Abstract
Coronary artery fistulae (CAF) are rare congenital or acquired in which a connection forms between one of the coronary arteries and a heart chamber or with other vessels. This paper describes three cases of CAF along with their initial presentation, imaging findings and management. The first case is a rare form of CAF in which the left circumflex coronary artery fistula empty into left ventricle. We discuss the different types of CAF along with their prevalence and the different imaging tools that could be utilized to identify CAF. There is no unifying consensus on treatment strategy for symptomatic fistulae and we proposed a management algorithm that could be used to make a decision for intervention versus observation. We discuss options for intervention- surgical, catheter-based and medical therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
Coronary Artery Fistulae (CAF) are rare congenital or acquired epicardial coronary communication with a cardiac chamber or a great vessel.
-
CAF are usually diagnosed incidentally.
-
Majority of CAF are asymptomatic.
-
CAF can cause heart failure, myocardial ischemia and arrhythmia.
-
Large or symptomatic CAF needs intervention.
-
Single, proximal and non-tortuous CAF can be treated percutaneously.
-
Tortuous, distal and multiple CAF need surgical closure.
Introduction
Coronary artery fistulae (CAF) are rare congenital or acquired epicardial coronary communications with a cardiac chamber coronary cameral fistula (CCF) or the great vessels of the heart e.g. pulmonary veins or arteries. They bypass the myocardial capillary network, hence the term coronary arterio-venous fistulae is also used [1,2,3]. The majority of CAF are incidentally found and rarely lead to symptoms. There is no consensus on the management of fistulae in symptomatic patients; thus, current strategies remain largely contingent on experience and discretion. Herein we discuss three cases of CAF, a brief review of the literature, as well a proposed algorithm for management. One of the cases is a rare form of CAF (left circumflex coronary artery fistula emptying into left ventricle). A unilateral/single left circumflex coronary fistula shunting into the left ventricle (LV) has rarely been described, though identified as part of multiple coronary artery fistulae [6]. According to Salah et al. this particular morphology constitutes only 4% of all published cases from 2000 to 2010. In this report we also discuss the management of CAF in the setting of unstable angina and non-ST segment elevated myocardial infarction (NSTEMI).
Classification
There are various classifications for coronary artery fistulae. The different forms of classification typically take into account the following variables: vessel of origin, segment of origin (Sakakibara type A vs. Sakakibara type B), site of termination (coronary arteriovenous fistulae vs. coronary cameral fistulas), fistula morphology (simple vs. complex fistula), fistulae number (single vs. multiple), and angiographic size. Sakakibara type A describes a fistula that originates from the proximal third of the native vessel, whereas Sakakibara type B describes a fistula that forms beyond the proximal third or the continuation of the native vessel. Arteries with proximal fistulae tend to be dilated, whereas those with distal fistulae are often more tortuous, thus posing a challenge for catheter based therapies [1,2,3,4]. A fistula that terminates into a great vessel such as the coronary sinus (CS), pulmonary artery (PA), pulmonary veins (PV), bronchial vessel, vena cava or other veins is called a coronary arteriovenous fistula. However, if it terminates into one of the four heart chamber then it is referred to as a coronary cameral fistula. The majority of these fistulae terminate in the right atrium or the pulmonary artery [5].
Morphology of fistulae is essential for their classification. A fistula is characterized as simple if it originates from a single vessel and has a single termination. Complex fistulae are characterized by plexiform variants. Although angiography plays an important role in the classification of fistulae by size, it is only applicable to simple macro-fistulae. A fistula is considered large if it is more than twice the size of the distal reference vessel diameter, medium if one to two times the size of the distal reference vessel diameter, and small when less than the distal reference vessel diameter.
