Abstract
Introduction
This study aimed to identify the imaging characteristics that can help differentiate intraparenchymal hemorrhage from benign contrast extravasation on post-procedural noncontrast CT scan in acute ischemic stroke patients after endovascular treatment.
Methods
We reviewed the clinical and imaging records of all acute ischemic stroke patients who underwent endovascular treatment in two hospitals over a 3.5-year period. The immediate post-procedural CT scan was evaluated for the presence of hyperdense lesion(s). The average attenuation of the lesion(s) was measured. Intraparenchymal hemorrhage was defined as a persistent hyperdensity visualized on follow-up CT scan, 24 h or greater after the procedure.
Results
Of the 135 patients studied, 74 (55 %) patients had hyperdense lesion(s) on immediate post-procedural CT scan. Follow-up scans confirmed the diagnosis of intraparenchymal hemorrhage in 20 of these 74 patients. A receiver operating characteristic analysis showed that the average attenuation of the most hyperdense lesion can differentiate intraparenchymal hemorrhage from contrast extravasation with an area under the curve of 0.78 (p = 0.001). An average attenuation of <50 Hounsfield units (HU) in the most visually hyperattenuating hyperdense lesion had 100 % specificity and 56 % sensitivity for identification of contrast extravasations. Petechial hyperdensity was seen in 46/54 (85 %) patients with contrast extravasation versus 9/20 (45 %) patients with intraparenchymal hemorrhage on the immediate post-procedural CT scan (p < 0.001).
Conclusion
An average attenuation <50 HU of the most hyperattenuating hyperdense parenchymal lesion on immediate post-procedural CT scan was very specific for differentiating contrast extravasation from intraparenchymal hemorrhage in acute ischemic stroke patients after endovascular treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
After completion of endovascular treatment, a noncontrast CT scan is usually performed to look for any parenchymal hyperdensity suggestive of hemorrhagic lesion. Parenchymal hyperdense areas are commonly seen on immediate post-endovascular CT scans. However, a significant fraction (39–57 %) of hyperdense lesions on a post-endovascular therapy CT scan represents contrast extravasation [1–3]. The imaging characteristics and clinical outcome of these hyperdense lesions have been described periodically, and different terms have been used to categorize these lesions [1–4]. Differentiation of hyperdensities caused by contrast extravasation from those caused by intraparenchymal hemorrhage is especially critical in the immediate post-procedural period, when the use of oral antiplatelets, intravenous (IV) glycoprotein IIB/IIIA inhibitors [5, 6], or IV anticoagulation is considered to prevent reocclusion [7, 8]. In addition, the National Institute of Neurological Disorders and Stroke (NINDS) recombinant tissue plasminogen activator (rt-PA) trial showed that 80 % of fatal hemorrhages occur within 12 h of thrombolytic administration, and the rest occurred within 24 h [9]. Thus, early detection of intraparenchymal hemorrhage can provide an opportunity to potentially limit hemorrhage growth.
Although the recent application of dual energy CT has shown promising results in the differentiation of intraparenchymal hemorrhage from iodinated contrast, the dual energy CT scanners are not widely available and the method has not been rigorously validated [10]. In this study, we aimed to find an easy-to-apply, reproducible, and objective method for differentiating hyperdensities caused by contrast extravasation from those caused by intraparenchymal hemorrhage on conventional monochromatic noncontrast CT scans obtained following endovascular treatment in patients with acute ischemic stroke.
Methods and materials
Patients
We reviewed the prospectively collected data registry of all patients with acute ischemic stroke who underwent endovascular treatment, from December 2006 to June 2010, at two university-affiliated hospitals, supplemented by retrospective chart review. Details of the data collection and ascertainment methods have been described in previous publications [11–13]. As part of our clinical data collection, the severity of ischemic stroke at the time of admission was graded using the National Institutes of Health Stroke Scale (NIHSS) scores. Clinical outcomes were assessed by the modified Rankin scale (mRs). Favorable outcomes were defined as scores of 0–2 on the mRs at the time of discharge.
