Abstract
Purpose of Review
The review provides an overview of current endovascular management of patients with acute ischemic stroke in the light of recent landmark trials proving unequivocal benefit of the intervention.
Recent Findings
Several randomized trials looking at selective groups of patients presenting after an acute ischemic stroke due to large vessel occlusion in the anterior circulation demonstrated an overwhelming benefit of the endovascular treatment compared to intravenous thrombolysis, leading to expedited changes in the American Heart Association/American Stroke Association guidelines. Nonetheless, there are a relative large number of patients that were not included in those trials that might still benefit from endovascular treatment (acute posterior circulation-related strokes or acute embolic occlusion of middle cerebral artery beyond the main trunk for instances) and in which further studies are needed. We also briefly discuss endovascular techniques, post-procedure care, and endovascular treatment delivery models to expedite stroke patient assessment and rapid transport using updated and improved workflow protocols to provide timely recanalization.
Summary
Endovascular treatment of acute occlusion of a proximal large artery in the anterior circulation is currently the standard of care. Time and quality of recanalization are the most important variables that determine the outcome. The indication for endovascular therapy in different scenarios (acute embolic occlusion in the posterior circulation or more distal branch occlusions) has to be individualized according to each patient’s particular characteristics until new evidence is provided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is the leading cause of permanent disability in the world and the fifth most common cause of death in the USA [1]. On an average, every 40 s, someone in the USA has a stroke resulting in ∼ 795,000 people experiencing a new or recurrent stroke per year [2]. For almost two decades, intravenous (IV) recombinant tissue plasminogen activator (r-tPA) was the only proven treatment for acute ischemic stroke. A key advantage of IV r-tPA is that it can be started rapidly after clinical assessment and computed tomography (CT) of the brain without the use of contrast material. Unfortunately, only few patients with acute ischemic stroke (< 10%) receive IV r-tPA, after symptom onset. Limitations of IV r-tPA include a narrow therapeutic time window (majority 3 and 4.5 h for a selective cohort), dependence on available serum plasminogen for efficacy, the resistance of an old or large thrombus to fibrinolysis, and the risks of systemic and cerebral hemorrhage [3]. Finally, most of the patients with a proximal vessel occlusion in the anterior circulation (60 to 80% of patients) die within 90 days after stroke onset or do not regain functional independence despite IV r-tPA treatment [4]. The major reason for the limited efficacy of IV r-tPA is the modest rate of effective reperfusion among patients with a large vessel occlusion [5].
The endovascular approach primarily started with intra-arterial (IA) delivery of fibrinolytic agents and led to fist successful prospective randomized trial with the use of IA recombinant pro-urokinase [6]. From 2002 to 2012, three randomized controlled trials of endovascular treatment of acute ischemic stroke with primarily IA fibrinolysis and/or first generation-mechanical embolectomy devices, Thrombolysis for Acute Ischemic Stroke (SYNTHESIS Expansion) [7], The Interventional Management of Stroke Trial III (IMS III) [3], and The Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) [8], failed to prove clinical efficacy of endovascular treatment. None of these studies showed a benefit of adjunct endovascular treatment over IV r-tPA alone. MR RESCUE also showed no benefit for patients treated within 8 h even if selected by multimodal neuroimaging criteria.
It was after the joint efforts of the neurointerventional community and the financial support from the medical device industry that in late 2014 and early 2015, five randomized clinical trials demonstrated the clinical benefit of newer generations of mechanical thrombectomy devices in acute ischemic stroke when used in timely fashion (Table 1). Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke (MR CLEAN) [9••]; Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion With Emphasis on Minimizing CT to Recanalization Times (ESCAPE) [4]; Solitaire FR With the Intention for Thrombectomy as Primary Endovascular Treatment of Acute Ischemic Stroke (SWIFT PRIME) [10]; Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial; IA, intra-arterial(EXTEND-IA) [11]; and Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within 8 Hours of Symptom Onset (REVASCAT trial) [12] formed the basis of updated and expedited American Heart Association (AHA)/American Stroke Association(ASA) guidelines published in 2015 and establishing the consideration of the stent retrievers as the standard of care in patient with a large vessel occlusion in the anterior circulation within 6 h from symptom onset and without early changes on non-contrast CT (ASPECTS ≥ 6). A systematic review and meta-analysis of randomized trials concluded that endovascular therapy significantly improved functional outcomes (without compromising safety) in patients with acute ischemic stroke compared with standard therapy [13].
Every or nearly every patient in the five stent retriever studies first received IV r-tPA thereby emphasizing that it should not be withheld if patient is considered for mechanical thrombectomy; however, in patients under consideration for mechanical thrombectomy, observation after IV alteplase to assess for clinical response should not be performed [14••]. All the studies enrolled subjects ≥ 18 years of age. There are no randomized trials of endovascular therapy in pediatric population. From these trials, there are insufficient data in patients with NIHSS scores < 6 to determine whether there is an overall net benefit from endovascular therapy in this population [15].