Case 1
An 85 year old female with a history of atrial fibrillation (AF) on coumadin therapy, hypertension, hyperlipidemia, papillary thyroid cancer with bone metastasis status post total thyroidectomy, radiation therapy (20 + years ago), and recent cholangitis, presented to the emergency department for sudden onset of moderate to severe substernal chest pain with radiation to the left shoulder and jaw that occurred while she was washing dishes. The pain was associated with diaphoresis and cold clammy extremities. She denied any previous episodes of chest pain. Her blood pressure was 234/64 mmHg and heart rate was 63 bpm. Cardiac and respiratory examinations were unremarkable except for a 2/6 early systolic murmur heard towards the apex. Initial EKGs revealed AF, new left axis deviation and new Q waves in V1-V3. Transthoracic echocardiography showed a left ventricular ejection fraction (LVEF) of 30%, akinetic septum, apex and anterior wall. There were moderately dilated left and right atria with trace aortic regurgitation and moderate mitral regurgitation. Cardiac troponin-I was 8.8 ng/ml and subsequently rose to > 50 (normal 0–0.03 ng/ml). Cardiac catheterization was performed and showed a 30% lesion in the mid left anterior descending artery (LAD), a 30% lesion in the distal LAD, and a 30% lesion in the proximal portion of the 1st diagonal branch. A large fistula originating from the left circumflex coronary artery and emptying into the left ventricle was visualized, as shown in Fig. 1. This patient had a large fistula and presented with NSTEMI and low LVEF, which are indications for fistula closure. The risks versus benefits of fistula closure were discussed with the patient; because of her advanced age, she opted to proceed with medical management going forward. Thus, she was managed medically with beta-blockers and aspirin, with a plan for periodic follow ups.
Case 2
A 64-year-old man with a history of coronary artery disease (CAD), percutaneous coronary intervention with drug eluting stents to the right coronary artery (RCA), Type 1 diabetes mellitus (DM), transient ischemic attack (TIA), hypertension (HTN), hyperlipidemia (HLD), chronic obstructive pulmonary disease (COPD), depression, and ongoing smoking presented with increasing dyspnea. He also had a history of snoring and possible sleep apnea, without formal diagnosis. He denied chest pain or symptoms of cardiac ischemia or arrhythmia. His heart rate was 75 bpm and regular, and his BP was 136/83 mmHg. Physical examination including cardiovascular examination was normal. EKG revealed normal sinus rhythm, left anterior fascicular block and normal ST-T pattern. The patient underwent nuclear SPECT myocardial perfusion stress testing which revealed mid inferior and inferior-lateral wall ischemia. He then underwent cardiac catheterization, which revealed 50% in-stent restenosis of mid RCA, 50% stenosis of obtuse marginal branch, and a small coronary cameral fistula arising off RV marginal branch emptying into RA as shown in Fig. 2. Transthoracic echocardiography revealed normal biventricular size and function. Estimated pulmonary arterial pressures were within normal limits. The patient was managed medically without further symptoms.
Case 3
A 60-year old female with a history of HTN, heart failure with preserved ejection fraction (HFpEF), COPD, cerebrovascular disease (CVA), HLD, breast cancer, and right partial mastectomy was admitted with intermittent pressure-like chest pain for 4 weeks. She had no other symptoms. Her heart rate was 71 bpm and regular, and her BP was 150/90 mmHg. Her physical examination including cardiovascular examination was normal except for partial right mastectomy. EKG revealed normal sinus rhythm with normal conduction and ST-T segment pattern, as shown in Fig. 3. Cardiac troponin-I was elevated at 0.48 ng/ml (normal 0–0.03 ng/ml). She underwent cardiac catheterization which revealed 40–50% stenosis of proximal right coronary artery (RCA). Coronary fractional flow reserve (FFR) did not reveal a significant pressure drop across the lesion in the RCA, suggesting a non-obstructive lesion. There was a small coronary arterial fistula from the LAD opening into left pulmonary circulation as shown in Fig. 4. Left ventricular end diastolic pressure was 13 mmHg. A transthoracic echocardiogram revealed mildly reduced left ventricular systolic function with estimated LVEF at 50% and features of early diastolic dysfunction. The patient’s presentation was thought to be secondary to uncontrolled hypertension. Her medical management was optimized, and she continued to do well on follow up.
