Abstract
Purpose of Review
Mitral annular calcification (MAC) is associated with cardiovascular comorbidities and events and in the presence of mitral stenosis (MS) represents a high-risk cohort with limited treatment options. Emerging hybrid, minimally invasive, and transcatheter therapies that use circumferential MAC as an anchor for mitral valve replacement are emerging, but none are consistently associated with ideal outcomes.
Recent Findings
In patients with MAC and nonrheumatic calcific mitral stenosis who are severely symptomatic, mitral intervention may be indicated. Surgical decalcification and replacement of the mitral valve remains the conventional therapy. Surgical techniques to avoid decalcification are being described including a left atrium to left ventricular apex graft conduit. Transcatheter balloon-expandable valves designed for the aortic valve have been implanted in the mitral position in MAC with a surgical direct transatrial transcatheter approach or transseptal transcatheter approach. Left ventricular outflow tract (LVOT) obstruction remains prevalent and associated with increased mortality. Direct transatrial approach allows for surgical resection of the anterior leaflet to mitigate this risk, and percutaneous therapies to lacerate the anterior leaflet or to ablate the basal septum are being developed. Cardiac computed tomography has emerged as a requisite for patient selection and procedural planning and has powerful predictive value for LVOT obstruction and valve embolization in valve-in-MAC. Novel transcatheter valves designed specifically for the mitral space are being studied in patients with MAC.
Summary
MAC with mitral stenosis remains a challenging disease. Advances in technique, technology, and imaging may create new and reproducible treatment options with low procedural mortality for this challenging disease entity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mitral valve (MV) annulus is a saddle-shaped fibrous structure prone to calcific degeneration. Mitral annular calcification (MAC) has been described as a chronic degenerative process localized to this fibrous tissue resulting in calcium deposition especially favoring the posterior annulus [1]. There is emerging evidence that the degenerative process is in fact active and metabolically regulated related not only to hemodynamic stress, but also inflammation, lipid, bone, and mineral metabolism [2], with evidence of active calcification with increased (18)F-fluorodeoxyglucose activity on positron emission tomography [3]. As the calcium deposits progress, MAC can be visualized by echocardiography, x-ray, and computed tomography (CT).
The prevalence of MAC is not only related to the age and demographics of the described population but also imaging modality used and ranges between 5 and 42% [2]. In the Multi-Ethnic Study of Atherosclerosis of 6,814 patients without cardiovascular disease at baseline, MAC was found by CT in 9% of patients and associated with age, female gender, BMI, diabetes, and current smoking and notably not associated with lipids or CRP—although their relationship may have been influenced by the higher prevalence of lipid lowering meds in patients with MAC [4]. In the Heart and Soul Study of 1,020 ambulatory patients with coronary artery disease, the prevalence of MAC was 19%. Patients with MAC were more likely to have inducible ischemia and cardiovascular events [5].
There is a clear association between MAC and MV disease, arrhythmias, and cardiovascular events. MAC is strongly associated with atrial fibrillation (AF) in part due to left atrial enlargement. Conduction abnormalities are common and not only include AF, but also atrioventricular block and bundle branch block. Patients with CKD are at risk for MAC due to tissue deposition of calcium from excess calcium-phosphorus product. MAC is also associated with congenital conditions such as Marfan’s syndrome and Hurler’s syndrome [1, 2]. MAC has also been associated with atherosclerosis [6], and patients with progression of MAC and/or aortic valve calcifications were more likely to develop heart failure in follow-up [7].
Mitral Annular Calcification with Nonrheumatic Mitral Stenosis
In patients with MAC diagnosed by transthoracic echocardiography (TTE), concomitant MS is uncommon, with severe MS being reported in only 0.2% of patients and 2.5% of patients greater than 90 years old [4, 8]. The pathophysiology is thought to be annular calcification extending into the mitral leaflets resulting impaired mobility and geometric distortion. Percutaneous mitral balloon valvotomy (PMBV) can be attempted in patients with a history of rheumatic MS and a low Wilkin’s score, but recently updated American College of Cardiology and American Heart Association (ACC/AHA) guidelines otherwise offer minimal guidance on this challenging patient population with MAC and calcific degenerative nonrheumatic MS [9].