Criteria for Patient Selection
According to latest AHA/ASA guidelines [14••] currently, the selected cohorts to be considered for endovascular treatment include the following:
-
Prestroke patient should be functionally independent (modified Rankin score (mRS) 0 to 1)
-
Patient should be an adult with significant stroke (age ≥ 18 years, NIHSS score of ≥ 6) and computed tomography (CT) brain without evidence of large infarct suggested by Alberta Stroke Program Early CT Score (ASPECTS) of ≥ 6 with imaging proven causative occlusion of the internal carotid artery or proximal middle cerebral artery (MCA) M1 segment.
-
The technical goal of the thrombectomy procedure should be reperfusion to a modified thrombolysis in cerebral infarction (mTICI) 2b/3 angiographic result to maximize the probability of a good functional clinical outcome.
Time for intervention with mechanical thrombectomy has been changing rapidly. Abovementioned landmark trials supported mechanical thrombectomy up to 6 h from last well-known, latest guidelines published in the light of two major trials DAWN (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo) [16•] and DEFUSE 3 (The Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) [17] support mechanical thrombectomy beyond 6 h. Now according to AHA guidelines, in selected patients with acute ischemic stroke within 6 to 16 h of last known normal who have large vessel occlusion in the anterior circulation and meet other DAWN or DEFUSE 3 eligibility criteria, mechanical thrombectomy is recommended (class 1, level of evidence A). In addition, it is reasonable to consider mechanical thrombectomy within 6 to 24 h of last known normal if patients meet DAWN eligibility criteria [14••]. The DEFUSE 3 trial used perfusion-core mismatch and maximum core size as imaging criteria to select patients with large anterior circulation occlusion 6 to 16 h from last seen well for mechanical thrombectomy. This trial showed a benefit in functional outcome at 90 days in the treated group (mRS score 0–2, 44.6 vs 16.7%; RR, 2.67; 95% CI, 1.60–4.48). The DAWN trial used clinical imaging mismatch (a combination of NIHSS score and imaging findings on CTP or DW-MRI) as eligibility criteria to select patients with large anterior circulation vessel occlusion for treatment with mechanical thrombectomy between 6 and 24 h from last known normal. This trial demonstrated an overall benefit in function outcome at 90 days in the treatment group (mRS score 0–2, 49 vs 13%; adjusted difference, 33%; 95% CI, 21–44; posterior probability of superiority > 0.999). Interestingly, there are a larger treatment effects in the DAWN and DEFUSE 3 trials than in the 6-h trials and probably are attributable to the low rate of favorable outcomes in the medical control group, which may be related to the small proportion of patients who presented in time to receive intravenous r-tPA. DEFUSE 3 trial enrolled patients who had larger core infarctions than those in the DAWN trial and also included patients with milder stroke symptoms. Because of these differences, the DEFUSE 3 trial enrolled a broader patient population than the DAWN trial (approximately 40% of the patients in the DEFUSE 3 trial would not have met the DAWN selection criteria). However, the efficacy of endovascular therapy in our trial was similar in patients who did meet the eligibility criteria used in the DAWN trial and in patients who did not meet those criteria [17].
Approximately 1 in 4 patients with ischemic stroke wake up with neurological deficits and only 0.3 to 2.1% of wake-up strokes (WUS) are treated with IV thrombolysis due to unknown time of stroke symptoms onset [18,19,20,21]. Neuroimaging modalities like contrast-enhanced CT or MR imaging play a vital role in treating such patients by estimating salvageable brain tissue. In CT studies, CT angiogram (CTA) is used to detect the location of occlusion in the intracranial arteries and CT perfusion (CTP) is used to detect the area of infarct core and the salvageable tissue. Based on CTP, the area of mismatch or the ischemic penumbra is calculated based on visual inspection by comparing the CBF (cerebral blood flow) and CBV (cerebral blood volume) deficit. The outcomes at hospital discharge after reperfusion using endovascular techniques of large areas of mismatch with a major arterial occlusion in wake-up stroke have been demonstrated to be comparable to known time of onset strokes [22]. Similarly on MR imaging diffusion-weighted imaging (DWI), fluid-attenuated inverse recovery (FLAIR) sequence mismatch helps in knowing the infarct age and on the other hand, perfusion-weighted and diffusion-weighted imaging (PWI-DWI) mismatch gives an estimate of the salvageable penumbra amenable for reperfusion with the help of endovascular therapy [23, 24]. The abovementioned radiological imaging concepts are also utilized in clinical trials to extend window beyond conventional time window for IV r-tPA such as WAKE-UP [25] study and Extending the Time for Thrombolysis in Emergency Neurological Deficits (EXTEND) study [26]. Results from abovementioned DAWN and DEFUSE 3 trials can be extended now to benefit patients with wake-up stroke as long as these trial eligibility criteria are followed.
