tend to have dilation of the entire vessel leading to coronary aneurysm formation.
CAFs, which can be both single or multiple in a given patient, are rare and found in ~ 0.9% of the population, with the majority being congenital in origin secondary to abnormal embryological development. If sinusoids connected to primitive myocardium fail to regress, such as in pulmonary atresia with intact ventricular septum, there may be resultant coronary-cameral fistulae. Persistent remnant connections between coronary arteries and mediastinal arteries may lead to coronary arteriovenous fistulas [2]. There is a smaller percentage of CAFs that are iatrogenic from etiologies such as direct chest trauma, myocardial biopsies, percutaneous coronary interventions, cardiac surgery, intracardiac device implantation, and radiofrequency ablation [1]. CAFs connecting to the pulmonary artery can also be acquired in patients with chronic thromboembolic disease. While the majority of CAFs are isolated, some are associated with other congenital anomalies such as atrial or ventricular septal defects, tetralogy of Fallot, and patent ductus arteriosus [2].
The right coronary artery is the most common site of CAFs which accounts for 50–55% of cases, followed by the left anterior descending artery. Most CAFs are simple with a single fistulous tract, but ~ 10% are complex with intertwined blood vessels and multiple fistulous tracts [2]. If the communication is large enough, CAFs can manifest clinically as myocardial ischemia, heart failure, and arrhythmias. Asymptomatic patients can become symptomatic with age-related variations in endothelial function, cardiac chamber and vascular compliance, and comorbid conditions, leading to increased risk of thrombosis. Still, most CAFs are discovered incidentally during noninvasive imaging or coronary angiography [3•]. Spontaneous closure of CAFs happens in 1–2% of cases, but repair may be warranted if fistulas are enlarging, have hemodynamic consequences, or are causing symptoms. Repair can be surgical or with percutaneous transcatheter closure, with the optimal approach determined by various patient and procedural factors [2].
Diagnosis
While most CAFs are discovered incidentally, some may present with unique features. Given the fistulous connection between two vascular structures with differences in pressure, there may be a continuous murmur on physical exam with a crescendo-decrescendo pattern. The differential for a continuous murmur also includes patent ductus arteriosus, ruptured sinus of Valsalva aneurysm, anomalous left coronary artery arising from the pulmonary artery (ALCAPA), vasculitis, and coronary ectasia secondary to atherosclerosis [1, 2].
Medium to large CAFs can present with chronic angina due to coronary steal with blood shunted through the low-pressure fistula and away from the distal native coronary artery. If large CAFs terminate in right-sided structures such as the right atrium, right ventricle, or pulmonary arteries, there can be left-to-right shunting of blood potentially leading to pulmonary hypertension secondary to high flow and biventricular heart failure. Large CAFs that terminate in the left atrium or left ventricle, create a left-to-left shunt at risk for left heart failure and secondary right heart failure given time. The cardiac chambers and vasculature at risk depends upon the location of CAF drainage.
Other clinical manifestations of large CAFs include thrombosis, embolism, and dissection which can present as acute coronary syndromes, hemopericardium, and tamponade if the fistula ruptures, symptoms (e.g., stridor, dysphagia, pain) due to physical compression of mediastinal structures from dilated coronaries or cardiovascular chambers, papillary muscle abnormalities causing valvular regurgitation, and atrial/ventricular arrhythmias from resultant cardiomyopathy [1, 3•]. While they are not part of the current endocarditis guidelines for routine prophylaxis, CAFs do portend an increased risk of infective endocarditis/endarteritis compared to the general population [2].
Various imaging modalities can be used to visualize CAFs. Larger CAFs can be diagnosed on echocardiography as large vascular anomalies, but the differential diagnosis for this finding should include ALCAPA. Echocardiography is also essential for determining baseline atrial and ventricular chamber dimensions and function, screening for pulmonary hypertension, visualizing flow at the origin or termination points of the fistula, shunting of blood flow, and proximity of the fistula with neighboring cardiac structures [1, 3•].
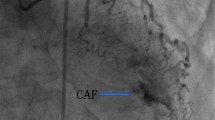
Coronary angiography is used to diagnose CAFs and can help determine which fluoroscopic views are ideal to guide potential intervention. Visualization of fistulas with angiography can be difficult due to incomplete opacification of contrast and overlap of aneurysmal, tortuous vessels, but can be improved with procedural techniques such as the use of larger catheters, power injections, balloon occlusion of the distal fistula, coronary wedge injections, and dual injection at the origin and termination of the fistula [3•].