Significance of Combined Disease
Patients with MAC and MS are at high risk for mortality. In a recently published TTE cohort of 1,004 patients with severe MAC and nonrheumatic MS with a mean mitral gradient (MMG) ≥2 mmHg, survival was <50% at 5 years despite only 8% of the cohort having MMG ≥9 mmHg with mortality driven by comorbid diseases such as older age, AF, renal insufficiency, mitral regurgitation, and non-mitral valvular disease [10]. In a separate cohort of 143 patients with severe MS and severe MAC with a mean age of 72.7 years, 59% of whom had valves suitable for and underwent PMBV, mortality at follow-up of the overall cohort was nonetheless 31%. Of patients who underwent mitral valve replacement (MVR), 30% had died with median time to death of 358 days after surgery. Patients who either had PMBV or periodic monitoring faired just as poorly with the same proportion deceased within 327 days from the first echocardiogram [11].
Noninvasive Treatment Options
Medical treatment options are limited. Heart rate control to minimize MV gradients is recommended, along with anticoagulation if otherwise indicated (e.g., patients with AF, left atrial clot, and/or history of stroke). Treatment of comorbidities may have some effect at delaying the progression of MAC, but the level of evidence is weak. In patients with osteoporosis, the use of bisphosphonates, denosumab, osteoprotegerin, and teriparatide may decrease valve calcification. In patients with CKD, the use of phosphate binders, vitamin D receptor agonists, calcimimetics, vitamin K, and sodium thiosulfate might also slow the progression of valve calcification [12]. Of particular interest is cinacalcet—a calcimimetic and treatment for secondary hyperparathyroidism in end-stage renal disease—which when added to vitamin D in a randomized trial did attenuate the progression of Agatston CAC scores in the aorta, aortic valve, and MV from baseline to 52 weeks (+24% versus +31%) as compared to vitamin D alone [13]. Atorvastatin has been studied in patients with calcific aortic valve disease, and despite reductions in low-density lipoprotein cholesterol, aortic jet velocities and valvular calcification were similar between groups [14]. Endothelin receptor-A has been shown to be upregulated in stenotic valves and calcified aortas, and endothelin receptor antagonists have been shown to reduce vascular calcification and inflammation, smooth muscle cell differentiation, and calcification in animal models [15,16,17]. As endothelin receptor antagonists are relatively well tolerated and an approved oral therapy for patients with pulmonary arterial hypertension, this pathway could hold therapeutic potential.
Mitral Annular Calcification and Preprocedural Imaging
MAC is readily detected by screening TTE best visualized on short axis of the MV and can be characterized as involving part of the posterior annulus, the entire posterior annulus, or circumferential involving the anterior annulus as well. However, the density and volume of MAC and its extension into the myocardium and other surrounding tissue can be difficult to appreciate by echocardiography. While transesophageal echocardiography (TEE) may offer a higher resolution and improved delineation of the mitral annulus and leaflets, cardiac CT with contrast has emerged as an essential imaging modality for MAC and can not only qualify but quantify the density, severity, and extension of MAC and its relationship to other cardiac structures. It can furthermore detect caseous calcification of the MAC which has surgical implications [18]. Staging of MAC severity has been proposed as illustrated in Figure 1 but lacks overall consensus [18, 19], with one recent trial of a novel transcatheter mitral valve replacement (TMVR) using CT volumetric quantification of MAC as a cutoff to determine severity [20••].
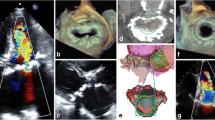
Proposed grading of mitral annular calcification. Short-axis parasternal transthoracic echocardiography images (left panels) and CT angiography images (right panels) show mild mitral annular calcification (arrow) with scattered calcification <180° (top), moderate mitral annular calcification with dense continuous calcification (arrow) <270° (middle), and severe mitral annular calcification with circumferential (arrow) >270° dense calcification (bottom) (reproduced from: Eleid MF et al. JACC Cardiovasc Imaging. 2016 Nov;9(11):1318-1337. 10.1016/j.jcmg.2016.09.001, with permission from Elsevier) [18].
Imaging of Concomitant Valve Disease
In patients with MAC, imaging of concomitant valve disease—either MS or MR—can be challenging. Careful interrogation for valvular disease including evaluating for thickened calcified leaflets, presence and severity of MS, and presence and severity of MR is necessary to determine whether patients have significant MV disease that can explain severe symptoms and indicate a potentially high-risk surgical or transcatheter intervention. Moreover, imaging could determine which interventions are feasible and used to guide the operators during intervention. For patients with or calcific MS, MV gradients by continuous wave Doppler are easily obtained but are influenced by heart rate and MR. MV area by continuity equation is recommended over pressure half time to determine severity of MS but can also be influenced by regurgitant aortic and mitral valves [9, 18]. Characterization of the MV leaflets assessing for extent of calcification and leaflet thickening could help with determination of MS severity, and the use of TEE and 3-dimensional (3D) planimetry of the MV can be helpful (Figure 2). When noninvasive imaging is insufficient, invasive hemodynamic assessment could be performed with direct measurement of simultaneous left atrial and left ventricular (LV) pressure. For patients with MAC and MS, assessing for rheumatic features, leaflet thickening, restriction, calcification, and subvalvular calcification helps with procedural planning. Finally, careful assessment for MR may require TEE imaging as annular calcium could shadow Doppler signals in and underappreciate MR with TTE imaging.
