Abstract
Purpose of Review
This review summarizes the pathophysiology of mitral annular calcification (MAC) with recent findings and current strategies for diagnosis and treatment.
Recent Findings
Major factors in MAC development seem to be shear stress of the flow past the mitral valve, local inflammation, and dysregulation in regulators of mineral metabolism. MAC itself poses daunting technical challenges. Implanting a valve on top of the calcium bar might lead to paravalvular leak (PVL) that is less likely to heal. Annular decalcification allows for better valve seating and potentially better healing and less PVL. This, however, comes with the risk for catastrophic atrioventricular groove disruption. MAC can be sharply dissected with the scalpel; the annulus can be reconstructed with the autologous pericardium. Transcatheter mitral valve replacement is a promising approach in the treatment of patients who are deemed high-risk surgical candidates with severe MAC.
Summary
MAC is a multifactorial disease that has some commonalities with atherosclerosis, mainly regarding lipid accumulation and calcium deposition. It is of great clinical importance, being a risk marker of cardiovascular events (including sudden death) and, with its progression, can have a negative impact on patients’ lives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitral annular calcification (MAC) is a pathological entity that was recognized in the early twentieth century [1, 2]. It is a chronic process in which microinjuries and endothelium dysfunction lead to lipid accumulation progressing to calcium deposition, involving the fibrous annulus of the mitral valve (MV) typically in the posterior region [3, 4].
This process may be facilitated by conditions that increase shear stress in the fibrous skeleton of the valve, such as aortic stenosis, hypertrophic cardiomegaly, systemic arterial hypertension [5], and altered mineral metabolism state, such as that in chronic kidney disease (CKD) [6,7,8,9].
It is strongly associated with atherosclerotic risks factors such as dyslipidemia, smoking, type II diabetes mellitus, systemic arterial hypertension, and obesity, and is frequently found together with calcification of the coronary arteries and aortic valve [7, 9•, 10,11,12].
It tends to be asymptomatic and is mostly an incidental finding, although symptoms may appear with its progression, such as palpitations (owing to atrial fibrillation), dyspnea (due to mitral regurgitation or stenosis), and focal deficits (after stroke) [5, 13, 14, 15•, 16].
Its prevalence in the general population is as high as 10%, according to large autopsy studies, tending to affect more women and the elderly [8, 9•, 13, 17, 18].
Pathophysiology and Association with Atherosclerosis
Due to its higher prevalence in the elderly population and its time-dependent character, MAC was thought to be a passive process of calcium deposition [19].
It is now understood that it has a more active character in a regulated process, akin to bone formation with lipoprotein deposition and foci of chronic inflammation, similar to atherosclerosis, and it is even debatable whether they are different diseases or a spectrum of the same disease [20•, 21].
Its pathophysiology is probably heterogeneous, with multiple variables playing their specific roles, but with some serving as key steps.
Microinjuries
The current understanding of the onset of MAC is probably a microinjury/endothelium dysfunction caused by shear stress in the mitral valve annulus. The connection between mitral leaflets and its annulus is under increased shear stress due to its conformation, particularly in the posterior region [22].
Conditions that increase mitral annular stress, such as aortic stenosis or systemic arterial hypertension, are also associated with MAC [23, 24].
Shear stress and turbulent flow are sufficient to activate the endothelium, leading to local inflammation. When sustained, they lead to proliferation and migration of inflammatory cells and lipid accumulation [25].
Whole blood viscosity (WBV) also plays an important role in MAC formation, as a major component in valve shear stress. WBV can be calculated using hematocrit (HCT, %) and total plasma protein (TP, g/l), dividing it into (a) high shear rate (HSR) and (b) low shear rate (LSR) equations:
Cetin et al. studied 184 patients with MAC and 133 patients without MAC to confirm the role of WBV using this formula and to measure early diastolic mitral annular velocity (Ea) and late diastolic mitral annular velocity (Aa) with pulse Doppler tissue echocardiography. After adjusting for known risk factors for MAC, WBV values were independent predictors of MAC, with a cutoff value of 70.1 for WBV in LSR, a sensitivity of 83.7%, and a specificity of 73.7% (AUC 0.785, P < 0.001); and a WBV cutoff value of 17.5 in HSR, a sensitivity of 79.6%, and a specificity of 71.4% (AUC 0.761, P < 0.001) for the prediction of MAC [26•].
Endothelial-Mesenchymal Transformation
In the embryonic heart, endothelial cells (ECs) in the cardiac cushions downregulate cellular adhesion molecules, transiently upregulating the contractile protein alpha-smooth muscle actin, differentiating in mesenchymal cells and then migrating to the valve interstitium to become valvular interstitial cells (VICs) [27].
VICs have multiple phenotypes, notably quiescent valvular interstitial cells—which are the most abundant type in healthy adult valve tissue and activated valvular interstitial cells. Quiescent valvular interstitial cells have mechanical sensors that allow them to respond to stress or trauma [27, 28].
Endothelial dysfunction caused by shear stress can lead to the transformation of quiescent valvular interstitial cells into activated valvular interstitial cells through changes in transforming growth factor and smooth muscle alpha-actin levels, as seen in mitral valves subjected to mechanical stress. Activated valvular interstitial cells produce higher levels of alkaline phosphatase, and start to express osteocalcin and CD 31 (markers for osteoblasts), which may lead to calcific deposition [27,28,29,30]. Over time, these deposits coalesce into a fibrotic, macroscopically rigid band, in a process similar to bone formation [23, 24].
The Role of Inflammation
It is still debatable if MAC is associated with systemic inflammatory markers [6]. Inflammation may play an important role in the development of MAC at the site of valvular calcification and in the early stages of the disease, but several studies have not found significant relationships between inflammatory markers and MAC after adjusting for cardiovascular disease risk factors [9•, 31].