Imaging Selection of Patients
It is becoming widely accepted that the two main variables affecting the outcome of patients with ischemic stroke are the time to recanalization and the collateral flow. Early changes in basic neuroimaging may indicate poor collateral flow and may place patients at risk of complications. According to the guidelines by the American Heart Association [14••], non-contrast CT will provide the necessary imaging information to make decisions about use of IV r-tPA. Multimodal CT and MRI, including perfusion imaging, should not delay administration of IV r-tPA. For patients who otherwise meet criteria for endovascular therapy, it is reasonable to proceed with CT angiogram (CTA) or MR angiogram (MRA) of the head and neck. Additional imaging such as perfusion studies for selecting patients for mechanical thrombectomy in < 6 h is not recommended. As discussed above in selected patients with AIS within 6 to 24 h of last known normal who have large vessel occlusion (LVO) in the anterior circulation, obtaining CTP, DW-MRI, or MRI perfusion is recommended to aid in patient selection for mechanical thrombectomy, but only when imaging and other eligibility criteria from DAWN trial [16•] and DEFUSE 3 trial [17] are being strictly applied in selecting patients for mechanical thrombectomy. It may also be reasonable to incorporate collateral flow status into clinical decision-making in some candidates to determine eligibility for mechanical thrombectomy.
Uncertain Indications for Endovascular Treatment
The abovementioned criteria, however, do not include other commonly seen stroke scenarios and raise questions about the clinical effectiveness of endovascular treatment beyond a selective cohort of the patients. We will try to present a few common scenarios routinely seen in clinical practice and discuss their management.
-
1.
According to AHA/ASA guidelines, in patients who have prestroke mRS score > 1 but still have prestroke reasonable quality of life, or patients presenting with ASPECTS less than 6 on initial head CT scan, or NIHSS score < 6 if disabling and a large vessel occlusion is documented by imaging such as occlusion of the internal carotid artery (ICA) or proximal MCA (M1), the use of endovascular therapy with stent retrievers may be reasonable when treatment can be initiated (groin puncture) within 6 h of symptom onset. Operators typically individualize endovascular treatment in these circumstances based on institutional and personal discretion in regard to patient selection criteria. Additional data from randomized trials are needed since benefit can still be questioned [14••].
-
2.
Posterior circulation stroke and distal MCA stroke: Although all of the landmark trials excluded posterior circulation strokes, noninvasive vessel imaging helps to identify thrombus location before deciding endovascular treatment in the posterior circulation especially with a large vessel occlusion, in the basilar artery (Fig. 1) and proximal posterior cerebral artery [27]. The AHA/ASA guidelines support this approach and suggest that endovascular therapy with stent retrievers may be reasonable for carefully selected patients with acute ischemic stroke in whom treatment can be initiated (groin puncture) within 6 h of symptom onset and who have causative occlusion of the M2/M3 portion of the MCA, anterior cerebral arteries, vertebral arteries, basilar artery, or posterior cerebral arteries [14••]. The benefits of thrombectomy of smaller caliber arteries in a more distal location must be carefully considered in relation to the risks of the procedure. The occlusion of these smaller and more distal arteries, such as the distal M2 and the M3 segments of the MCA, pericallosal artery, or the posterior cerebral artery sometimes causes ischemic strokes with pronounced clinical impact on the patient. The clinical benefit that can be obtained by recanalization is also based on the eloquence of the dependent territory of the artery occluded [28].There is some experience with smaller 3-mm stent retrievers and aspiration devices [29]. A recent study of 3MAX reperfusion system (Penumbra, Alameda, California) demonstrated safety and efficacy in terms of both revascularization and functional independence for patients with acute ischemic stroke secondary to M2 and M3 occlusions using direct first pass aspiration technique (ADAPT), either as frontline monotherapy or in combination with adjunctive devices. However, larger prospective studies are necessary to provide more evidence of the benefits and safety of these procedures [28].
-
3.
Tandem occlusion: Approximately 25% of patients with MCA occlusion will have a concomitant internal carotid artery (ICA) occlusion, and 50% of patients with an ICA occlusion will have a proximal MCA occlusion. Cervical ICA occlusion with MCA embolic occlusion is associated with a low rate of recanalization and poor outcome after IV r-tPA [30, 31]. Outcomes for the subgroup of patients with cervical carotid occlusion were reported in ESCAPE [4] (OR, 8.7; 95% CI, 1.9–39.4) and MR CLEAN [9] (adjusted OR, 1.43; 95% CI, 0.78–2.64). Although thrombectomy for patients with cervical ICA occlusion is beneficial based on these data, the optimal management of the underlying stenosis is not clear. Carotid stenting and intracranial thrombectomy for the treatment of acute stroke resulting from tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage [32, 33]. In addition, there is some risk for thromboembolic stroke at the time of stenting [15]. Based on the Prognostic Factors Related to Clinical Outcome Following Thrombectomy in Ischemic Stroke (RECOST) study, the use of stent angioplasty in internal carotid dissection tandem occlusion is safe and effective after intracranial revascularization when compared to isolated anterior circulation occlusion stroke therapy. The best modality to treat proximal stenosis is based on an individual case basis; however, one meta-analysis suggested distal to proximal revascularization if possible appears to be a more practical and effective approach and may offer the advantage of decreased time to reperfusion, which is associated with better functional outcome [34, 35]. Major limitation of this analysis was that no study evaluated the difference in time to reperfusion for the anterograde and retrograde approach and its association with clinical outcome. Randomized, multicenter trials are required to potentially confirm these results and to further identify clinical and radiologic factors contributing to the good outcome. The acute use of antithrombotic medication in concomitant carotid artery stenting and intracranial mechanical thrombectomy is not yet defined. The need of antiplatelet loading doses and the route of administration (parenteral vs oral loading) especially when combined to simultaneous IV thrombolytic medication are particular challenging situations that have to be analyzed on specific case to case basis.