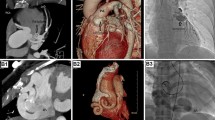
While coronary angiography has been the gold standard historically, multidetector cardiac computed tomographic (CT) imaging is now being used more commonly to visualize CAFs. This modality has the advantages of being a noninvasive procedure with high spatial and temporal resolution, and with shorter acquisition times. While CT alone may not provide adequate visual understanding of size, number of fistulous connections, and anatomic path, multiplanar CT-guided reconstruction can greatly enhance procedural planning with true appreciation of the complexities of these vascular anomalies with 3-D-printed models [2]. 3-D reconstruction involves volumetric image acquisition from CT, data postprocessing with segmentation and modeling, selection of the type of printer and material to be used, and finally the actual process of printing. 3-D-printed models can be used for manual bench testing of different sized and shaped occluder devices to confirm adequate seal and stable rims for closure [4, 5•].
MR angiography can be an alternative to CT if ionizing radiation and iodinated contrast are of concern, but has lower spatial resolution, longer acquisition times, and is less effective in portraying the distal coronary arteries and extracardiac structures [2].
The most common types of CAFs identified on imaging are coronary-cameral fistulas, coronary artery-to-pulmonary artery fistulas, coronary artery-to-coronary sinus fistulas, and coronary artery-bronchial artery fistulas.
-
1.
Coronary-cameral fistulas usually drain into the right ventricle followed by the right atrium and are either directly connected to the cardiac chamber or indirectly through a sinusoidal network of vessels.
-
2.
Coronary artery-to-pulmonary artery fistulas are diagnosed more often now due to frequent usage of CT angiography, and they appear as abnormal contrast blush on imaging. These fistulas can be comprised of either a single fistulous connection, or small multiple fistulous connections between the coronary artery and the main pulmonary trunk. The left anterior descending artery is more commonly involved, followed by the right coronary artery [2].
-
3.
Coronary artery-to-coronary sinus fistulas are the third most common type of CAF presenting as dilated and tortuous coronary arteries attached to a dilated coronary sinus or one of the other cardiac veins. If a dilated coronary sinus is seen on imaging, other causes of this finding should be eliminated such as unroofed coronary sinus, anomalous pulmonary venous return, persistent left-sided superior vena cava, and tricuspid valve abnormalities. Patients with fistulas to the coronary sinus are at high risk for thrombosis and MI [2].
-
4.
Coronary artery-bronchial artery fistulas are much less common and usually originate from the left circumflex artery. These fistulas tend to be silent but can be more prominent in the setting of higher coronary artery pressure (e.g., supravalvular aortic stenosis), or lower bronchial artery pressure (e.g., pulmonary atresia or tetralogy of Fallot). If patients are symptomatic, they can present with dyspnea, hemoptysis, or other clinical manifestations of bronchiectasis [2].
Management
There is a gap in the evidence base on how to best manage patients with CAFs. The 2018 ACC/AHA Guidelines for the Management of Adults with Congenital Heart do not go into specific recommendations of therapy but rather accentuate the importance of a knowledgeable multidisciplinary team to determine the role of medical therapy and/or percutaneous or surgical closure. Based on expert opinion, the 2008 Guidelines attempted to be more granular in terms of when to intervene in CAF, giving a class I indication for closure of a large CAF via surgical or transcatheter techniques after defining the anatomic course of the anomaly, and a class I recommendation for closure of small to medium CAFs with symptoms including myocardial ischemia, arrhythmia, otherwise unexplained ventricular systolic or diastolic dysfunction or enlargement, or endarteritis [6] (Table 1).
The 2008 ACC/AHA guidelines mention a class IIa recommendation to follow patients with small asymptomatic CAFs with echocardiography every 3–5 years to monitor for progression of size or chamber enlargement [6]. These patients are commonly placed on prophylactic long-term antiplatelet therapy. Anticoagulation is also considered for patients with large distal fistulas given the risk of thrombus formation [2].
Medium-sized CAFs have been defined as a vessel diameter \(\ge\) 1 to 2 times the largest diameter of the coronary vessel not feeding the fistula, and large CAFs are > 2 times the largest diameter of the coronary vessel not feeding the fistula [3•]. If patients are low surgical risk and transcatheter closure is not logistically achievable, surgical closure should be considered [3•]. Anatomic features that support surgical CAF ligation include large fistulas with high flow, fistulas with multiple communications and drainage sites, very tortuous and aneurysmal fistulas, fistulas where the distal portion is inaccessible with a closure device, if termination of the fistula is too close to normal coronary segments, if there are large branches at risk for embolization, and if there is need for surgical repair of other congenital abnormalities. In the setting of very large fistulas ≥ 10 mm in size, ligation of the coronary artery proximal and distal to the CAF with placement of a distal coronary bypass graft, in addition to internal closure of the CAF, may be warranted [2, 3•].