There is a lack of studies to confirm the role of paracrine signaling in MAC formation, but recently the association between MAC and epicardial fat thickness (EFT) has been demonstrated by Guler and Varol [32]. In their study, 78 patients with MAC were compared to a control group and found that EFT was significantly higher, with no differences in cardiovascular risk factors between groups. They postulate that EFT may play a role in MAC development by secreting inflammatory cytokines that would act locally.
Rezaeian et al. studied 631 HIV+ men to verify whether there was an association between HIV and MAC. They found that HIV+ patients are 3 times more likely to have MAC, but no relationship between inflammatory markers and presence of MAC. They ascribed this finding to the chronic use of protease inhibitors with their resulting metabolic disorders [31, 33].
The Role of Altered Mineral Metabolism
CKD is a strong risk factor for MAC. Part of this association may be due to the fact that in both diseases, patients tend to have more atherosclerotic risk factors [6], but also owing to an altered state in body minerals, notably the calcium-phosphorus metabolism and the role of endothelial-mesenchymal transformation (EMT) of VICs in osteoblastic-like cells [29, 34].
Blood levels of calcium and phosphorus are kept adequate by a delicate balance between parathyroid hormone and calcitriol—converted from 25-hydroxyvitamin D3 (25(OH)D) in the kidneys. Most of the body’s calcium and phosphorus is trapped in the bones: less than 1% is circulating in the bloodstream. Parathyroid hormone signalizes for calcium reabsorption from bones and kidneys, increasing blood calcium levels and stimulating renal phosphate excretion; while calcitriol increases serum calcium and phosphate levels by stimulating intestinal absorption. Renal failure leads to a decrease in serum calcium and an increase in serum phosphate. Parathyroid tries to achieve homeostasis by elevating parathyroid hormone secretion, thus promoting calcium reabsorption from bones [6].
Tibuakuu et al. performed a large population analysis of 5530 patients from MESA (Multiethnic European Study of Atherosclerosis) to study the association of high or low serum 25(OH)D with valvular calcification. They found that patients with 25(OH)D ≥ 20 ng/ml were more likely to have MAC. However, after adjusting to other variables, no statistically significant association was found [34].
Other regulators of mineral metabolism also seem to influence MAC development. Fetuin-A is a binding protein that inhibits crystallization of calcium-phosphorus in soft tissue. In a study of 3585 patients from CHS (Cardiovascular Health Study), Bortnick et al. found a strong inverse association of fetuin-A and MAC, questioning if increased fetuin-A levels could decrease MAC progression [9•].
Fibroblast growth factor 23 (FGF-23) controls serum phosphate by inhibiting renal tubular transport and calcitriol synthesis, and high levels of this protein are being associated with MAC [9•, 35].
Magnesium also appears to play a role in MAC formation. It protects against calcification by negatively regulating osteogenic differentiation through a transient receptor potential melastatin (TRPM7) activity and increased expression of osteopontin and matrix Gla protein (both anticalcification proteins) [36].
Studying 150 diabetic patients with mild to moderate CKD in a prospective study, Silva et al. observed that serum magnesium levels below 1.7 mg/dl were independent predictors of mitral valve calcification, adding that in these populations magnesium should be considered therapeutic to prevent mitral calcification [35].
The Genetic Modulation
Afshar et al. demonstrated the relationship between genetic predisposition to high triglyceride levels and their association with MAC. They reviewed three large patient cohorts—the Framingham Health Study, MESA, and the AGE-RS (Age, Gene/Environment Susceptibility-Reykjavik Study), and noted that triglyceride genetic risk score (GRS) is strongly associated with MAC (odds ratio [OR] per triglyceride GRS unit: 1.73; 95% confidence interval [CI]: 1.24–2.41; P = 0.0013). Since triglyceride GRS is highly correlated with circulating triglyceride levels, they postulated that deposition of triglycerides in the mitral annulus may influence the development of MAC [20•].
Thanassoulis et al. performed a genome-wide investigation among 3975 patients diagnosed with MAC by CT, and identified two single nucleotide polymorphisms (SNPs) on chromosome 2 near IL1F9 gene cluster associated with MAC, designated as rs17659543 and rs13415097, with P = 1.5 × 10−8 and P = 1.8 × 10−8, respectively, for MAC association [37]. These aforementioned P values mean genome-wide significance (GWS), which is a specific threshold for determining the statistical significance of a reported association between a given SNP and a given trait (in this case, MAC).
Diagnoses/Imaging
Chest X-ray (Fig. 1) may show a C-, J-, U-, or O-shaped radiopaque image in the mitral annulus, usually with its opening directed to the aortic valve. Because of the left pulmonary veins, sometimes it is better visualized in lateral projections [5].
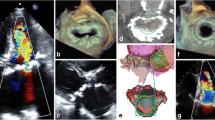
On M-mode echocardiography, MAC is seen as an echo-dense band near the mitral valve, usually beneath the posterior leaflet, anterior and parallel to the left ventricular free wall. On 2D echocardiogram (Fig. 2), it is seen as an irregular, echo-dense shell-like structure that produces acoustic shadowing [11, 38]. The calcific deposits are most often seen in the posterior part of the annulus, evenly distributed. When it occurs in the anterior region, it tends to be in the medial segment [39].
3D transesophageal echocardiography (Fig. 3) can be used to determine the effective orifice area of the mitral valve and mitral annular area by measuring the inner and outer mitral annulus and the posterior leaflet’s angle opening to calculate the reduction of the mitral valve area; thus, it can also be used to determine if the mitral valve should be approached in patients undergoing aortic valve surgery [40].