An 82-year-old man with history of atrial fibrillation presented with left-sided weakness and diminished consciousness requiring intubation, NIHSS > 25 (estimated) last seen normal was greater than 10 h. Head CT scan demonstrated hyperdense basilar artery (a) and confirmed occlusion of intracranial vertebral artery and basilar artery (b). Basilar artery thrombus visualized as filling defect (c). After one pass of stent retriever (Solitaire 4 mm × 20 mm) with aspiration and intra-arterial thrombolytic infusion (12 mg r-tPA), complete recanalization was achieved (d). Follow-up MRI brain (e) demonstrated small scattered strokes in bilateral cerebellar hemispheres. Visible clot fragments after retrieval (f). Patient was extubated after the procedure and NIHSS was 1 post procedure
Endovascular Techniques
The major difference leading to positive outcome in recent mechanical thrombectomy trials was the use of self-expandable, fully retrievable stent in the majority of the patients leading to higher recanalization rates in addition to shorter time to treatment in imaging proven large vessel occlusion patients (Table 1). Stent retrievers lead to immediate flow restoration after deployment and mechanical thrombectomy during withdrawal. The usefulness of mechanical thrombectomy devices other than stent retrievers is not well established for either technical efficacy or clinical benefit. These trials were not designed to demonstrate the superiority of stent retrievers over other devices such as snares or aspiration systems. Clinical experience has shown the combination of balloon guide catheters or distal-access/aspiration catheters, and stent retrievers provide rapid, effective, and safe recanalization. The use of a proximal balloon guide catheter or a large-bore distal-access catheter rather than a cervical guide catheter alone in conjunction with stent retrievers appears to be beneficial [15, 36, 37].
Another technique called “The technique of A Direct Aspiration, First Pass Technique for the Endovascular Treatment of Stroke” (ADAPT) appears promising with a high rate of recanalization. A recent study of 243 consecutive patients with large intracranial artery occlusions of the anterior circulation, treated within 6 h, compared ADAPT with the Solitaire FR stent retriever group. Patients treated with ADAPT achieved higher final recanalization rates (82.3 vs 68.9%; adjusted relative risk, 1.18; 95% CI, 1.02–1.37; P = .022), though differences in clinical outcomes between the cohorts were not significant. Use of an adjunctive device was more frequent in the ADAPT group (45.2 vs 13.5%, P < .0001). The rate of embolization in new territories or symptomatic hemorrhage did not differ significantly between the two groups [38].
The ASTER trial [39] (Contact Aspiration vs Stent Retriever for Successful Revascularization) compared the contact aspiration technique and the standard stent retriever technique as first-line endovascular therapy for successful revascularization within 6 h among patients with acute anterior circulation ischemic stroke and LVO. The proportion of patients with successful revascularization at the end of all interventions was 85.4% (n = 164) in the contact aspiration group versus 83.1% (n = 157) in the stent retriever group (OR, 1.20; 95% CI, 0.68–2.10; P = 0.53; difference, 2.4%; 95% CI, − 5.4 to 9.7%). The secondary clinical end point of mRS score of 0 to 2 at 90 days was achieved by 82 of 181 (45.3%) in the contact aspiration group versus 91 of 182 (50.0%) in the stent retriever group (OR, 0.83; 95% CI, 0.54–1.26; P = 0.38). According to the latest AHA guidelines, the use of mechanical thrombectomy devices other than stent retrievers as first-line devices for mechanical thrombectomy may be reasonable in some circumstances, but stent retrievers remain the first choice [14••].
In general, the technical goal of the thrombectomy procedure should be a TICI grade 2b/3 angiographic result to maximize the probability of a good functional clinical outcome. The use of adjunctive salvage techniques, including IA fibrinolysis, may be reasonable to achieve these angiographic results if completed within 6 h of symptom onset. However, it should be kept in mind that endovascular therapy with stent retrievers is recommended over IA fibrinolysis as first-line therapy whenever feasible [14••].