The next branchpoint when considering closure is where the fistula originates from (Fig. 1). While comprehensive identification and closure of all feeder vessels is important for all CAFs, those that originate from distal beds are more likely to be associated with the post-procedural complication of MI. This can be due to various mechanisms such as thrombus formation secondary to stasis in the aneurysmal segment of the feeding coronary artery after CAF closure, pre-existing thrombus that embolizes from the proximal aneurysmal vessel to distal side branches, or unintentional jailing of distal side vessels with closure devices. If the risk of these complications is substantial, surgical closure may need to be considered [3•].
Interventional Approach of CAF Closure
Careful procedural planning is necessary to determine if transcatheter closure is feasible, and then, a suitable technical approach needs to be designated (Table 1). Adequate visualization of the CAF anatomy, typically by CT and coronary angiography, is fundamental to delineate fistulous tract and normal coronary artery anatomy by the techniques described above. Procedural planning should include:
-
1.
Proximal versus distal device closure based on CAF location.
-
2.
Retrograde arterial approach typically using coaxial catheter systems or a prograde venous approach using an arteriovenous guidewire “rail.”
-
3.
Choice of closure device depending upon the anatomy.
CAFs originating from proximal coronary beds should have a transarterial approach where the fistula is accessed antegrade through the parent coronary artery, due to a shorter distance to travel. A 7- or 8-French guide catheter can be used via radial or femoral arterial access to engage the coronary artery, after which a wire can be used to enter the fistula. While larger fistulas can be approached with 0.035 hydrophilic wires, smaller fistulas should be traversed with 0.014 coronary wires to prevent vascular injury. Depending on the size of the fistula, either microcatheters or delivery catheters can be advanced over the wires to transport occluder devices for deployment.
A transvenous approach is most commonly used for CAFs originating from distal coronary beds, given a lower risk of injury to the parent vessel with retrograde access. Femoral or internal jugular veins are cannulated and the termination of the fistula is accessed with various sheaths or guiding catheters over an extra support angled hydrophilic 0.035 wire, followed by delivery of the occluder device [3•]. Very tortuous or aneurysmal distal fistulous attachments can be technically challenging for closure especially if the connection is small in diameter, often requiring the creation of an arteriovenous rail. Through either a transarterial or transvenous approach, the wire advanced through either the proximal or distal end of the fistula can be snared and exteriorized, therefore forming an arteriovenous rail that provides extra support for catheter and device delivery [3•].
The devices used for fistula closure include vascular occluders, embolization coils, and stent grafts (Table 2). Vascular occluders are more suitable for large fistulas and are made with braided Nitinol mesh which allows for minimal flow through the device and therefore increased thrombogenicity. These plugs are malleable to vessel anatomy which allows for flexibility and less hemolysis. However, they require a large catheter and more support for delivery. Embolization coils are usually made of steel or platinum to easily conform to the vessel shape and are coated with fibers to increase thrombogenicity. Coils are more suitable for small and tortuous fistulas, but multiple coils may be needed to achieve complete seal and they cannot be easily repositioned unlike vascular occluders. Covered stent grafts are not the first intervention of choice due to risk of stent thrombosis, MI, and occlusion of parent coronary artery side branches, but can be considered for fistulas with multiple origins or if coil or vascular occluders are not feasible.
The need for concomitant medical therapy depends on the type of percutaneous intervention performed. Patients who receive stent grafts will likely have to be on oral anticoagulation or prolonged courses of dual antiplatelet therapy to promote stent patency [3•]. Patients with coronary sinus-type CAFs generally need long-term anticoagulation to prevent adverse events after the reparative procedure, given high risk of thrombosis in these anomalies [2].
Outcomes
The natural history of patients with CAFs is overall benign, with the majority of adults remaining asymptomatic throughout life. However, older longitudinal studies of children with unoperated CAFs suggest a small risk of sudden death. Despite the lack of robust data in this population, the signal for premature mortality associated with this condition has motivated the consideration of CAF interventions to improve long-term outcomes, in addition to providing symptom relief [7,8,9].