Two- and three-dimensional echocardiograms displaying caseous calcification of the mitral annulus. CCMA, caseous calcification of the mitral annulus. a 2D echo, b 3D echo (reproduced from Pala et al. [81])
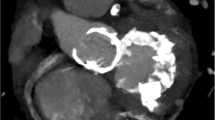
MAC can be visualized and quantified from a single computed tomography (CT) image acquisition (Fig. 4) without additional contrast agents [41]. Electrocardiogram (ECG)-gated cardiac CT is a useful tool to measure the extent and location of MAC and to plan the surgery when indicated. It can accurately show areas where there is more calcium, where it does not allow suture, and where it is safe to do. It can also predict the need for decalcification and reconstruction of the annulus, providing vital anatomical information reducing periprocedural risk of complications [21, 42]. In patients undergoing transcatheter mitral valve replacement (TMVR), cardiac CT is crucial to evaluate the left ventricle outflow tract (LVOT) to predict obstruction after valve implantation [43•].
CT for the evaluation of MAC should ideally have high-resolution images of the left atrium (LA)–left ventricle (LV) annular junction, to clearly assess the full extent of the disease, which in some cases can infiltrate the LV, interventricular septum, and right ventricle.
Due to its pattern, some areas of the annulus that may appear to be heavily calcified in CT scan are in truth fibrotic tissue [44].
MAC can be classified as mild (focal, soft echodensity of the mitral annulus, < 180° of total annular circumference), moderate (more prominent echodensity of the annulus, < 270° of total annular circumference), or severe (marked echodensity involving more than 270° of circumference of the mitral annulus or progressing to LV inflow tract or thickness > 4 mm) [45, 46••].
Caseous calcification of the mitral annulus (CCMA) is a relatively uncommon progression of MAC, with prevalence ranging around 0.6% of patients with MAC [47]. The precise mechanism of transformation is not yet fully understood.
As with MAC, patients with CCMA are usually asymptomatic. In rare cases, it can lead to significant pathologies. In one post-mortem examination of a patient with CCMA, Curl et al. found that the caseous calcification extended to most of the interventricular septum, a large portion of the left ventricular wall, and a portion of the right ventricular wall. It also passed through the myocardium emerging in the epicardium. Histopathological findings of epicardial plaque had diffuse calcification with mixed necrotic eosinophilic material, and in the intracardiac mass there were areas of amorphous necrotic material, and sparse chronic inflammation including lymphocytes and histiocytes [48].
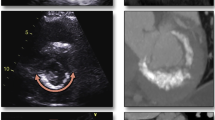
CCMA is seen on transthoracic echocardiography as a round, echo-dense mass in the periannular region, usually close to the posterior leaflet, without acoustic shadowing artifacts with central areas of echolucencies resembling liquefaction [49], almost always presenting in the posterior leaflet, with some rare exceptions [50].
In some cases, it can be a challenging differential diagnosis with cardiac tumors and myocardial abscesses [48, 51]. Although rare, both CCMA and endocarditis can coexist [52].
The diagnosis of CCMA by echocardiography can sometimes be difficult, especially in patients with poor acoustic windows. In such cases, cardiac CT can help in the diagnosis. CCMA appears in CT as an oval- or crescent-shaped hyperdense mass with a calcified rim [53].
Clinical Implications
Although patients with MAC are asymptomatic in most cases, it is associated with CVD, including increased risk of sudden death [54], and several complications can arise with its progression.
Cerebrovascular Events
MAC is related to cerebrovascular events (CVEs), with risk ranging from 4.8 to 24.1% in several studies [15•]. Possibly, the mechanism is embolization of caseous necrotic debris from CCMA lesion and embolization of calcium and cholesterol particles [15•, 55, 56]. CCMA may impose a high risk of CVEs. In a review of 86 publications with a total of 130 patients, Dietl et al. found a prevalence of CVE in patients with CCMA of 19.2%, giving us some reason to considerate the possibility of elective surgical resection of CCMA in low-risk asymptomatic patients to prevent CVE [15•].
Mitral Valve Disease
Mitral regurgitation (MR) is often associated with MAC (15–55%), mainly explained by the fact that the annulus may be impaired in reducing its diameter during systole, and when the calcified mass extends beneath the posterior leaflet, causing distortion of the posterior leaflet and making coaptation of the leaflets less effective [5].
Mitral stenosis (MS) can also occur as caused by MAC, almost always when it is severe [39, 57]. The main mechanism appears to be a reduction in the effective orifice area and an impairment of the posterior leaflet opening [40]. Due to its apparent mechanism—microinjury and endothelium dysfunction, facilitated by increased stress on the fibrous skeleton of the valve—it is reasonable to assume that it is more often a consequence of MS than the cause [58].
Endocarditis
Although MAC was thought to be the result of bacterial endocarditis, it is now known that this is not the case; in fact, patients with MAC are at risk of developing endocarditis [5]. In a small autopsy report, a high prevalence of Staphylococcus aureus in these patients was emphasized [59]. It is most often described in autopsy studies, rarely reported during life [60].
Eicher et al. studied 62 cases of native mitral valve infective endocarditis diagnosed with transesophageal echocardiography (TEE) and found that 24% had vegetations originating from a calcified mitral annulus. This group of patients had significantly larger vegetations, a high frequency of ring abscess and a high frequency of para-annular ventriculoatrial leakage, and worse clinical outcomes when compared with patients who had endocarditis originating from the mitral leaflets [61, 62].
Conduction System Disease
The association between MAC and cardiac conduction disorders has been well reported since the early twentieth century [1]. Patients with MAC may have a wide variety of related arrythmias, with prevalence ranging from 55 to 70%, mainly atrial fibrillation (AF) and atrioventricular block [58, 63].