Discussion
The prevalence of CAF is variable, and has been reported to be 0.1–0.22%. Zanchin et al. estimated 0.26%, whilst a Turkish registry of patients undergoing cardiac catheterization showed 0.08% [7]. The largest reported series comprised 33,600 patients who had diagnostic cardiac catheterization and showed a 0.1% prevalence [8]. In addition to being classified on the basis of where they terminate and originate, CAFs should also be described whether they are multilateral or unilateral [9]. Said et al. noted that 69% of unilateral fistulae originated from the left coronary artery (LCA) and 31% from the right coronary artery (RCA). About 29% of LCA originating fistulae are from the LCx [7, 8]. Only 4% of LCx originating fistulae terminate in the left ventricle as with our first case [7], while 81% terminate in PA/RA/RV according to Said et al. [9]. There is no racial or gender predilection; about 47% are male [9], and the mean age is 51.4 years.
The majority of CAFs are congenital, however there are few reports of acquired fistulae. Acquired fistulae could result from acute myocardial infarction, tumor, percutaneous coronary angioplasty, coronary artery bypass grafting, cardiac transplant, cardiac biopsy, and pacemaker placement or ablation of accessory pathways [10]. Coronary steal phenomenon is believed to be the primary pathophysiological problem in coronary fistulae [11]. Symptoms depend on the degree of steal or shunt. Shunt flow depends on the size of fistula and the receiving chambers. Significant runoff from a high-pressure coronary vessel to a low resistance receiving cavity (RA/RV/PA/CS) could cause ischemia. However, Angelini et al. concluded that few fistulae cause critical ischemia [12].
Large CAF can present with signs and symptoms of heart failure depending on the amount of left to right flow shunt into the RA/RV/PA; they may also present as angina depending on the amount of steal [1, 9, 15, 16]. Symptoms may include fatigue, orthopnea, dyspnea on exertion and chest pain. Three main syndromes arise; CHF reported in 8% of patients with CAF, MI in 2%, and 4% present with infective endocarditis [9]. Our patient in case one presented with NSTEMI with normal coronaries on coronary angiography. Hence, coronary artery steal phenomenon could explain the ischemia, though this is an uncommon presentation, especially for unilateral fistulae [13].
Coronary arterial fistulae are usually asymptomatic and are found incidentally on routine angiography. Clinical examination may not be revealing, or a continuous murmur may be audible. A continuous murmur usually results from a shunt flow between two vascular systems, and among the differential diagnoses are coronary arterial fistulae, pulmonary arterial fistulae, ruptured sinus of Valsalva aneurysm, or patent ductal arteriosus (PDA). Most patients will be referred for asymptomatic continuous murmur, which mimics a patent ductal arteriosus (PDA). The typical murmur is over the mid-chest or even lower, compared with below the left clavicle for PDA [13, 14].
Young people usually present with exertional dyspnea (60%), endocarditis in fistula (20%), or angina (3–7%). Fistulae that terminate in the right sided chamber cause left-to-right shunts that could lead to development of pulmonary hypertension. On the other hand, fistulae that terminate in a left sided chamber could cause left ventricular overload, and these patients typically present with symptoms and signs of heart failure. They can also present with palpitations, syncope, myocardial ischemia or infarction. Maron et al. reported that 13% of sudden cardiac deaths in young athletes were due to coronary arterial malformations. Congestive heart failure (19%) and arrhythmias are the usual presentations in older populations [1, 9, 15].
The gold standard for diagnosis is cardiac catheterization and angiography. Noninvasive techniques include cardiac computed tomography (CT), magnetic resonance imaging (MRI), and transesophageal and transthoracic echocardiography. Cardiac catheterization is usually used to assess the significance of the fistula and provide information regarding its anatomy. The use of a transesophageal echocardiography is essential intraoperatively to assess the precise location of the drainage. 3D images of MRI or cardiac CT (as shown in Fig. 6) can be reconstructed to better assess the anatomy of the fistulous tract (as shown in Fig. 5) [14, 16].