Treatment
The protocol for endovascular treatment has been described previously [13, 14]. Patients who met the criteria for receiving IV thrombolytic therapy were administered 0.9 mg/kg of IV rt-PA [15, 16]. Additional mechanical thrombectomy was considered in patients with severe ischemic deficits (NIHSS score of ≥10) or demonstration of occlusion on CT angiogram in large arteries such as M1 or M2 segments of the middle cerebral artery, internal carotid artery, basilar artery, or bilateral vertebral arteries [14]. Patients who were not candidates for IV rt-PA and presented within 8 h of symptom onset were selected for endovascular treatment based on qualitative and quantitative analyses demonstrating preserved cerebral blood volume, decreased cerebral blood flow, and increased mean transit time in ≥20 % of the affected region and involving the cortex, on admission CT perfusion [13]. Patients on chronic anticoagulation and INR <1.7 were treated with IV and/or endovascular treatment [17]. Either Omnipaque 240 (iohexol, Nycomed) or Visipaque 320 (iodixanol, GE Amersham Health) was used as contrast medium for the procedures.
Image acquisition
Following the endovascular procedure, all patients underwent immediate noncontrast CT scans as part of our institutional protocol. Follow-up noncontrast CT scans were obtained in the next 24 h and repeated as per clinical condition. The admission and post-procedural follow-up noncontrast CT scans were performed using a 64-slice multidetector CT (Brilliance CT; Philips Medical Systems, Best, the Netherlands) in helical mode: 120 kV, mA/slice 400, field of view 25 cm, pitch 0.4, rotation speed 0.5 s, reconstructed in 5-mm-thickness axial and coronal images. Details of CT angiography/perfusion scan acquisition protocols have been described previously [11–13].
Image analysis
Each follow-up CT scan was reviewed and assessed by two independent reviewers on the picture archiving and communications system (PACS; Philips iSite PACS, Amsterdam, Netherlands). The areas of hyperdensity were segmented using a free-hand region-of-interest marker on all CT scan slices and correlated with the official report. For the immediate post-procedural CT scan, two different image analysis methods were used: (1) the whole hyperdense lesion(s) were segmented and the average Hounsfield unit (HU) attenuation and volume were determined in each patient; and (2) the average attenuation of the single most (visually) hyperattenuating lesion, on just one CT slice, was calculated for each patient. For statistical analysis, we used the average of the values obtained by the two reviewers. Contrast extravasation was defined as hyperdense lesions that disappeared or prominently cleared within 24 h with no mass effect on follow-up CT scans. If hyperdensity persisted longer than 24 h and/or developed a mass effect or characteristic hypoattenuation rim, it was classified as intraparenchymal hemorrhage. We also categorized all hyperdensities, regardless of being hemorrhage or extravasation as per the European Cooperative Acute Stroke Study 2 (ECASS-2) classification as follows: hemorrhagic infarct (HI): petechial hemorrhage without space-occupying effect (HI1: small petechia versus HI2: more confluent petechia) and parenchymal hemorrhage (PH): hemorrhage with mass effect [PH1 (<30 % of the infarcted area) versus PH2 (>30 % of the infarcted area)] [18]. Symptomatic hemorrhage was defined as intraparenchymal hemorrhage that resulted in deterioration of four points or greater on the NIHSS score within 24 h.
Statistical analysis
In this study, continuous variables are presented as mean ± standard error of the mean, ordinal variables as median (interquartile), and categorical variables as numbers (frequencies). The chi-square test or Fisher’s exact test was used to compare categorical variables, and analysis of variance (ANOVA) with Tukey’s b post hoc test was employed to compare continuous variables in our analysis. We also evaluated the correlation between the calculated average attenuations between the two reviewers using the rho (r) correlation coefficient. We also determined the effect of contrast volume and concentration as well as their interaction terms on hyperdense lesion attenuation using regression analysis. In addition, area under the curve (AUC) for receiver operating characteristic (ROC) analysis was used to evaluate the predictive value of the average HU attenuation of whole lesion(s) and the most hyperattenuating lesion for identifying intraparenchymal hemorrhage. All statistical analyses were performed using SPSS for Windows software (version 19.0, SPSS Inc., Chicago, IL).
Results
A total of 135 patients were included in our study with a median age of 68 years (54–80 years). Most of the patients (124/135, 92 %) had anterior circulation ischemic stroke with distal internal carotid, middle cerebral, and anterior cerebral artery occlusions in 28 (20.7 %), 94 (69.6 %), and 2 (1.5 %) patients, respectively. In addition, seven (5.2 %) patients with basilar artery and four (3.0 %) with posterior cerebral artery occlusion were included in our study.