The association between AF and MAC is largely demonstrated by Wesley et al., where 6641 patients from MESA study were evaluated for the presence of MAC: 9.3% had MAC and, at an average follow-up of 8.5 years, 4.6% developed AF, with MAC associated with an increased risk (HR 1.9, 95% CI 1.5–2.5) [64].
Possible explanations are that calcium may deposit in the membranous portion of the intraventricular septum close to the bundle of His and its branches, causing delays in the interatrial and intra-atrial electromechanical coupling intervals. In addition, patients with MAC have a large left atrium when compared with the normal population [65].
Current Strategies
Facing MAC in a symptomatic patient is not an easy task. When calcification in the mitral annulus becomes symptomatic, the patient is usually elderly and with comorbidities that make the treatment exceedingly difficult. Moreover, when it presents symptoms, MAC is already at an advanced stage, especially in cases of anterior annular calcification and progression to the left ventricle [66•].
Over the past years, several surgical techniques had emerged to deal with MAC and its possible complications, usually divided into two categories: with mitral annulus reconstruction or without mitral annulus reconstruction.
Surgery with Mitral Annulus Reconstruction
As previously described, MAC can extend in some cases involving the whole annulus, passing through LVOT and penetrating various areas of the myocardium, sometimes not allowing valve repair or even replacement. In those cases, debridement of the calcified areas may be necessary. With extensive debridement, the mitral annulus may be compromised and need reconstruction.
Some surgical techniques have been performed over the years, trying to decalcify the mitral annulus, allowing valve repair or replacement in the native annulus [3, 44, 67,68,69].
En bloc decalcification and reconstruction of the annulus was first described by Carpentier. In this technique, the calcified annular bar was removed and the exposed myocardium was covered with atrium flap or autologous/bovine pericardium patch. Results range from originally 2.9% in-hospital mortality to 20% in recent studies [3, 67, 68], and 5-year survival around 68% in older series [69].
When done en bloc, this type of decalcification is said to reduce the risk of embolic events rather than taking piece by piece of calcified areas. Even so, special attention should be paid to removing all debris [44].
Over time, it has been observed that aggressive annular debridement greatly increases operative complexity [67] and can lead to atrioventricular groove rupture, circumflex coronary artery injury, and thromboembolic events [70, 71].
Surgery without Mitral Annulus Reconstruction
Several studies have demonstrated the safety of replacing the mitral valve in a MAC patient, with little debridement of the calcified area, achieving satisfactory results, with no difference in mortality compared with patients who underwent mitral valve replacement without MAC [66•].
Techniques vary from implanting the prosthetic valve in intra-atrial position and in intra-annular position.
Intra-atrial techniques in which the mitral prosthesis would be inserted over the mitral annulus to avoid implantation in the heavy calcified annulus have been described several times. Nataf et al. proposed enlarging the sewing ring of the prosthesis with a Dacron collar and then implant it 0.5–1.5 cm above the mitral annulus. Atoui et al. performed a variation of this technique in which they inserted the prosthesis above the mitral annulus with pledgeted suture in the atrial free wall. In both procedures, due to exposure of the left atrium to ventricular pressures, aneurysmal dilatation and prothesis dehiscence can occur [70, 72].
To avoid complications of implanting a mitral prosthesis in an atrial position, some techniques have emerged trying to place the valve inside the calcified annulus. Disadvantages of this type of technique are the risks of paravalvular leakage, valve dehiscence, and friable calcified annulus embolism. In addition, passing sutures through the calcified annulus can be challenging if not all calcified areas are well known [68, 73, 74]. Note that implanting a mitral prosthesis into a heavily calcified annulus can sometimes force the surgeon to place a smaller prosthesis than if decalcification techniques were applied.
Before choosing this type of implantation, the surgeon should know preoperatively which valve is to be implanted, being careful to choose one with an adequate orifice area, and not allowing prosthesis-patient mismatch, which increases perioperative and long-term mortality [75].
Lafrenière-Bessi et al. [76] described recently a new surgical approach to manage MAC and avoid debridement by using a transseptal approach and a mitral valve anchoring strategy inside the coronary sinus. According to the authors [76], this new surgical method was devised to anchor the prosthetic valve, using the native uncalcified coronary sinus, to avoid postoperative sequelae such as paravalvular leak and valve dehiscence caused by suture placement through calcified or debrided structures. The idea behind this technique came from the approach extending up to the opening of the coronary sinus into the right atrium. The concept of opening the sinus would allow for enough tissue to anchor a mitral prosthesis slightly over the plane of the annulus for approximately one-third of the circumference of the valve in its posterior aspect.
Management of CCMA
Chauvette et al. [77] described a transseptal approach to deal with CCMA extending from the left atrioventricular groove into the left ventricular posterior wall. An incision is made on the atrial endocardium contiguous to the posterior portion of the mitral annulus toward the posterior commissure. After resection of the toothpaste-like material, circular stiches like purse strings from within are performed and then tied to obliterate the dead space left by the resection.
Transcatheter Mitral Valve Replacement
With the advancement of transcatheter devices to replace heart valves, patients with MAC who are at high risk for conventional mitral replacement have another option. Transcatheter mitral valve replacement (TMVR) is still at its first stages and is not offered outside tertiary/specialized centers.
In 2018, Guerrero et al. published the 1-year outcomes of a multicenter experience of 116 extreme surgical risk patients with severe MAC who underwent TMVR. The mean age was 73 ± 12 years, 68% were female, the mean Society of Thoracic Surgeons (STS) score was 15.3 ± 11.6%, and 90% were in New York Heart Association (NYHA) functional class III or IV. Despite 100% technical success (according to Mitral Valve Academic Research Consortium), high 30-day mortality rates (13% cardiovascular deaths and 12% non-cardiac deaths) and 1-year mortality rates (23.5% cardiovascular deaths and 30.2% non-cardiac deaths) were observed, and LVOT obstruction was the most important and independent predictor [78••].