Limited case series have shown that transcatheter CAF closure is effective in the setting of favorable anatomy, with ~ 80–90% success rates. Potential rare complications include device migration, MI, recanalization, coronary spasm, fistula dissection, and transient arrhythmias. Device migration is more common in patients with embolization coils, and MI occurs in the setting of stent grafts or large fistulas with stagnant flow after closure. Risk of MI from retrograde thrombus promulgation after transcatheter closure can be mitigated by placing the device > 1 cm away from the origin of the fistula, but these patients can also be referred for surgical closure with simultaneous bypass grafting and started on indefinite oral anticoagulation after closure [3•].
Due to ~ 10% potential risk of CAF residual leakage or recanalization post procedure, it is recommended to follow patients with coronary CT angiography or angiography 1–5 years after CAF closure, or in the setting of recurrent symptoms [2, 3•].
In order to minimize adverse outcomes, transcatheter or surgical CAF closure should be performed at experienced tertiary care centers with the involvement of a comprehensive team including general cardiology, interventional cardiology, and cardiothoracic surgery. Multicenter longitudinal registries are needed to help elucidate the national progression of CAFs and how these patients fare after various transcatheter and surgical interventions. Advancements in cardiac imaging such as the use of 3-D-reconstructed models can hopefully continue to improve outcomes with more optimal peri-procedural planning and device selection [3•].
Conclusion
CAFs are rare cardiac anomalies with variable presentations and complex anatomy. Medium to large or symptomatic CAFs should be closed, with symptoms generally manifested as ischemia, heart failure, and arrhythmias. Transcatheter closure devices include vascular occluders, embolization coils, and stent grafts. Patients with very large fistulas may require surgical ligation with bypass. In repaired CAFs, long-term oral anticoagulation is recommended to minimize the risk of MI. Given the complexity of CAFs, cardiac CT with multiplanar 3-D reconstruction can be utilized to enhance procedural planning. Closure should be considered at experienced tertiary care centers with appropriate specialists and access to equipment.
Data Availability
The authors confirm that the data supporting the findings of this review are available within the article.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rao SS, Agasthi P. Coronary artery fistula. StatPearls. 2023 June 5; https://www.ncbi.nlm.nih.gov/books/NBK559191/
Yun G, Nam TH, Chun EJ. Coronary artery fistulas: pathophysiology, imaging findings, and management. Radiographics. 2018;38(3):688–703.
• Al-Hijji M, El Sabbagh A, El Hajj S, AlKhouli M, El Sabawi B, Cabalka A, et al. Coronary artery fistulas: indications, techniques, outcomes, and complications of transcatheter fistula closure. JACC: Cardiovasc Interv. 2021 July 12; 14(13):1393–406. This study highlights contemporary procedural techniques of transcatheter coronary artery fistula closure, as well as indications, outcomes, and complications of this procedure.
Brantner P, Madaffari A, Fahrni G, Zellweger MJ, Haaf P. 3D-printed visualization of a complex coronary-venous fistula with additional feeders from the descending aorta. J Am Coll Cardiol Case Rep. 2020;2(11):1736–8.
• Kanduri J, Truong QA, Wong SC, Bergman G, Kim L, Holzer R, et al. Percutaneous closure of giant aneurysmal coronary artery-to-coronary sinus fistulae with guidance from three-dimensional printed models: a case series. Eur Heart J Case Rep. 2023 Jan 10; 7:1–6. This case series highlights the use of computed tomography to generate 3D reconstructed models to help augment visuospatial understanding of coronary artery fistula, and to facilitate manual bench testing of devices for closure.
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: executive summary. Circulation. 2008;118(23):2395–451.
Hobbs RE, Millit HD, Raghavan PV, Moodie DS, Sheldon WC. Coronary artery fistulae: a 10-year review. Cleveland Clinic Quarterly. 1982 Winter; 49(4):191–7.
Sunder S, Balakrishnan KG, Tharakan JA, Titus T, Pillai VR, Francis B, et al. Coronary artery fistula in children and adults: a review of 25 cases with long-term observations. Int J Cardiol. 1997;58(1):47–53.
Davis JT, Allen HD, Whellar JJ, Chan DP, Cohen DM, Teske DW, et al. Coronary artery fistula in the pediatric age group: a 19-year institutional experience. Ann Thorac Surg. 1994;58(3):760–3.
Author information
Authors and Affiliations
Contributions
J.K. and H.S. wrote the main manuscript text and Z.F. prepared the figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanduri, J., Falk, Z. & Singh, H.S. Diagnosis and Management of Congenital Coronary Artery Fistulas in Adults. Curr Cardiol Rep 26, 373–379 (2024). https://doi.org/10.1007/s11886-024-02038-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-024-02038-1