Preprocedural Planning
In patients being considered for surgery, the extent of leaflet calcification can determine whether MV repair is feasible usually for MAC with MR. The extension of MAC into the myocardium and other surrounding tissue or vertically towards the AV may determine the amount of MAC debulking necessary, and whether patch repair and reconstruction of the posterior annulus is necessary. For patients who are at intermediate risk for surgery with unrepairable leaflets, the amount and location of MAC and determining the aorto-mitral relationship determine the feasibility mainly to avoid left ventricular outflow tract (LVOT) obstruction of a valve-in-MAC intervention with a transcatheter balloon-expandable valve originally designed for the aortic valve (ViMAC) or for a novel TMVR therapy. Finally, septal shape and presence of thickening can be described to determine whether alcohol septal ablation (ASA) or other related procedures might help prevent LVOT obstruction.
CT can further help guide eligibility for ViMAC taking into account CT-derived mitral annulus area, mean diameter, aorto-mitral angulation, and annulus-to-apex distance [21]. CT has also been used to develop novel risk scores such as to predict risk of valve embolization in ViMAC, using average calcium thickness, degrees of annulus circumference involved, calcification at one or both fibrous trigones, and calcification of one or both leaflets to predict valve embolization. Higher scores are associated with less embolization and/or migration with MAC scores ≤6 associated with a 5.86 (95% CI 1 to 34.3) odds ratio for embolization on multivariate analysis [22]. Cardiac CT is a requisite for preprocedural planning, while TEE imaging continues to guide most surgical and structural heart interventions [18, 23, 24].
Treatment Guidelines
Rheumatic MS
Severity and indication for intervention in patients with rheumatic MS is predicated on mitral valve area (MVA) and patient symptoms. Progressive MS or stage B is defined as planimetered MVA >1.5 cm2 without symptoms. Asymptomatic severe MS or stage C is defined as planimetered MVA ≤1.5 cm2 supported by diastolic pressure half time ≥150 ms with severe enlargement of the LA or PASP >50 mmHg without symptoms. Finally, symptomatic severe MS or stage D meets the same hemodynamic criteria as stage C but also with presence of patient symptoms. In patients with severe symptomatic MS with favorable valve morphology and less than moderate MR, PMBV is recommended, with MV surgery as an alternative. In asymptomatic severe MS with pulmonary artery systolic pressures >50 mmHg, PMBV is recommended as well [9]. Patients with MAC but also a history of rheumatic MS with favorable morphology can still be considered for PMBV although outcome data for PMBV for rheumatic MS in MAC is scarce.
Nonrheumatic Calcific MS
In patients with nonrheumatic calcific MS, the leaflets are usually affected with no commissural fusion, and there is no role for PMBV. The prognosis of this patient population is poor, and the usual echocardiographic methods for determining severity of stenosis are prone to error. Intervention whether by surgery or by a transcatheter approach is reserved for the highly symptomatic patient refractory to diuresis and heart rate control [9]. A proposed diagnostic and therapeutic workflow is illustrated in Figure 3. Potential treatment options in patients with indicated mitral intervention for MAC and severe symptomatic nonrheumatic MS are explored in Table 1.
How to manage stenosis due to mitral annular calcification. Abbreviations: PMBV, percutaneous mitral balloon valvotomy; ACC, American College of Cardiology; AHA, American Heart Association; MS, mitral stenosis; MVA, mitral valve area; TEE, transesophageal echocardiogram; 3D, 3-dimensional; MAC, mitral annular calcification; CT, computed tomography; CKD, chronic kidney disease; ERA, endothelin receptor antagonist; PAH, pulmonary artery hypertension.
Mitral Valve Surgery in Patients with Mitral Annular Calcium
Surgical mitral valve repair or replacement can be challenging with severe MAC as the standard approach requires increased operating time for extensive decalcification and reconstruction of the mitral annulus to allow a well-seated prosthesis, minimize periprosthetic leak, and minimize stroke risk. In this approach, the annulus is first decalcified which may weaken the annulus and potentially lead to catastrophic AV groove disruption and circumflex injury. After decalcification a continuous or figure-of-8 suture is used to close the annular defect. In some cases of extensive decalcification, the annulus would need to be reconstructed either with an atrial muscle flap, bovine pericardium, or, when repair is not possible, the anterior mitral leaflet.