In patients at high risk of LVOT obstruction, open atrial transcatheter implantation is another possibility, with early studies showing mortality rates ranging from 0 to 27%. In this modality, after cardiopulmonary bypass, the mitral valve is exposed by left atriotomy. To avoid LVOT obstruction, resection of the anterior mitral valve leaflet is performed. CT simulation of the neo-LVOT after valve implantation is used to try to avoid the risk of LVOT obstruction [79]. Pratz et al. published an experience of 26 patients, with a mean age of 78 ± 7 years: 92% were female and 96% were in NYHA III or IV. The 30-day mortality was still high (27%). In a single center, Russel et al. published a study of 8 patients with mean STS score of 8%. They reported zero in-hospital or 30-day mortality. Both achieved 100% technical success with very low paravalvular leakage [79, 80].
Conclusion
MAC is a complex disease that affects the elderly or patients with comorbidities that already impair their life condition, thus imposing a great challenge in follow-up and treatment.
Advances in cardiovascular calcification mechanism studies may provide physicians with insights into different approaches in the future, acting when the disease is not yet symptomatic, preventing its progression.
Once MAC advances to cause symptoms, decisions must be made to choose the best type of interventional therapy. Surgeons and interventional cardiologists must balance the pros and cons of surgical and transcatheter procedures to ensure the lowest possible risk of adverse events.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yater WM, Cornell VH. The heart block due to calcareous lesions in the bundle of His. Ann Intern Med. 1935;8:777. https://doi.org/10.7326/0003-4819-8-7-777.
Bedeir K, Kaneko T, Aranki S. Current and evolving strategies in the management of severe mitral annular calcification. The Journal of Thoracic and Cardiovascular Surgery 2018 volume 157, number 2. DOI: https://doi.org/10.1016/j.jtcvs.2018.05.099.
Carpentier AF, Pellerin M, Fuzellier JF, Relland JY. Extensive calcification of the mitral valve anulus: pathology and surgical management. J Thorac Cardiovasc Surg. 1996;111(4):718–30. https://doi.org/10.1016/S0022-5223(96)70332-X.
Roberts WC, Perloff JK. Mitral valvular disease: a clinicopathologic survey of the conditions causing the mitral valve to function abnormally. Ann Intern Med. 1972;77(939–975):939–75. https://doi.org/10.7326/0003-4819-77-6-939.
Nestico PF, Depace NL, Morganroth J, Kotler MN, Ross J. Mitral annular calcification: clinical, pathophysiology, and echocardiographic review. Am Heart J. 1984;107:989–96. https://doi.org/10.1016/0002-8703(84)90840-8.
Fox CS, Guo CY, Larson MG, Vasan RS, Parise H, O'Donnell CJ, et al. Relations of inflammation and novel risk factors to valvular calcification. Am J Cardiol. 2006;97:1502–5. https://doi.org/10.1016/j.amjcard.2005.11.086.
Maher ER, Young G, Smyth-Walsh B, Pugh S, Curtis JR. Aortic and mitral valve calcification in patients with end-stage renal disease. Lancet. 1987;2:875–7. https://doi.org/10.1016/s0140-6736(87)91370-5.
Abd Alamir M, Radulescu V, Goyfman M, et al. Prevalence and correlates of mitral annular calcification in adults with chronic kidney disease: results from CRIC study, 117e122. Atherosclerosis, DOI. 2015;242. https://doi.org/10.1016/j.atherosclerosis.2015.07.013.
• Bortnick AE, Bartz TM, Ix JH, et al. Association of inflammatory, lipid and mineral markers with cardiac calcification in older adults. Heart. 2016;102:1826–34. https://doi.org/10.1136/heartjnl-2016-309404 It shows the association between fetuin-A and FGF-23 in MAC development.
Roberts WC. The senile cardiac calcification syndrome. Am J Cardiol. 1986;58:572–4. https://doi.org/10.1016/0002-9149(86)90045-7.
Adler Y, Fink N, Spector D, Wiser I, Sagie A. Mitral annulus calcification - a window to diffuse atherosclerosis of the vascular system. Atherosclerosis. March 2001;155(1):1–8. https://doi.org/10.1016/s0021-9150(00)00737-1.
Sharma R, Pellerin D, Gaze DC, Mehta RL, Gregson H, Streather CP, et al. Mitral annular calcification predicts mortality and coronary artery disease in end stage renal disease. Atherosclerosis. 2007;191:348–54. https://doi.org/10.1016/j.atherosclerosis.2006.03.033.
O’Neal WT, Efird JT, Nazarian S, et al. Mitral annular calcification progression and the risk of atrial fibrillation: results from MESA. European Heart Journal - Cardiovascular Imaging (2017) 0, 1–6. DOI: https://doi.org/10.1093/ehjci/jex093.
Benjamin EJ, Plehn JF, D’Agostino RB, Belanger AJ, Comai K, Fuller DL, et al. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327:374–9. https://doi.org/10.1056/NEJM199208063270602.
• Dietl C, Hawthorn C, Raizada V. Risk of cerebral embolization with caseous calcification of the mitral annulus: review article. The Open Cardiovascular Medicine Journal. 2016;10(1):221–32. https://doi.org/10.2174/1874192401610010221 It alerts for the high risk of CVE in patients with CCMA.
Labovitz AJ, Nelson JG, Windhorst DM, Kennedy HL, Williams GA. Frequency of mitral valve dysfunction from mitral anular calcium as detected by Doppler echocardiography. Am J Cardiol. 1985;55:133–7. https://doi.org/10.1016/0002-9149(85)90314-5.