Choice of Anesthesia
Retrospective studies have found that patients receiving general anesthesia for endovascular treatment in acute ischemic stroke have worse neurological outcome compared with patients receiving conscious sedation. According to AHA/ASA guidelines, it may be reasonable to favor conscious sedation over general anesthesia during endovascular therapy for acute ischemic stroke. However, the ultimate selection of anesthetic technique during endovascular therapy for acute ischemic stroke should be individualized on the basis of patient risk factors, tolerance of the procedure, and other clinical characteristics [14••, 37]. In a recent randomized trial involving ninety patients with acute ischemic stroke, there was no difference between general anesthesia and conscious sedation in neurological outcome; however, important point to be made in this trial is that there was no difference noted between the two groups in regard to blood pressure levels, during the endovascular treatment or post-procedure care [40]. We believe that any significant drop in blood pressure should be avoided if general anesthesia is used for endovascular procedure.
Post-procedure Care
All patients should ideally be admitted to a stroke unit or neurologic critical care unit. Organized care in stroke units results in improved survival, independence, and probability of living at home. [41].
Post-procedural imaging has been a matter of debate due to no common consensus among the operators. Usually, the preferred imaging technique is non-contrast head CT to detect early hemorrhage. The timing of performing the follow-up head CT varies among institutions, from immediately post procedure to 24 h or up until it is usually associated with clinical deterioration. Often, we run into a predicament if the hyperdensity on the head CT scan represents early hematologic transformation or contrast extravasation or both [42]. Such instances may need follow-up serial imaging as 30–50% of cases may just be due to contrast extravasation but if present at 24 h are usually associated with hemorrhagic transformation and poor outcomes especially in parenchymal hematoma (PH2) category rather than hemorrhagic infarction (HI) [43, 44]. As early detection and management of hemorrhagic transformation helps in better clinical outcomes, flat panel detector CT scan (FPCT) can be performed on the patient on the same angiography table. It quickly processes fluoroscopic images into the same format as traditional CT scanners and can detect ICH with 91–100% sensitivity and negative predictive value (NPV) but has only 88% specificity and 44–84.2% positive predictive value (PPV) [45,46,47]. Dual-energy CT scans have been able to distinguish hemorrhage from contrast extravasation by image reconstruction from the particle attenuation characteristics using two different X-ray spectra at different kilovoltages [48]. This may help in earlier detection of hemorrhage as well as help in patients in particular who may need antithrombotic agents after endovascular therapy. Limitations are duration and presence of metallic artifacts which may interfere in image processing [49, 50].
Transient elevation of arterial blood pressure (BP) is frequent in acute ischemic stroke and may help to increase perfusion of tissue jeopardized by ischemia. The course of elevated systolic blood pressure after acute ischemic stroke was found to be inversely associated with the degree of vessel recanalization. When recanalization failed, systolic BP remained elevated longer than when it succeeded [51]. It has also been suggested by a retrospective analysis that higher peak values of systolic blood pressure independently correlated with worse 90-day modified Rankin scale and a higher rate of hemorrhagic complications [52]. Decreasing post-recanalization blood pressure is reasonable and may limit reperfusion injury, though evidence is lacking [53]. The ESCAPE [4] protocol mentions that systolic BP ≥ 150 mmHg is probably useful in promoting and keeping collateral flow adequate while the artery remains occluded and that controlling BP once reperfusion has been achieved and aiming for a normal BP for that individual are sensible. The DAWN [16•] protocol recommends maintaining systolic BP < 140 mmHg in the first 24 h in subjects who are perfused with mechanical thrombectomy. According to latest AHA guidelines, in patients who undergo mechanical thrombectomy with successful reperfusion, it might be reasonable to maintain BP at a level < 180/105 [14••].
The routine use of large bore sheaths for use of multiple catheters and balloon guide catheters in mechanical thrombectomy for ischemic stroke has led to concerns for vascular groin complications. Usage of vascular closure devices are non-inferior to manual compression in terms of vascular access site complications and also reduced time to hemostasis. Nursing education plays a very important role and they should be trained to distinguish skin wound bleeding from significant arterial bleeding and retroperitoneal hemorrhage. Standardized protocols should be followed diligently in maintenance of hemostasis at the puncture site and assessment for vascular complications [54, 55].
Early evaluation and participation for therapies are ideally initiated in the acute care environment if feasible. The effectiveness with which this need is met correlates with outcomes [56]. A 3-month outcome should be assessed for all treated patients by telephone or clinic visit to measure treatment results and participate in a registry to document and compare outcomes. A balanced and comprehensive approach to these post-procedure considerations is crucial to ensure an optimized patient outcome [53].