Avoiding Decalcification
Alternatively, techniques have been described to avoid decalcification of MAC by suturing through and otherwise working around the MAC [25] and other intra-annular implantation techniques prone to significant downsizing of the valve, with intra-atrial implantation of valves (prone to paravalvular leak), or hybrid approaches most suitable for large annuluses [26,27,28]. A novel avoidance strategy is to bypass the valve altogether with a left atrium to LV apex graft conduit. In this approach, a 23-mm mechanical aortic-valved conduit is used with the valve portion excised, rotated, and implanted into the LV apex. The conduit is implanted one end onto the left atrial appendage, and the other end onto the valve [29]. This technique is reserved for patients with mild-or-less MR, and more data is needed to determine its safety and efficacy.
While MAC can represent a challenge for surgery, appropriate patient selection could still result in favorable outcomes. In a recent cohort of 64 patients with MAC without leaflet calcification undergoing endoscopic robotic MV repair (84% for Barlow’s disease), MAC was excised and AV groove reconstruction with 19/64 requiring patch repair. 30-day mortality was a favorable 3.1% [30]. Albeit such decalcification and valve repair is mostly reserved for patients with MAC and MR, nonetheless evaluation by a surgeon with experience operating on patients with MAC is an essential component of determining the appropriate therapy for this high-risk MAC and MS cohort.
Early Transcatheter Mitral Valve Replacement Experience for Valve-in-Mitral Annular Calcification
Transcatheter therapies for valvular disease in patients with significant MAC is a rapidly developing field. Patients with severe MR and MAC with relative sparing of mitral leaflets could have durable results with percutaneous mitral valve repair [19], with appropriate patient selection [31]. MAC can also be used as an anchor for bioprosthetic valves crimped and mounted on the ends of catheters. These valves can be delivered by a surgeon via a transatrial approach, by an interventional cardiologist via a transseptal approach, or in joint fashion via a transapical approach. In patients with MAC and MS, balloon-expandable transcatheter valves designed for the aortic valve can be implanted in the MV with MAC serving as an anchor. The major limitation of this approach is LVOT obstruction from the ventricular edge of the implanted valve overhanging into the LVOT—an anterior structure—and pushing the native anterior MV leaflet into that space. Other major concerns are the potential for valve embolization based on the degree and distribution of leaflet and annular calcification necessary for valve anchoring and paravalvular leak due to the appropriation of a circular valve for the D-shaped MV annulus. Major advances have been made for the latter concerns with appropriate oversizing of implanted valves, and with newer generation valves with outer skirts allowing for tighter seals [32]. However, LVOT obstruction remains a major limitation of the therapy.
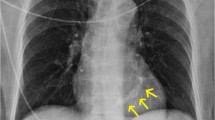
Early experience from a multicenter valve-in-mitral annular calcification (ViMAC) registry of 116 patients with a mean age of 73 and mean STS score of 15.3% demonstrated fair technical success of 76.7% but a high incidence, 11.2%, of LVOT obstruction with hemodynamic compromise. Thirty-day and 1-year mortalities were 25% and 53%, respectively [33•]. In the 13 patients with LVOT obstruction with hemodynamic compromise, only 4/13 survived to hospital discharge, and only 2/13 were alive at 1-year follow-up. An analysis of a larger STS/ACC transcatheter valve therapy registry of 100 ViMAC cases demonstrated similar findings of 30-day mortality of 21.8% and 10% LVOT obstruction with hemodynamic compromise [34]. An example of transcatheter ViMAC is illustrated in Figure 4.
Fluoroscopy of transcatheter mitral valve in mitral annular calcification (arrows) RAO view with left ventriculography demonstrating during cardiac systole no perivalvular leak and the potential interaction of the ventricular edge of the valve stent with the left ventricular outflow tract (asterisk).