The average time interval between symptom onset and endovascular treatment (onset-to-microcatheterization time) was 5.1 ± 0.3 h; the immediate post-procedural CT scan was performed after a mean interval of 4.9 ± 0.6 h after initiating the endovascular treatment. The mean time interval between the immediate and the follow-up CT scans was 16.2 ± 1.3 h; all patients had a second post-procedural follow-up CT scan. Additional follow-up cerebral imaging (CT and/or MRI) was performed in 33 patients during the hospital stay as per their clinical care.
On the immediate post-procedural CT scan, 74 (55 %) patients had hyperdense lesion(s). Follow-up scans confirmed the diagnosis of intraparenchymal hemorrhage in 20 of 74 patients. Table 1 summarizes the patients’ characteristics and treatments in patients with no immediate hyperdense lesion, those with contrast extravasation, and those with intraparenchymal hemorrhage based on the results of the follow-up CT scans. There were no significant differences identified between patients with intraparenchymal hemorrhage or those with contrast extravasation (Table 1).
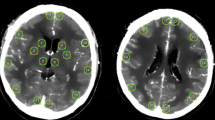
Between the two reviewers, there were good correlations between the calculated average attenuation of the whole lesion (r = 0.951, p < 0.001) and the most hyperattenuating lesion (r = 0.981, p < 0.001). The average attenuation of the most hyperattenuating lesion was significantly higher in patients with intraparenchymal hemorrhage (74.7 ± 8.2 HU) compared to those with contrast extravasation (56.6 ± 2.4 HU, p = 0.005), whereas higher average attenuation of the whole hyperdense lesion(s) in patients with intraparenchymal hemorrhage (52.7 ± 2.6 HU) compared to those who had contrast extravasation (47.3 ± 1.3 HU) did not reach statistical significance (p = 0.07). The ROC analysis also showed that the average attenuation of the most hyperattenuating lesion can better differentiate intraparenchymal hemorrhage from extravasation (AUC = 0.78, p = 0.001) compared to the average attenuation of the whole hyperdense lesion(s) (AUC = 0.66, p = 0.06) (Fig. 1). We also found that an average attenuation <50 HU of the “most hyperattenuating lesion,” found in 24/54 (44 %) patients with contrast extravasation, had 100 % specificity and 56 % sensitivity for distinguishing contrast extravasation from intraparenchymal hemorrhage (Figs. 2 and 3). The hyperdense lesion(s) volume and the product of volume and average attenuation could not differentiate intraparenchymal hemorrhage from contrast extravasation based on the ROC analysis results.
Receiver operating characteristic plots for the average Hounsfield unit attenuation of the most hyperattenuating lesion (continuous line with an area under the curve of 0.78, p = 0.001) compared with the average attenuation of the whole hyperdense lesion(s) (dotted line with an area under the curve of 0.66, p = 0.06), in differentiation of the intraparenchymal hemorrhage from contrast extravasation
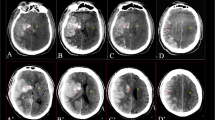
Post-procedural intraparenchymal hemorrhage in a 72-year-old woman with acute occlusion of the left middle cerebral artery inferior branch. There was near-complete recanalization after mechanical thrombectomy and intra-arterial thrombolytic infusion. The first post-procedural CT scan (a) shows petechial shape (HI1) enhancement in the left temporal lobe (outlined) with an average attenuation of 52 HU (2.9 h after microcatheterization), which persisted on follow-up scans obtained 7.4 h (b) and 38 h (c) after the procedure with peripheral parenchymal edema
Post-procedural contrast extravasation in a 42-year-old woman with acute occlusion of the left middle cerebral artery inferior branch. There was partial recanalization after mechanical thrombectomy, angioplasty, and intra-arterial thrombolytic infusion. The first post-procedural CT scan (a) obtained 2.7 h after the microcatheterization showed contrast extravasation in the left temporal lobe with an average attenuation of 46 HU in the most hyperattenuating lesion (outlined), which was resolved on follow-up scans obtained 8.1 h (b) and 16.9 h (c) after the intervention
Overall, 45/135 (33.3 %) patients had favorable outcome with mRs of ≤2 at discharge. The rate of favorable outcome was not significantly different among patients who had no hyperdensity on the immediate post-procedural noncontrast CT (39 %), those with intraparenchymal hemorrhage (20 %), and patients with contrast extravasation (32 %) (p = 0.262). Overall, the rate of early symptomatic intraparenchymal hemorrhage in our series was 9.6 % (13/135).