Thoracic computed tomogram showing course of a fistula (black arrows) originating from left main coronary artery, coursing parallel to left circumflex artery (white arrow), posterior and above the pulmonary artery (PA), and draining into the right atrium (The right atrium not visualized in these sections)
There are no guideline-based medical therapies approved for treatment of CAF. Medications are often used to treat the sequelae of fistulae including heart failure and arrhythmia. So far, there is no unifying evidence-based consensus on treatment strategies for symptomatic fistulae [16]. Medical management, surgical repair, and catheter closure have all been used. Indications for interventions on CAF include large fistulas, increasing left-to-right shunt, congestive heart failure, left ventricular dysfunction (diastolic or systolic), history of endocarditis, and myocardial ischemia [14].
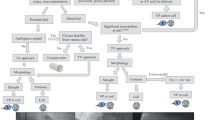
Coronary fistulae close spontaneously in only 1–2% of cases. The 2008 ACC/AHA ACHD Management Guidelines give a Class I recommendation for closure of large coronary artery fistulas (as shown in Fig. 6), regardless of symptom status, by transcatheter or surgical routes [19]. Transcatheter approaches are often feasible, especially if the fistulous communication takes off proximally from the coronary artery [17, 18]. Surgical repair is preferred in cases of large or tortuous fistulae [17, 18]. Another Class I recommendation is for mild to moderate coronary artery fistulae closure in the following settings: myocardial ischemia, arrhythmia, unexplained systolic or diastolic dysfunction, or endarteritis. On the other hand, for patients with asymptomatic, small to moderate, coronary artery fistulae, follow up with echocardiography every 3–5 years is reasonable [18, 19]. All the recommendations are level C of evidence as shown in Table 1. We have proposed an algorithm for management of coronary fistulae as shown in Fig. 7.
Coronary angiogram of the LMCA showing a large CAF originating near the LMCA bifurcation and connecting to the right atrium. Noted previous coiling, which failed to obliterate CAF. LMCA = left main coronary artery, LCX = left circumflex artery, LAD = left anterior descending artery CAF = coronary arteriovenous fistula
It is critical to understand the anatomy of the fistula once selected for transcatheter closure. The body of the fistula is usually the landing zone for the device that will be used. It is essential to avoid particular sites, including the native coronary vessel, fistulae ostium, or the fistulae termination. For example, if the device is landed in or interferes with the native coronary vessel, it could cause dissection, spasm or in situ thrombosis. Also, it is important that the fistula narrows as it terminates, otherwise there is a high risk for device embolization. The size of the fistula leads to the selection of devices that will be used for closure. Usually hydrophilic vascular coils are used as the first line device when the fistula measures < 5 mm in diameter. Patent ductus occluders or vascular coils are used when the fistula measures > 5 mm in diameter [20, 21].
Abbreviations
- AF:
-
Atrial fibrillation
- CAD:
-
Coronary artery disease
- CAF:
-
Coronary artery fistula
- CCF:
-
Coronary cameral fistula
- CHF:
-
Congestive heart failure
- COPD:
-
Chronic obstructive pulmonary disease
- CS:
-
Coronary sinus
- CVA:
-
Cerebrovascular disease
- DM:
-
Diabetes mellitus
- FFR:
-
Fractional flow reserve
- HLD:
-
Hyperlipidemia
- HTN:
-
Hypertension
- LA:
-
Left atrium
- LAD:
-
Left anterior descending artery
- LCA:
-
Left coronary artery
- LCX:
-
Left circumflex artery
- LM:
-
Left main
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- NSTEMI:
-
Non-ST segment elevated myocardial infarction
- PA:
-
Pulmonary artery
- PDA:
-
Patent ductus arteriosus
- PV:
-
Pulmonary vein
- RA:
-
Right atrium
- RCA:
-
Right coronary artery
- RV:
-
Right ventricle
- TIA:
-
Transient ischemic attack
References