Left Ventricular Outflow Tract Obstruction
An analysis of a TMVR registry of 521 patients undergoing TMVR for valve-in-valve, valve-in-ring, and ViMAC demonstrated adequate overall technical success of 87.1%. In the 58 patients who underwent ViMAC procedures, however technical success was only 62.1%, with higher 30-day and 1-year mortalities of 34.5% and 62.8%, respectively. LVOT obstruction occurred in 37/521 patients (7.1%) but 23/58 (39.7%) of patients who underwent ViMAC procedures. LVOT obstruction on univariate analysis was associated with mortality with a hazard ratio of 2.87 (95% CI 1.66–4.96) [35]. A subsequent analysis of the registry including 194 patients who underwent preprocedural CT planning showed a 13.4% incidence of LVOT obstruction again with a predilection for patients undergoing ViMAC procedures (37 patients, 54.1%). Patients with LVOT obstruction had a 34.6% procedural mortality compared with 2.4% for patients without obstruction. Importantly the authors found a neo-LVOT area cutoff of 170 mm2 to be highly predictive of LVOT obstruction and that preprocedural CT estimated neo-LVOT area had strong correlation with post-implant CT measured neo-LVOT area (r=0.83). Distance between mitral annulus and interventricular septum was predictive as well. Both were more predictive of LVOT obstruction than aorto-mitral angle [36] and illustrate the importance of CT in both preprocedural planning and patient selection.
Evolving Approaches to Mitral Annular Calcification and Novel Valves
Strategies are being developed to avoid or rescue LVOT obstruction caused by ViMAC. A direct transatrial transcatheter approach has been developed with a median sternotomy or right thoracotomy approach, followed by cardiopulmonary bypass, exposure of the left atrium, and resection of the anterior MV leaflet to avoid LVOT obstruction. Surgical septal myomectomy can be performed as well. A balloon-expandable valve is delivered with TEE and fluoroscopic guidance across the MV and deployed. Pledgeted sutures can first be placed and later used to anchor the sealing skirt of the implanted valve to prevent valve embolization and minimize paravalvular leak with the larger published experiences of 8 and 26 patients associated with 100% technical success, and 30-day mortality of 0% and 27%, respectively [37, 38•, 39, 40]. Only 1 patient had significant LVOT obstruction. This strategy is being studied prospectively in a single-arm SITRAL trial [41].
Transcatheter Approach to Avoid LVOT Obstruction
Fully transcatheter approaches are being developed as well. Operators have developed a technique to intentionally lacerate the anterior MV leaflet to prevent LVOT obstruction using catheters placed in the left atrium and LV to puncture the anterior leaflet and lacerate it with electrocautery in a fully transcatheter manner. Early experience of this technique—abbreviated LAMPOON—on 30 patients achieved midline laceration of the anterior leaflet in 100% of patients and 30-day survival of 93%. This technique can be performed ahead of valve-in-ring and for ViMAC implants and is being studied in a prospective single-arm trial [42]. An alternative strategy is alcohol septal ablation (ASA) as a bail-out option for patients with LVOT obstruction from a thick basal septum during TMVR. In patients at high risk of LVOT obstruction as predicted by preprocedural planning CT, 30 patients underwent pre-emptive ASA. Median increase in neo-LVOT area was 111.2 mm2 with the maneuver. Two patients died before TMVR, and overall mortality at 30 days was 10% [43]. An alternative investigational approach in patients where anterior laceration of the MV leaflet is not feasible or coronary anatomy is not favorable for ASA is percutaneous cardiac ablation of a thickened basal septum with 3D electroanatomic mapping and guidance to avoid ablation of conduction tissue [44].
The MITRAL II trial will incorporate some of the above techniques in a prospective nonrandomized multicenter trial of transseptal ViMAC in 200 patients with severe MAC and symptomatic MV disease [45]. The results of the SITRAL, LAMPOON, and MITRAL II trials will help determine the reproducibility and optimal approach for using balloon-expandable valves originally designed for the aortic valve for native mitral disease in patients with severe MAC.
Novel Technologies and Valves
Ongoing technological advances may create new options for patients with MAC. As an example, in patients with MAC and MR, NeoChord (NeoChord, Louis Park, MN) has been used successfully in 13 patients avoiding the mitral annulus altogether, although recurrent moderate or more MR appeared more prevalent in patients with MAC than patients with other pathologies [46]. The Tendyne transcatheter bioprosthetic valve (Abbott Structural, Santa Clara, CA) is a trileaflet porcine pericardial valve within a 2 self-expanding nitinol frames contoured to fit the mitral annulus without need for significant oversizing. The prothesis is secured by a tether to an epicardial pad at the LV apex. The placement is done via a left lateral thoracotomy without cardiopulmonary bypass via a transapical approach through the LV apex. In a recent experience in treatment of MR in the setting of severe MAC—defined as myocardial invasion or total volume by CT ≥750 mm3—9 patients were implanted with no deaths. LVOT obstruction occurred in 1 patient due to malrotation of the prosthesis and was rescued with ASA [20••]. A second valve designed for the mitral position with a MAC trial arm is the Intrepid valve (Medtronic, Minneapolis, MN) [47]. While both trials are for treatment of MR not MS, they may be appropriated for treatment of MS in MAC in the future.