Endovascular Treatment Delivery Models
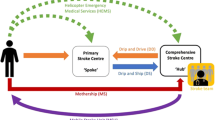
A focused approach is needed to ensure delivery of endovascular treatment to the right patients. Typical setup includes a comprehensive stroke center as “central hub” for endovascular therapy with surrounding “spokes” referring hospitals [57]. Transferring patients to an endovascular-capable stroke center commonly delays endovascular treatment. A pre-hospital clinical tool to help detect large vessel related ischemic stroke, e.g., Rapid Arterial oCclusion Evaluation (RACE) scale, which also assesses cortical signs like head/gaze deviation and aphasia/agnosia in addition to extremity and face weakness, may help facilitate rapid transport directly to a endovascular-capable stroke center by emergency medical services (EMS) especially if it is located within comparable driving time to a nearby smaller community hospital [58].
Once transfer has been decided for a patient, pre-hospital notification to the receiving hospital should be made. It is important to have a well-coordinated multidisciplinary team to receive the patient, including ER, neurology, and neuroendovascular physicians. It is important to move the patient through a rapid evaluation to confirm the occlusion with neuroimaging and then to the operating room as quickly as possible [59]. The use of telemedicine has revolutionized the treatment of stroke by improving the patient outcomes in regions where there is lack of neurologists with stroke expertise [60]. Half of patients arrive at a hospital after 2 h from symptom onset [61]. The largest, and most important, window for reducing delays to treatment is therefore prior to hospital arrival. In an effort to bring acute stroke thrombolytic treatment to the emergency site, the concept of mobile stroke units (MSUs) was introduced. This concept utilizes a specialized ambulance which is equipped with CT scanner, medications, point-of-care lab, and teleneurology. Preliminary studies have shown that MSUs have shown increased rates of treatments within 60 min of symptom onset and certain communities may benefit from these strategies [27, 62]. Leveraging these technological advances is important given the outcomes of latest endovascular trials which support the use of mechanical thrombectomy in selected patients with large vessel occlusions after IV thrombolysis is also time dependent in majority of the patients [27, 63].
Developing regional systems of stroke care should be considered. Healthcare facilities that provide initial emergency care, including administration of IV r-tPA, may consider emergency noninvasive intracranial vascular imaging to appropriately selected patients and may consider transferring if there is large vessel occlusion amenable for mechanical thrombectomy. One important point to consider for these hospitals is that observing patients after IV r-tPA to assess for clinical response before pursuing endovascular therapy is also not recommended. Centers capable of providing endovascular therapy should have 24/7 rapid access to cerebral angiography and qualified neurointerventionalists. Systems should be designed, executed, and monitored to emphasize expeditious assessment and treatment. Outcomes for all patients should be tracked. Facilities are encouraged to define criteria that can be used to credential individuals who can perform safe and timely IA revascularization procedures [15]. In addition, it is essential that all institutions construct a parallel processing method which best fits their resources and capabilities and participate in outcome registries if possible [59].
Conclusion
Acute ischemic stroke patient management has evolved tremendously wherein large vessel-related stroke management once having limited options is now having highly effective endovascular techniques not only in rates of recanalization but also decreased rates of disability. Recognition of large vessel-related stroke including pre-hospital triage, better interhospital transfer protocols, and technological advances including telestroke and mobile stroke units hopefully will improve the rate and timing of endovascular treatment for acute ischemic stroke patients and further decrease the stroke-related disability burden in the USA.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet (Lond). 2005;366(9499):1773–83.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322.
Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893–903.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30.
Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41(10):2254–8.
del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke. 1998;29(1):4–11.
Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368(10):904–13.
Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368(10):914–23.
•• Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. This first landmark study which demonstrated efficacy of mechanical thromectomy up to 6 hours using stent retrivers.
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306.
Sardar P, Chatterjee S, Giri J, Kundu A, Tandar A, Sen P, et al. Endovascular therapy for acute ischaemic stroke: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2015;36(35):2373–80.
•• Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. It covers previous landmark trials and other pertinenet management issues.
Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020–35.
• Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. This study was first randomized study to demonstrate efficacy of mechanical thrombectomy after 6 hours up to 24 hours from symptoms onset using perfusion imaging.
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New Engl J Med. 2018;378(8):708–18.
Nadeau JO, Shi S, Fang J, Kapral MK, Richards JA, Silver FL, et al. TPA use for stroke in the Registry of the Canadian Stroke Network. Can J Neurol Sci. 2005;32(4):433–9.
Marler JR, et al. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–7.
Moradiya Y, Janjua N. Presentation and outcomes of “wake-up strokes” in a large randomized stroke trial: analysis of data from the International Stroke Trial. J Stroke Cerebrovasc Dis. 2013;22(8):e286–92.
Costa R, Pinho J, Alves JN, Amorim JM, Ribeiro M, Ferreira C. Wake-up stroke and stroke within the therapeutic window for thrombolysis have similar clinical severity, imaging characteristics, and outcome. J Stroke Cerebrovasc Dis. 2016;25(3):511–4.
Silva GS, Lima FO, Camargo EC, Smith WS, Singhal AB, Greer DM, et al. Wake-up stroke: clinical and neuroimaging characteristics. Cerebrovasc Dis (Basel). 2010;29(4):336–42.
Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508–17.