Table 2 summarizes the hyperdensity appearance on the early post-procedural noncontrast CT based on the ECASS-2 classification. Notably, all hemorrhages were ipsilateral to endovascular treatment. The PH2 appearance was much more common among patients with intraparenchymal hemorrhage (50 %) compared to those with contrast extravasation (4 %, p = 0.001). Moreover, while petechial enhancement (HI1/HI2) was seen in 46/54 (85 %) patients with contrast extravasation, only 9/20 (45 %) patients with intraparenchymal hemorrhage had petechial hyperdensity (p < 0.001). Additionally, among the intraparenchymal hemorrhage patients with hematoma type hemorrhage (PH1/PH2), the lowest average attenuation of the “most hyperattenuating lesion” was 58 HU. None of the 24 patients with an average attenuation <50 HU had PH1/PH2 appearance on their immediate post-procedural noncontrast CT scan.
Of note, among those 61 patients who had no hyperdensity on their immediate post-endovascular treatment noncontrast CT scan, six patients developed intraparenchymal hemorrhage on their follow-up CT scans obtained 1 to 5 days after the procedure, comprising two HI1, one PH1, and three PH2 lesions.
The data regarding the volume, type, and concentration of the contrast media administered during endovascular treatment were available in 59/135 (44 %) patients. Of these, 28 patients received Omnipaque 240, and 31 patients received Visipaque 320. The average volume of contrast administered was 137.9 ± 36 mL (n = 7) in patients with no hyperdensity, 130 ± 48 mL (n = 12) in those with intraparenchymal hemorrhage, and 129.7 ± 52 mL (n = 40) in those with contrast extravasation. The multivariate regression analysis showed that the contrast volume, type, and product of contrast volume and type had no significant effect on the average attenuation of the most hyperattenuating lesion among patients with hyperdense lesion (either hemorrhage or extravasation) on their post-procedural CT scan (n = 52, p = 0.459) or those with contrast extravasation only (n = 40, p = 0.083).
Discussion
Angiographically confirmed persistent reocclusion within the first 24 h after endovascular treatment can be seen in 9 % of acute ischemic stroke patients and is associated with a higher rate of neurological deterioration [8]. Early use of IV antiplatelet and/or anticoagulation treatment in these patients may prevent reocclusion [5, 6]. However, the risk of new hemorrhage and exacerbation of existing intraparenchymal hemorrhage is high with such treatments [5, 19]. Therefore, differentiation of contrast extravasation from intraparenchymal hemorrhage is crucial in the immediate post-procedural period in order to start antiplatelet and/or anticoagulation therapy in these patients.
Hyperdense lesions found on post-procedural CT scan represent varying degrees of microvascular integrity disruption. When the ischemic injury is limited to the endothelial cell permeability barrier, hyperdensities may represent contrast extravasation with no hemorrhagic product, whereas when the ischemic injury degrades the basal lamina, hyperdense lesions may be associated with some degree of hemorrhage [20]. Similarly, on digital subtraction angiography, the angiographic blush visualized during thrombolysis is suggestive of blood-brain barrier damage, and prolonged angiographic blush has been shown to predict hemorrhagic complications [21, 22].
In the current study, a value below 50 HU average attenuation of the most hyperattenuating lesion on the immediate post-procedural noncontrast CT scan was exclusively found in contrast extravasation cases (versus intraparenchymal hemorrhage). Previous studies have also shown that lesions with higher densities (HU values) on the post-endovascular noncontrast CT scan are associated with symptomatic intraparenchymal hemorrhage [3, 23]. In patients with hematoma formation (PH1/PH2), the lowest average attenuation was 58 HU; therefore, the “50 HU average attenuation” value provided an adequate threshold to exclude symptomatic intraparenchymal hemorrhage although it is only 56 % sensitive for detecting benign contrast extravasation on immediate post-procedural CT scans. The possibility that hyperdense lesions with low average HU attenuation had a component of minor hemorrhage that resolved within 24 h cannot be entirely excluded; however, the neurological consequences of such a hemorrhagic component are expected to be minimal. Interestingly, in our cohort, this cutoff could exclude intraparenchymal hemorrhage in almost one third (24/74) of the patients who had hyperdense lesions on their immediate post-procedural CT scan.