Conclusion
MAC with mitral stenosis remains a challenging disease. Advances in technique, technology, and imaging may create new and reproducible treatment options with low procedural mortality for this challenging disease entity.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral annulus calcification. J Am Coll Cardiol. 2015;66(17):1934–41. https://doi.org/10.1016/j.jacc.2015.08.872.
Massera D, Kizer JR, Dweck MR. Mechanisms of mitral annular calcification. Trends Cardiovasc Med. 2020;30(5):289–95. https://doi.org/10.1016/j.tcm.2019.07.011.
Massera D, Trivieri MG, Andrews JPM, Sartori S, Abgral R, Chapman AR, et al. Disease activity in mitral annular calcification. Circ Cardiovasc Imaging. 2019;12(2):e008513. https://doi.org/10.1161/CIRCIMAGING.118.008513.
Kanjanauthai S, Nasir K, Katz R, Rivera JJ, Takasu J, Blumenthal RS, et al. Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2010;213(2):558–62. https://doi.org/10.1016/j.atherosclerosis.2010.08.072.
Holtz JE, Upadhyaya DS, Cohen BE, Na B, Schiller NB, Whooley MA. Mitral annular calcium, inducible myocardial ischemia, and cardiovascular events in outpatients with coronary heart disease (from the Heart and Soul Study). Am J Cardiol. 2012;109(8):1092–6. https://doi.org/10.1016/j.amjcard.2011.11.043.
Koulaouzidis G, Nicoll R, MacArthur T, Jenkins PJ, Henein MY. Coronary artery calcification correlates with the presence and severity of valve calcification. Int J Cardiol. 2013;168(6):5263–6. https://doi.org/10.1016/j.ijcard.2013.08.019.
Fashanu OE, Upadhrasta S, Zhao D, Budoff MJ, Pandey A, Lima JAC, et al. Effect of progression of valvular calcification on left ventricular structure and frequency of incident heart failure (from the Multiethnic Study of Atherosclerosis). Am J Cardiol. 2020;134:99–107. https://doi.org/10.1016/j.amjcard.2020.08.017.
Akram MR, Chan T, McAuliffe S, Chenzbraun A. Non-rheumatic annular mitral stenosis: prevalence and characteristics. Eur J Echocardiogr. 2009;10(1):103–5. https://doi.org/10.1093/ejechocard/jen179.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72–e227. https://doi.org/10.1161/CIR.0000000000000923.
Pasca I, Dang P, Tyagi G, Pai RG. Survival in patients with degenerative mitral stenosis: results from a large retrospective cohort study. J Am Soc Echocardiogr. 2016;29(5):461–9. https://doi.org/10.1016/j.echo.2015.12.012.
Tsutsui RS, Simsolo E, Saijo Y, Gentry J, Puri R, Reed G, et al. Severe mitral stenosis in patients with severe mitral annular calcification: an area of unmet need. JACC Cardiovasc Interv. 2019;12(24):2566–8. https://doi.org/10.1016/j.jcin.2019.08.030.
Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int. 2013;93(4):365–73. https://doi.org/10.1007/s00223-013-9712-z.
Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26(4):1327–39. https://doi.org/10.1093/ndt/gfq725.
Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352(23):2389–97. https://doi.org/10.1056/NEJMoa043876.
Lariviere R, Gauthier-Bastien A, Ung RV, St-Hilaire J, Mac-Way F, Richard DE, et al. Endothelin type A receptor blockade reduces vascular calcification and inflammation in rats with chronic kidney disease. J Hypertens. 2017;35(2):376–84. https://doi.org/10.1097/HJH.0000000000001161.
Wu SY, Zhang BH, Pan CS, Jiang HF, Pang YZ, Tang CS, et al. Endothelin-1 is a potent regulator in vivo in vascular calcification and in vitro in calcification of vascular smooth muscle cells. Peptides. 2003;24(8):1149–56. https://doi.org/10.1016/j.peptides.2003.07.008.
Peltonen T, Taskinen P, Napankangas J, Leskinen H, Ohtonen P, Soini Y, et al. Increase in tissue endothelin-1 and ETA receptor levels in human aortic valve stenosis. Eur Heart J. 2009;30(2):242–9. https://doi.org/10.1093/eurheartj/ehn482.
Eleid MF, Foley TA, Said SM, Pislaru SV, Rihal CS. Severe mitral annular calcification: multimodality imaging for therapeutic strategies and interventions. JACC Cardiovasc Imaging. 2016;9(11):1318–37. https://doi.org/10.1016/j.jcmg.2016.09.001.