Challoumas D, Pericleous A, Dimitrakaki IA, Danelatos C, Dimitrakakis G (2014) Coronary arteriovenous fistulae: a review. Int J Angiol 23(1):1–10. https://doi.org/10.1055/s-0033-1349162
Burns KE, Ferguson KA, Spouge A, Brown JE (2001) Massive congenital coronary arteriovenous malformation presenting with exertional dyspnea and desaturation in an adult: a case report and review of the literature. Can J Cardiol 17(1):85–89
Dodge-Khatami A, Mavroudis C, Backer CL (2000) Congenital heart surgery nomenclature and database project: anomalies of the coronary arteries. Ann Thorac Surg 69(4 Suppl):S270–S297
Sakakibara S, Yokoyama M, Takao A, Nogi M, Gomi H (1966) Coronary arteriovenous fistula nine operated cases. Am Heart J 72(3):307–314
Agostini D, Grollier G, Scanu P, Lognone T, Potier JC (1992) Left coronaro-ventricular fistula after myocardial infarction. Apropos of a case. Arch Mal Coeur Vaiss 85(3):373–376
Kuo CT, Chiang CW, Chern MS, Lee YS, Chang CH (1992) Imaging of multiple coronary artery fistulas to right ventricle by transthoracic and transesophageal echocardiography. Chest 102(5):1623–1625
Serçelik A, Mavi A, Ayalp R, Pestamalci T, Gümüsburun E, Batiraliev T (2003) Congenital coronary artery fistulas in Turkish patients undergoing diagnostic cardiac angiography. Int J Clin Pract 57(4):280–283
Gowda RM, Vasavada BC, Khan IA (2006) Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol 107(1):7–10. https://doi.org/10.1016/j.ijcard.2005.01.067
Said SA (2011) Current characteristics of congenital coronary artery fistulas in adults: a decade of global experience. World J Cardiol 3(8):267–277. https://doi.org/10.4330/wjc.v3.i8.267
Said SA, van der Werf T (1999) Acquired coronary cameral fistulas: are these collaterals losing their destination? Clin Cardiol 22(4):297–302
Sunkara A, Chebrolu LH, Chang SM, Barker C (2017) Coronary artery fistula. Methodist Debakey Cardiovasc J 13(2):78–80. https://doi.org/10.14797/mdcj-13-2-78
Angelini P (1999) Functionally significant versus intriguingly different coronary artery anatomy: anatomo-clinical correlations in coronary anomalies. G Ital Cardiol 29(6):607–615
Brooks CH, Bates PD (1983) Coronary artery-left ventricular fistula with angina pectoris. Am Heart J 106(2):404–406
Qureshi SA (2006) Coronary arterial fistulas. Orphanet J Rare Dis 1(1):51. https://doi.org/10.1186/1750-1172-1-51
Maleszka A, Kleikamp G, Minami K, Peterschröder A, Körfer R (2005) Giant coronary arteriovenous fistula. A case report and review of the literature. Z Kardiol 94(1):38–43
Nagpal P, Khandelwal A, Saboo SS, Garg G, Steigner ML (2015) Symptomatic coronary cameral fistula. Heart Views 16(2):65–67. https://doi.org/10.4103/1995-705X.159225
Kiefer TL, Crowley AL, Jaggers J, Harrison JK (2012) Coronary arteriovenous fistulae: the complexity of coronary artery-to-coronary sinus connections. Tex Heart Inst J 39(2):218–222
Kochar A, Kiefer T (2017) Coronary artery anomalies: when you need to worry. Curr Cardiol Rep 19(5):39. https://doi.org/10.1007/s11886-017-0854-x
Warnes CA, Williams RG, Bashore TM et al (2008) ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease): developed in collaboration With the American society of echocardiography, heart rhythm society, international society for adult congenital heart disease, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation 118(23):e714–e833. https://doi.org/10.1161/CIRCULATIONAHA.108.190690
Bruckheimer E, Harris M, Kornowski R, Dagan T, Birk E (2010) Transcatheter closure of large congenital coronary-cameral fistulae with Amplatzer devices. Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.22365
Reddy G, Davies JE, Holmes DR, Schaff HV, Singh SP, Alli OO (2015) Coronary artery fistulae. Circulation 8(11):850. https://doi.org/10.1161/CIRCINTERVENTIONS.115.003062
Acknowledgements
We are grateful to the editorial work by Emily Dobbs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, M., Kassem, K.M., Osei, K. et al. Coronary artery fistulae. J Thromb Thrombolysis 48, 345–351 (2019). https://doi.org/10.1007/s11239-019-01897-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-01897-8