To identify contrast extravasation and contrast enhancement as mutually exclusive entities on CT scan is not possible. Contrast enhancement is predominantly attributed to neovascularization with new capillaries lacking integrity of the blood-brain barrier, usually manifesting between the 10th day and 1 month after ischemic stroke onset [24]. However, a component of contrast enhancement is related to extravasation of blood and contrast through disrupted blood-brain barrier [24]. Contrast extravasation is a more specific term that denotes only hyperdensity related to contrast accumulation in the extravascular and parenchymal compartment. Yoon et al. defined contrast extravasation as a hyperdense lesion with maximum Hounsfield unit >90 that persisted on a follow-up CT scan [3]. However, six of seven patients with contrast extravasation (as per definition) had concomitant hemorrhage on immediate post-procedure CT scans preventing adequate delineation of contrast extravasation from blood extravasation [3]. We defined “contrast extravasation” as a hyperdensity that resolves (or significantly clear) within 24 h of endovascular intervention with no peripheral edema on follow-up CT scan. We think that the differentiation between contrast-related and blood-related hyperdensities on CT scan has more therapeutic implications rather than the differentiation between extravasation and enhancement.
Although prior studies have shown the imaging evolution and clinical correlations of hyperdense lesions on post-procedural CT [1–4], none of them have identified a reproducible and objective method for differentiating intraparenchymal hemorrhage from contrast extravasation. Moreover, unlike prior studies [2–4], we used a free-hand region-of-interest selection tool to segment the hyperdense lesions and measure the average attenuation (HU). This is an easy-to-apply method and readily available to all PACS viewers. In addition, our analysis showed that the average HU values of the most hyperattenuating lesion (with the highest visual density) on a single CT slice can better differentiate intraparenchymal hemorrhage from contrast extravasation compared with the whole lesion(s) average HU. We think that such a method can be more practical and less time consuming, compared with determining the maximum HU or measuring the whole lesion volume average attenuation.
It is plausible that the amount and concentration of the injected contrast media affect the development and attenuation of post-procedural hyperdense lesions. In our series, the type (concentration) and volume of the contrast media administered during endovascular intervention were not available in all patients which made the evaluation limited. However, the multivariate regression analysis showed no significant effect of the injected contrast volume and concentration or their interaction on post-procedural hyperdense lesion densities. In addition, an average attenuation below 50 HU in the most hyperattenuating lesion could exclude intraparenchymal hemorrhage regardless of the administered contrast volume and concentration.
Recently, dual energy CT scanners have been introduced for differentiation of iodinated contrast from hemorrhage (iron deposits) in the brain following endovascular treatment [25]. With dual energy CT, images are reconstructed from two different X-ray spectra at different kilovoltages either from two separate X-ray sources or one X-ray source with rapid kilovoltage switching [10]. This acquisition method allows decomposition of CT into virtual noncontrast and iodine overlay images, which allows differentiation of parenchymal iodinated contrast staining from hemorrhagic iron deposits and calcification. However, these scanners are not yet widely available and the acquisition protocols require adjustment to the higher image quality for optimal gray–white matter differentiation.
The results of our study are subject to some limitations. This is a retrospective study with a small sample size; for example, our cohort might not have been large enough to demonstrate that PH1/PH2 type intraparenchymal hemorrhage is a significant risk factor for poor clinical outcome, or to identify other potential confounders. Moreover, reliable and consistent records regarding the presence of contrast blush during the procedures were not available in all patients; such data could have been correlated with post-procedural CT findings. In addition, a separate cohort may be required to validate our prediction model. In our series, the patients were treated with a variety of endovascular treatments including IA thrombolytics, mechanical thrombectomy, and primary angioplasty, and some patients received IV rt-PA before endovascular thrombolysis/thrombectomy. The varying effects of these modalities on blood-brain barrier breakdown and hemorrhage may have introduced heterogeneity in our observations.
Conclusion
Although the single source (monochromatic) brain CT scan is limited in the differentiation of hemorrhagic product deposition from iodinated contrast, we described a practical and time-sensitive method to increase the ascertainment of intraparenchymal hemorrhage. Our findings suggest that an average attenuation below 50 HU of the most hyperattenuating hyperdense lesion on immediate post-procedure CT scan can effectively exclude intraparenchymal hemorrhage in acute ischemic stroke patients, although it is modestly sensitive for detecting contrast extravasation. Such an approach represents an easy-to-use method for exclusion of intraparenchymal hemorrhage in patients who may benefit from antiplatelet/anticoagulation treatment after endovascular treatment.