Nagakane Y, Christensen S, Brekenfeld C, Ma H, Churilov L, Parsons MW, et al. EPITHET: positive result after reanalysis using baseline diffusion-weighted imaging/perfusion-weighted imaging co-registration. Stroke. 2011;42(1):59–64.
Barreto AD, Fanale CV, Alexandrov AV, Gaffney KC, Vahidy FS, Nguyen CB, et al. Prospective, open-label safety study of intravenous recombinant tissue plasminogen activator in wake-up stroke. Ann Neurol. 2016;80(2):211–8.
Amiri H, Bluhmki E, Bendszus M, Eschenfelder CC, Donnan GA, Leys D, et al. European Cooperative Acute Stroke Study-4: extending the time for thrombolysis in emergency neurological deficits ECASS-4: ExTEND. Int J Stroke. 2016;11(2):260–7.
Smith EE, Schwamm LH. Endovascular clot retrieval therapy: implications for the organization of stroke systems of care in North America. Stroke. 2015;46(6):1462–7.
Altenbernd J, Kuhnt O, Hennigs S, Hilker R, Loehr C. Frontline ADAPT therapy to treat patients with symptomatic M2 and M3 occlusions in acute ischemic stroke: initial experience with the Penumbra ACE and 3MAX reperfusion system. J Neurointerv Surg 2018;10(5)434–9.
Haussen DC, Lima A, Nogueira RG. The Trevo XP 3x20 mm retriever (‘Baby Trevo’) for the treatment of distal intracranial occlusions. J Neurointerv Surg. 2016;8(3):295–9.
Dababneh H, Guerrero WR, Khanna A, Hoh BL, Mocco J. Management of tandem occlusion stroke with endovascular therapy. Neurosurg Focus. 2012;32(5):E16.
Kistler JP, Ropper AH, Heros RC. Therapy of ischemic cerebral vascular disease due to atherothrombosis. (2). N Engl J Med. 1984;311(2):100–5.
Heck DV, Brown MD. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J Neurointerv Surg. 2015;7(3):170–5.
Aghaebrahim A, Jovin T, Jadhav AP, Noorian A, Gupta R, Nogueira RG. Endovascular recanalization of complete subacute to chronic atherosclerotic occlusions of intracranial arteries. J Neurointerv Surg. 2014;6(9):645–8.
Mbabuike N, Gassie K, Brown B, Miller DA, Tawk RG. Revascularization of tandem occlusions in acute ischemic stroke: review of the literature and illustrative case. Neurosurg Focus. 2017;42(4):E15.
Sivan-Hoffmann R, Gory B, Armoiry X, Goyal M, Riva R, Labeyrie PE, et al. Stent-retriever thrombectomy for acute anterior ischemic stroke with tandem occlusion: a systematic review and meta-analysis. Eur Radiol. 2017;27(1):247–54.
Nguyen TN, Malisch T, Castonguay AC, Gupta R, Sun CH, Martin CO, et al. Balloon guide catheter improves revascularization and clinical outcomes with the Solitaire device: analysis of the North American Solitaire Acute Stroke Registry. Stroke. 2014;45(1):141–5.
Dorn F, Stehle S, Lockau H, Zimmer C, Liebig T. Endovascular treatment of acute intracerebral artery occlusions with the solitaire stent: single-centre experience with 108 recanalization procedures. Cerebrovasc Dis (Basel). 2012;34(1):70–7.
Lapergue B, Blanc R, Guedin P, Decroix JP, Labreuche J, Preda C, et al. A direct aspiration, first pass technique (ADAPT) versus stent retrievers for acute stroke therapy: an observational comparative Study. AJNR Am J Neuroradiol. 2016;37(10):1860–5.
Lapergue B, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA. 2017;318(5):443–52.
Lowhagen Henden P, Rentzos A, Karlsson JE, Rosengren L, Leiram B, Sundeman H, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke trial (Anesthesia During Stroke). Stroke. 2017;48(6):1601–7.
Nikolaus T, Jamour M. Effectiveness of special stroke units in treatment of acute stroke. Z Gerontol Geriatr. 2000;33(2):96–101.
Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004;35(4):876–81.
Payabvash S, Qureshi MH, Khan SM, Khan M, Majidi S, Pawar S, et al. Differentiating intraparenchymal hemorrhage from contrast extravasation on post-procedural noncontrast CT scan in acute ischemic stroke patients undergoing endovascular treatment. Neuroradiology. 2014;56(9):737–44.
Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30(11):2280–4.
Gupta R, Cheung AC, Bartling SH, Lisauskas J, Grasruck M, Leidecker C, et al. Flat-panel volume CT: fundamental principles, technology, and applications. Radiographics Rev Publ Radiol Soc North Am Inc. 2008;28(7):2009–22.
Payabvash S, Khan AA, Qureshi MH, Saeed O, Suri MF, Qureshi AI. Detection of intraparenchymal hemorrhage after endovascular therapy in patients with acute ischemic stroke using immediate postprocedural flat-panel computed tomography scan. J Neuroimaging Off J Am Soc Neuroimaging. 2016;26(2):213–8.