Simon MA, Liu SF. Calcification of the mitral valve annulus and its relation to functional valvular disturbance. Am Heart J. 1954;48:497–505. https://doi.org/10.1016/0002-8703(54)90116-7.
Lung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Eur Heart J. 2003;24:1231–43. https://doi.org/10.1016/s0195-668x(03)00201-x.
Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–26. https://doi.org/10.1161/CIRCULATIONAHA.104.486738.
• Afshar M, Luk K, Do R, et al. Association of triglyceride-related genetic variants with mitral annular calcification. Am J Cardiol. 2017;69:24. https://doi.org/10.1016/j.jacc.2017.04.051 Genetic predisposition to high triglyceride levels might lead to MAC development.
Shekar C, Budoff M. Calcification of the heart: mechanisms and therapeutic avenues. Expert Rev Cardiovasc Ther. 2018. https://doi.org/10.1080/14779072.2018.1484282.
Zekry SB, Freeman J, Jajoo A, et al. Patient-specific quantitation of mitral valve strain by computer analysis of three-dimensional echocardiography a pilot study. Circ Cardiovasc Imaging. 2016;9:e003254. https://doi.org/10.1161/CIRCIMAGING.115.003254.
Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation. 2006;113:861–6. https://doi.org/10.1161/CIRCULATIONAHA.105.552844.
Johnson R, Leopold JA, Loscalzo J. Vascular calcification pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–59. https://doi.org/10.1161/01.RES.0000249379.55535.21.
Albanese I, Khan K, Barratt B, et al. Atherosclerotic calcification: Wnt is the hint. J Am Heart Assoc. 2018;7:e007356. https://doi.org/10.1161/JAHA.117.007356.
• Cetin EHO, Cetin MS, Canpolat U, et al. The forgotten variable of shear stress in mitral annular calcification: whole blood viscosity. Med Princ Pract. 2015;24:444–50. https://doi.org/10.1159/000431362 Shear stress as an important role in MAC physiopathology.
Gomel MA, Lee RK, Grande-Allen KJ. Comparing the role of mechanical forces in vascular and valvular calcification progression. Front Cardiovasc Med. 2018;5:197. https://doi.org/10.3389/fcvm.2018.00197.
Pagnozzi LA, Butcher JT. Mechanotransduction mechanisms in mitral valve physiology and disease pathogenesis. Front Cardiovasc Med. 2017;4:83. https://doi.org/10.3389/fcvm.2017.00083.
Wylie-Sears J, Aikawa A, Levine RA, et al. Mitral valve endothelial cells with osteogenic differentiation potential. Arterioscler Thromb Vasc Biol. 2011;31:598–607. https://doi.org/10.1161/ATVBAHA.110.216184.
Schoen FJ. Evolving concepts of cardiac valve dynamics. Circulation. 2008;118(18):1864–80. https://doi.org/10.1161/CIRCULATIONAHA.108.805911.
Rezaeian P, Miller PE, Haberlen SA, Razipour A, Bahrami H, Castillo R, et al. Extra-coronary calcification (aortic valve calcification, mitral annular calcification, aortic valve ring calcification and thoracic aortic calcification) in HIV seropositive and seronegative men: multicenter AIDS cohort study. J Cardiovasc Comput Tomogr. 2016;10(3):229–36. https://doi.org/10.1016/j.jcct.2016.02.002.
Guler S, Varol E. The relation between echocardiographic epicardial fat thickness and mitral annular calcification. Afri Health Sci. 2019;19(1):1657–64. https://doi.org/10.4314/ahs.v19i1.41.
Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–8. https://doi.org/10.1016/S0140-6736(02)11672-2.
Tibuakuu M, Zhao D, Boer IH, et al. Relation of serum vitamin D to risk of mitral annular and aortic valve calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2017 August 01;120(3):473–8. https://doi.org/10.1016/j.amjcard.2017.05.004.
Silva AP, Gundlach K, Büchel J, et al. Low magnesium levels and FGF-23 dysregulation predict mitral valve calcification as well as intima media thickness in predialysis diabetic patients. International Journal of Endocrinology. 2015;308190:10. https://doi.org/10.1155/2015/308190.
Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, et al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56:453–62. https://doi.org/10.1161/HYPERTENSIONAHA.110.152058.
Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–12. https://doi.org/10.1056/NEJMoa1109034.
Fox CS, Vasan RS, Parise H, Levy D, O'Donnell CJ, D'Agostino RB, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality. Circulation. 2003;107:1492–6. https://doi.org/10.1161/01.cir.0000058168.26163.bc.
Movva R, Murthy K, Romero-Corral A, Seetha Rammohan HR, Fumo P, Pressman GS. Calcification of the mitral valve and annulus: systematic evaluation of effects on valve anatomy and function. J Am Soc Echocardiogr. 2013;26(10):1135–42. https://doi.org/10.1016/j.echo.2013.06.014.
Iwataki M, Takeuchi M, Otani K, Kuwaki H, Yoshitani H, Abe H, et al. Calcific extension towards the mitral valve causes non-rheumatic mitral stenosis in degenerative aortic stenosis: real-time 3D transoesophageal echocardiography study. Open Heart. 2014;1:e000136. https://doi.org/10.1136/openhrt-2014-000136.
Yeboah J, Carr JJ, Terry JG, Ding J, Zeb I, Liu S, et al. Computed tomography-derived cardiovascular risk markers, incident cardiovascular events, and all-cause mortality in non-diabetics. The Multi-Ethnic Study of Atherosclerosis. Eur J Prev Cardiol. 2014 October;21(10):1233–41. https://doi.org/10.1177/2047487313492065.