Cheng R, Tat E, Siegel RJ, Arsanjani R, Hussaini A, Makar M, et al. Mitral annular calcification is not associated with decreased procedural success, durability of repair, or left ventricular remodelling in percutaneous edge-to-edge repair of mitral regurgitation. EuroIntervention. 2016;12(9):1176–84. https://doi.org/10.4244/EIJV12I9A191.
Sorajja P, Gossl M, Babaliaros V, Rizik D, Conradi L, Bae R, et al. Novel transcatheter mitral valve prosthesis for patients with severe mitral annular calcification. J Am Coll Cardiol. 2019;74(11):1431–40. https://doi.org/10.1016/j.jacc.2019.07.069Novel transcatheter valve designed for the mitral valve with a severe mitral annular calcification investigational arm.
Ludwig S, Ruebsamen N, Deuschl F, Schofer N, Kalbacher D, Schaefer A, et al. Screening for transcatheter mitral valve replacement: a decision tree algorithm. EuroIntervention. 2020;16(3):251–8. https://doi.org/10.4244/EIJ-D-19-01051.
Guerrero M, Wang DD, Pursnani A, Eleid M, Khalique O, Urena M, et al. A cardiac computed tomography-based score to categorize mitral annular calcification severity and predict valve embolization. JACC Cardiovasc Imaging. 2020;13(9):1945–57. https://doi.org/10.1016/j.jcmg.2020.03.013.
Wunderlich NC, Beigel R, Ho SY, Nietlispach F, Cheng R, Agricola E, et al. Imaging for mitral interventions: methods and efficacy. JACC Cardiovasc Imaging. 2018;11(6):872–901. https://doi.org/10.1016/j.jcmg.2018.02.024.
Little SH, Bapat V, Blanke P, Guerrero M, Rajagopal V, Siegel R. Imaging guidance for transcatheter mitral valve intervention on prosthetic valves, rings, and annular calcification. JACC Cardiovasc Imaging. 2021;14(1):22–40. https://doi.org/10.1016/j.jcmg.2019.10.027.
Salhiyyah K, Kattach H, Ashoub A, Patrick D, Miskolczi S, Tsang G, et al. Mitral valve replacement in severely calcified mitral valve annulus: a 10-year experience. Eur J Cardiothorac Surg. 2017;52(3):440–4. https://doi.org/10.1093/ejcts/ezx086.
Bedeir K, Kaneko T, Aranki S. Current and evolving strategies in the management of severe mitral annular calcification. J Thorac Cardiovasc Surg. 2019;157(2):555–66. https://doi.org/10.1016/j.jtcvs.2018.05.099.
Lafreniere-Bessi V, Cameron-Gagne M, Perron J, Levesque MH, Laflamme M, Charbonneau E, et al. Mitral annular calcification and mitral valve replacement: a new approach. Ann Thorac Surg. 2018;105(2):e55–e7. https://doi.org/10.1016/j.athoracsur.2017.09.028.
Edelman JJ, Badhwar V, Larbalestier R, Yadav P, Thourani VH. Contemporary surgical and transcatheter management of mitral annular calcification. Ann Thorac Surg. 2021;111(2):390–7. https://doi.org/10.1016/j.athoracsur.2020.04.148.
Said SM, Schaff HV. An alternate approach to valve replacement in patients with mitral stenosis and severely calcified annulus. J Thorac Cardiovasc Surg. 2014;147(6):e76–8. https://doi.org/10.1016/j.jtcvs.2014.02.039.
Loulmet DF, Ranganath NK, Neragi-Miandoab S, Koeckert MS, Galloway AC, Grossi EA. Advanced experience allows robotic mitral valve repair in the presence of extensive mitral annular calcification. J Thorac Cardiovasc Surg. 2019;161:80–8. https://doi.org/10.1016/j.jtcvs.2019.10.099.
Oguz D, Padang R, Nina R, Pislaru SV, Nkomo VT, Mankad SV, et al. Risk of increased mean diastolic gradient after transcatheter edge-to-edge mitral valve repair: a quantitative 3D TEE analysis. J Am Soc Echocardiogr. 2021;34:595–603.e2. https://doi.org/10.1016/j.echo.2021.01.018.
Yoon SH, Makkar R. Transcatheter mitral valve replacement in patients with severe mitral annular calcification. Interv Cardiol Clin. 2019;8(3):301–12. https://doi.org/10.1016/j.iccl.2019.02.008.
Guerrero M, Urena M, Himbert D, Wang DD, Eleid M, Kodali S, et al. 1-year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol. 2018;71(17):1841–53. https://doi.org/10.1016/j.jacc.2018.02.054Early experience using balloon-expadable aortic transcaheter valves in the mitral position anchored by mitral annular calcification demonstrating high 30-day and 1-year mortality.