Jang YM, Lee DH, Kim HS, Ryu CW, Lee JH, Choi CG, et al. The fate of high-density lesions on the non-contrast CT obtained immediately after intra-arterial thrombolysis in ischemic stroke patients. Korean J Radiol. 2006;7(4):221–8.
Ferda J, Novak M, Mirka H, Baxa J, Ferdova E, Bednarova A, et al. The assessment of intracranial bleeding with virtual unenhanced imaging by means of dual-energy CT angiography. Eur Radiol. 2009;19(10):2518–22.
Renu A, Amaro S, Laredo C, Roman LS, Llull L, Lopez A, et al. Relevance of blood-brain barrier disruption after endovascular treatment of ischemic stroke: dual-energy computed tomographic study. Stroke. 2015;46(3):673–9.
Dinkel J, Khalilzadeh O, Phan CM, Goenka AH, Yoo AJ, Hirsch JA, et al. Technical limitations of dual-energy CT in neuroradiology: 30-month institutional experience and review of literature. J Neurointerv Surg. 2015;7(8):596–602.
Mattle HP, Kappeler L, Arnold M, Fischer U, Nedeltchev K, Remonda L, et al. Blood pressure and vessel recanalization in the first hours after ischemic stroke. Stroke. 2005;36(2):264–8.
Mistry EA, Mistry AM, Nakawah MO, Khattar NK, Fortuny EM, Cruz AS, et al. Systolic blood pressure within 24 hours after thrombectomy for acute ischemic stroke correlates with outcome. J Am Heart Assoc. 2017;6(5):e006167.
Leslie-Mazwi T, Rabinov J, Hirsch JA. Endovascular treatment of acute ischemic stroke. Handb Clin Neurol. 2016;136:1293–302.
Schulz-Schupke S, Helde S, Gewalt S, Ibrahim T, Linhardt M, Haas K, et al. Comparison of vascular closure devices vs manual compression after femoral artery puncture: the ISAR-CLOSURE randomized clinical trial. JAMA. 2014;312(19):1981–7.
Elmasri MA, Kee ST, Moriarty JM, Gomes A, Lee EW, McWilliams JP. Single-center comparison of the efficacy and complications of arterial vascular closure devices in interventional radiology. J Vascular Access. 2017;18(4):339–44.
Belagaje SR, Zander K, Thackeray L, Gupta R. Disposition to home or acute rehabilitation is associated with a favorable clinical outcome in the SENTIS trial. J Neurointerv Surg. 2015;7(5):322–5.
Gorelick PB. Primary and comprehensive stroke centers: history, value and certification criteria. J Stroke. 2013;15(2):78–89.
Carrera D, Campbell BC, Cortes J, Gorchs M, Querol M, Jimenez X, et al. Predictive value of modifications of the prehospital rapid arterial occlusion evaluation scale for large vessel occlusion in patients with acute stroke. J Stroke Cerebrovasc Dis. 2017;26(1):74–7.
Mehta BP, Leslie-Mazwi TM, Chandra RV, Bell DL, Sun CH, Hirsch JA, et al. Reducing door-to-puncture times for intra-arterial stroke therapy: a pilot quality improvement project. J Am Heart Assoc. 2014;3(6):e000963.
Kleindorfer D, Xu Y, Moomaw CJ, Khatri P, Adeoye O, Hornung R. US geographic distribution of rt-PA utilization by hospital for acute ischemic stroke. Stroke. 2009;40(11):3580–4.
Fassbender K, Balucani C, Walter S, Levine SR, Haass A, Grotta J. Streamlining of prehospital stroke management: the golden hour. Lancet Neurol. 2013;12(6):585–96.
Parker SA, Bowry R, Wu TC, Noser EA, Jackson K, Richardson L, et al. Establishing the first mobile stroke unit in the United States. Stroke. 2015;46(5):1384–91.
Higashida R, Alberts MJ, Alexander DN, Crocco TJ, Demaerschalk BM, Derdeyn CP, et al. Interactions within stroke systems of care: a policy statement from the American Heart Association/American Stroke Association. Stroke. 2013;44(10):2961–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rakesh Khatri, Anantha R. Vellipuram, Alberto Maud, and Gustavo J. Rodriguez declare that they have no conflict of interest.
Salvador Cruz-Flores declares that he is a consultant for Novo Nordisk, Lilly, and Sunovion as clinical event adjudicator.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Ischemic Heart Disease
Rights and permissions
About this article
Cite this article
Khatri, R., Vellipuram, A.R., Maud, A. et al. Current Endovascular Approach to the Management of Acute Ischemic Stroke. Curr Cardiol Rep 20, 46 (2018). https://doi.org/10.1007/s11886-018-0989-4
Published:
DOI: https://doi.org/10.1007/s11886-018-0989-4