Higgins J, Mayo J, Skarsgard P. Cardiac computed tomography facilitates operative planning in patients with mitral calcification. Ann Thorac Surg. 2013;95:e9–11. https://doi.org/10.1016/j.athoracsur.2012.07.059.
• Blanke P, Naoum C, Dvir D, et al. Predicting LVOT obstruction in Transcatheter mitral valve implantation concept of the neo-LVOT. JACC Cardiovasc Imaging. 2017;10(4):482–5. https://doi.org/10.1016/j.jcmg.2016.01.005 The concept of simulating a new LVOT to predict obstruction after TMVR.
Kurazumi H, Mikamo A, Suzuki R, Hamano K. Mitral-valve replacement for a severely calcified mitral annulus: a simple and novel technique. Eur J Cardiothorac Surg. 2011 Mar;39(3):407–9. https://doi.org/10.1016/j.ejcts.2010.06.017.
Kohsaka S, Jin Z, Rundek T, et al. Impact of mitral annular calcification on cardiovascular events in a multiethnic community: the northern Manhattan study. J Am Coll Cardiol Img. 2008;1:617–23. https://doi.org/10.1016/j.jcmg.2008.07.006.
•• Eleid MF, Foley TA, Said SM, Pislaru SV, Rihal CS. Severe mitral annular calcification: multimodality imaging for therapeutic strategies and interventions. JACC Cardiovasc Imaging. 2016;9:1318–37. https://doi.org/10.1016/j.jcmg.2016.09.001 Imaging assessment, classification and importance to interventions in MAC.
Deluca G, Correale M, Ieva R, del Salvatore B, Gramenzi S, di Biase M. The incidence and clinical course of caseous calcification of the mitral annulus: a prospective echocardiographic study. J Am Soc Echocardiogr. 2008;21:828–33. https://doi.org/10.1016/j.echo.2007.12.004.
Curl E, Riemer E. Caseous calcification of the mitral annulus: case report and brief review. European Heart Journal - Case Reports. 2018;2(4). https://doi.org/10.1093/ehjcr/yty124.
Harpaz D, Auerbach I, Vered Z, et al. Caseous calcification of the mitral annulus: a neglected, unrecognized diagnosis. J Am Soc Echocardiogr. 2001;14:825–31. https://doi.org/10.1067/mje.2001.111877.
Mazzucco A, Abbasciano R, Onorati F, Brognoli G, Fanti D, Gottin L. Anterior mitral annulus caseoma: as benign as posterior counterparts? Cardiovasc Pathol. 2016;25:336–8. https://doi.org/10.1016/j.carpath.2015.10.002.
Dingli P, Felice H, Mizzi A, Montefort S. Caseous mitral annular calcification mimicking a lung tumor on chest X-ray. J Family Med Prim Care. 2017;6:442–4. https://doi.org/10.4103/jfmpc.jfmpc_416_16.
Ono M, Mizuno A, Masuda K, Suzuki K, Abe K, Kawazoe K, et al. Infective endocarditis on caseous calcification of the mitral annulus involving both the anterior and posterior annulus: a rare case report. Intern Med. 2018 Apr 1;57(7):965–9. https://doi.org/10.2169/internalmedicine.9520-17.
Michałowska I, Szymański P, Kwiatek P, Spałek M, Furmanek M, Zieliński P, et al. Caseous calcification of the mitral annulus – the complementary role of computed tomography and transthoracic echocardiogram. Pol J Radiol. 2018;83:537–42. https://doi.org/10.5114/pjr.2018.81148.
Konety SH, Koene RJ, Norby FL, et al. Echocardiographic predictors of sudden cardiac death the Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. Circ Cardiovasc Imaging. 2016;9:e004431. https://doi.org/10.1161/CIRCIMAGING.115.004431.
Matsuyama TA, Ishibashi-Ueda H, Ikeda Y, Nagatsuka K, Miyashita K, Amaki M, et al. Critical multi-organ emboli originating from collapsed, vulnerable caseous mitral annular calcification. Pathol Int. 2012;62(7):496–9. https://doi.org/10.1111/j.1440-1827.2012.02826.x.
Sequeira A, Morris L, Patel B, et al. Calcific mitral stenosis in the hemodialysis patient. Hemodial Int. 2014;18(1):212–4. https://doi.org/10.1111/hdi.12094.
Labovitz AJ, Nelson JG, Windhorst DM, Kennedy HL, Williams GA. Frequency of mitral valve dysfunction from mitral anular calcium as detected by Doppler echocardiography. Am J Cardiol. 1985;55:133–7. https://doi.org/10.1016/0002-9149(85)90314-5.
Fulkerson PK, Beaver BM, Auseon JC, Graber HL. Calcification of the mitral annulus: etiology, clinical associations, complications and therapy. Am J Med. 1979;66(6):967–77. https://doi.org/10.1016/0002-9343(79)90452-2.
Hospital TG, Hospital M. Bacterial endocarditis of the mitral valve associated with annular calcification 119: 323–326, 1978.
Minardi G, Pino PG, Sordi M, Pavaci H, Manzara C, Pulignano G, et al. Infective endocarditis on mitral annular calcification: a case report. Cases Journal. 2009;2:9072. https://doi.org/10.1186/1757-1626-2-9072.
Eicher JC, De Nadai L, Soto FX, et al. Bacterial endocarditis complicating mitral annular calcification: a clinical and echocardiographic study. J Heart Valve Dis. 2004;13:217–27.
Vistarini N, d’Alessandro C, Aubert S, et al. Surgery for infective endocarditis on mitral annulus calcification. J Heart Valve Dis. 2007;16:611–6.
Nair CK, Runco V, Everson GT, Boghairi A, Mooss AN, Mohiuddin SM, et al. Conduction defects and mitral annulus calcification. Br Heart J. 1980;44:162–7. https://doi.org/10.1136/hrt.44.2.162.