Guerrero M, Vemulapalli S, Xiang Q, Wang DD, Eleid M, Cabalka AK, et al. Thirty-day outcomes of transcatheter mitral valve replacement for degenerated mitral bioprostheses (valve-in-valve), failed surgical rings (valve-in-ring), and native valve with severe mitral annular calcification (valve-in-mitral annular calcification) in the United States: data from the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2020;13(3):e008425. https://doi.org/10.1161/CIRCINTERVENTIONS.119.008425.
Yoon SH, Whisenant BK, Bleiziffer S, Delgado V, Dhoble A, Schofer N, et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J. 2019;40(5):441–51. https://doi.org/10.1093/eurheartj/ehy590.
Yoon SH, Bleiziffer S, Latib A, Eschenbach L, Ancona M, Vincent F, et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2019;12(2):182–93. https://doi.org/10.1016/j.jcin.2018.12.001.
Brener MI, George I. Direct transcatheter mitral valve implantation in severe mitral annular calcification: technique and evidence. Ann Cardiothorac Surg. 2021;10(1):183–5. https://doi.org/10.21037/acs-2020-mv-12.
Russell HM, Guerrero ME, Salinger MH, Manzuk MA, Pursnani AK, Wang D, et al. Open atrial transcatheter mitral valve replacement in patients with mitral annular calcification. J Am Coll Cardiol. 2018;72(13):1437–48. https://doi.org/10.1016/j.jacc.2018.07.033Open atrial transcatheter mitral valve replacement technique with anterior leaflet resection associated with low mortality and high procedural success.
Kassar M, Khalique OK, Pilgrim T, Reineke D, Carrel T, Windecker S, et al. Surgical transatrial implantation of transcatheter heart valves in severe mitral annular calcification. Interv Cardiol Clin. 2019;8(3):313–9. https://doi.org/10.1016/j.iccl.2019.02.006.
Praz F, Khalique OK, Lee R, Veeragandham R, Russell H, Guerrero M, et al. Transatrial implantation of a transcatheter heart valve for severe mitral annular calcification. J Thorac Cardiovasc Surg. 2018;156(1):132–42. https://doi.org/10.1016/j.jtcvs.2018.03.016.
ClinicalTrials.gov. Surgical implantation of transcatheter valve in native mitral annular calcification (SITRAL) study (SITRAL). https://clinicaltrials.gov/ct2/show/nct02830204. Accessed 3/1/2021.
Khan JM, Babaliaros VC, Greenbaum AB, Foerst JR, Yazdani S, McCabe JM, et al. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol. 2019;73(20):2521–34. https://doi.org/10.1016/j.jacc.2019.02.076.
Wang DD, Guerrero M, Eng MH, Eleid MF, Meduri CU, Rajagopal V, et al. Alcohol septal ablation to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: first-in-man study. JACC Cardiovasc Interv. 2019;12(13):1268–79. https://doi.org/10.1016/j.jcin.2019.02.034.
Killu AM, Guerrero M, Siontis KC, El Sabbagh A, Eleid MF, Alkhouli M, et al. A novel technique-prophylactic septal radiofrequency ablation to prevent left ventricular outflow tract obstruction with transcatheter mitral valve replacement (RADIO-TMVR). J Cardiovasc Electrophysiol. 2020;31(11):3048–55. https://doi.org/10.1111/jce.14720.
ClinicalTrials.gov. The MITRAL II pivotal trial (Mitral Implantation of TRAnscatheter vaLves). (MITRAL-II). https://clinicaltrials.gov/ct2/show/NCT04408430. Accessed 3/1/2021.
Gerosa G, Nadali M, Longinotti L, Ponzoni M, Caraffa R, Fiocco A, et al. Transapical off-pump echo-guided mitral valve repair with neochordae implantation mid-term outcomes. Ann Cardiothorac Surg. 2021;10(1):131–40. https://doi.org/10.21037/acs-2020-mv-86.
ClinicalTrials.gov. Transcatheter mitral valve replacement with the Medtronic Intrepid™ TMVR system in patients with severe symptomatic mitral regurgitation (APOLLO). https://clinicaltrials.gov/ct2/show/NCT03242642. Accessed 3/1/2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Richard Cheng declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Structural Heart Disease
Rights and permissions
About this article
Cite this article
Cheng, R. How to Manage Mitral Stenosis Due to Mitral Annular Calcification. Curr Cardiol Rep 23, 148 (2021). https://doi.org/10.1007/s11886-021-01567-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01567-3