O’Neal WT, Efird JT, Nazarian S, et al. Mitral annular calcification and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis. Europace. 2015;17:358–63. https://doi.org/10.1093/europace/euu265.
Pekdemir H, Cansel M, Yagmur J, et al. Assessment of atrial conduction time by tissue Doppler echocardiography and P-wave dispersion in patients with mitral annulus calcification. J Electrocardiol. 2010;43:339–43. https://doi.org/10.1016/j.jelectrocard.2010.02.013.
• Saran N, Greason KL, Schaff HV, et al. Does mitral valve calcium in patients undergoing mitral valve replacement portend worse survival? Ann Thorac Surg 2018. 2019;107(2):444–52. https://doi.org/10.1016/j.athoracsur.2018.07.098 A less aggressive surgery achieves satisfactory results in treating MAC.
Price J, Glineur D, De Kerchove L, El Khoury G. Mitral valve repair is feasible following extensive decalcification and reconstruction of the atrioventricular groove. J Heart Valve Dis. 2015 Jan;24(1):46–52.
Kurazumi H, Mikamo A, Suzuki R, Hamano K. Mitral valve replacement in patients with severely calcified mitral valve annulus: surgical technique. Eur J Cardiothorac Surg. 2011;39(3):407–9. https://doi.org/10.1016/j.ejcts.2010.06.017.
David TE, Feindel CM, Armstrong S, Sun Z. Reconstruction of the mitral anulus: a ten-year experience. J Thorac Cardiovasc Surg. 1995;110(5):1323–32. https://doi.org/10.1016/S0022-5223(95)70055-2.
Atoui R, Lash V, Mohammadi S, et al. Intra-atrial implantation of a mitral valve prosthesis in a heavily calcified mitral annulus. Eur J Cardiothorac Surg. 2009;36:776–8. https://doi.org/10.1016/j.ejcts.2009.05.035.
Cammack PL, Edie RN, Edmunds LH Jr. Bar calcification of the mitral annulus. A risk factor in mitral valve replacement. J Thorac Cardiovasc Surg. 1987;94:399–404.
Nataf P, Pavie A, Jault F, et al. Intraatrial insertion of a mitral prosthesis in a destroyed or calcified mitral annulus. Ann Thorac Surg. 1994;58:163–7. https://doi.org/10.1016/0003-4975(94)91092-8.
Coselli JS, Crawford ES. Calcified mitral valve annulus: prosthesis insertion. Ann Thorac Surg. 1988;46:584–6. https://doi.org/10.1016/s0003-4975(10)64709-1.
Stefano SD, Lopez J, Florez S, et al. Building a new annulus: a technique for mitral valve replacement in heavily calcified annulus. Ann Thorac Surg. 2009;87:1625–7. https://doi.org/10.1016/j.athoracsur.2008.09.014.
MPBO S, LRP C, Rayol SC, RGS D, Menezes AM, Clavel MA, et al. Prothesis-patient mismatch negatively affects outcomes after mitral valve replacement: meta-analysis of 10.239 patients. Braz J Cardiovasc Surg. 2019;34(2):203–12. https://doi.org/10.21470/1678-9741-2019-0069.
Lafrenière-Bessi V, Cameron-Gagné M, Perron J, Lévesque MH, Laflamme M, Charbonneau É, et al. Mitral annular calcification and mitral valve replacement: a new approach. Ann Thorac Surg. 2018 Feb;105(2):e55–7. https://doi.org/10.1016/j.athoracsur.2017.09.028.
Chauvette V, Laflamme É, Lafrenière-Bessi V, Marzouk M, Bertrand O, O'Connor K, et al. Caseous calcification of the mitral annulus: a role for surgery. Ann Thorac Surg. 2019;S0003-4975(19)31566–8. https://doi.org/10.1016/j.athoracsur.2019.08.110.
•• Guerrero M, Urena M, Himbert D, Wang DD, et al. 1-Year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol. 2018;71:1841–53. https://doi.org/10.1016/j.jacc.2018.02.054 A promise for future treatment of severe MAC.
Praz F, Khalique OK, Lee R, Veeragandham R, Russell H, Guerrero M, et al. Transatrial implantation of a transcatheter heart valve for severe mitral annular calcification. J Thorac Cardiovasc Surg. 2018. https://doi.org/10.1016/j.jtcvs.2018.03.016.
Russell HM, Guerrero ME, Salinger MH, et al. Open atrial transcatheter mitral valve replacement in patients with mitral annular calcification. J Am Coll Cardiol. 2018;72:1437–48. https://doi.org/10.1016/j.jacc.2018.07.033.
Pala AA, Iner H, Ercisli MA. Approach to an unusual cardiac mass: mitral annulus caseoma. Braz J Cardiovasc Surg. 2019. https://doi.org/10.21470/1678-9741-2018-0361 Creative Commons user license https://creativecommons.org/licenses/by/4.0/.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
Luiz Rafael P. Cavalcanti, Michel Pompeu B. O. Sá, Álvaro M. Perazzo, Antonio C. Escorel Neto, Rafael A. F. Gomes, Alexander Weymann, Konstantin Zhigalov, Arjang Ruhparwar, and Ricardo C. Lima each declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evidence-Based Medicine, Clinical Trials and Their Interpretations
Rights and permissions
About this article
Cite this article
Cavalcanti, L.R.P., Sá, M.P.B.O., Perazzo, Á.M. et al. Mitral Annular Calcification: Association with Atherosclerosis and Clinical Implications. Curr Atheroscler Rep 22, 9 (2020). https://doi.org/10.1007/s11883-020-0825-3
Published:
DOI: https://doi.org/10.1007/s11883-020-0825-3