Abbreviations
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under the curve
- ECASS:
-
European Cooperative Acute Stroke Study
- HU:
-
Hounsfield unit
- IA:
-
Intra-arterial
- IV:
-
Intravenous
- mRs:
-
Modified Rankin scale
- NIHSS:
-
National Institutes of Health Stroke Scale
- NINDS:
-
National Institute of Neurological Disorders and Stroke
- PACS:
-
Picture archiving and communications system
- ROC:
-
Receiver operating characteristic
- rt-PA:
-
Recombinant tissue plasminogen activator
References
Parrilla G, Garcia-Villalba B, Espinosa de Rueda M et al (2012) Hemorrhage/contrast staining areas after mechanical intra-arterial thrombectomy in acute ischemic stroke: imaging findings and clinical significance. AJNR Am J Neuroradiol 33:1791–1796
Kim JT, Heo SH, Cho BH et al (2012) Hyperdensity on non-contrast CT immediately after intra-arterial revascularization. J Neurol 259:936–943
Yoon W, Seo JJ, Kim JK et al (2004) Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke 35:876–881
Mericle RA, Lopes DK, Fronckowiak MD et al (2000) A grading scale to predict outcomes after intra-arterial thrombolysis for stroke complicated by contrast extravasation. Neurosurgery 46:1307–1314, discussion 1314-1305
Qureshi AI, Harris-Lane P, Kirmani JF et al (2006) Intra-arterial reteplase and intravenous abciximab in patients with acute ischemic stroke: an open-label, dose-ranging, phase I study. Neurosurgery 59:789–796, discussion 796-787
Qureshi AI, Hussein HM, Janjua N et al (2008) Postprocedure intravenous eptifibatide following intra-arterial reteplase in patients with acute ischemic stroke. J Neuroimaging 18:50–55
Janjua N, Alkawi A, Suri MF et al (2008) Impact of arterial reocclusion and distal fragmentation during thrombolysis among patients with acute ischemic stroke. AJNR Am J Neuroradiol 29:253–258
Qureshi AI, Hussein HM, Abdelmoula M et al (2009) Subacute recanalization and reocclusion in patients with acute ischemic stroke following endovascular treatment. Neurocrit Care 10:195–203
Anonymous (1997) Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke 28:2109-2118
Phan CM, Yoo AJ, Hirsch JA et al (2012) Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. AJNR Am J Neuroradiol 33:1088–1094
Georgiadis AL, Memon MZ, Shah QA et al (2012) Intra-arterial tenecteplase for treatment of acute ischemic stroke: feasibility and comparative outcomes. J Neuroimaging 22:249–254
Hassan AE, Chaudhry SA, Miley JT et al (2013) Microcatheter to recanalization (procedure time) predicts outcomes in endovascular treatment in patients with acute ischemic stroke: when do we stop? AJNR Am J Neuroradiol 34:354–359
Hassan AE, Zacharatos H, Rodriguez GJ et al (2010) A comparison of computed tomography perfusion-guided and time-guided endovascular treatments for patients with acute ischemic stroke. Stroke 41:1673–1678
Hassan AE, Aman MM, Chauhdry SA et al (2013) Value of other endovascular techniques among patients with MERCI device failure during the treatment of acute ischemic stroke: what to do when MERCI fails? J Vasc Interv Neurol 5:9–13
Tekle WG, Chaudhry SA, Fatima Z et al (2012) Intravenous thrombolysis in expanded time window (3–4.5 hours) in general practice with concurrent availability of endovascular treatment. J Vasc Interv Neurol 5:22–26
Asaithambi G, Hassan AE, Chaudhry SA et al (2011) Comparison of time to treatment between intravenous and endovascular thrombolytic treatments for acute ischemic stroke. J Vasc Interv Neurol 4:15–20
Aggarwal HR, Hassan AE, Rodriguez GJ et al (2013) Use of intravenous recombinant tissue plasminogen activator in patients with borderline elevation of international normalized ratio. J Vasc Interv Neurol 6:1–8
Trouillas P, von Kummer R (2006) Classification and pathogenesis of cerebral hemorrhages after thrombolysis in ischemic stroke. Stroke 37:556–561
Qureshi AI, Saad M, Zaidat OO et al (2002) Intracerebral hemorrhages associated with neurointerventional procedures using a combination of antithrombotic agents including abciximab. Stroke 33:1916–1919
del Zoppo GJ, von Kummer R, Hamann GF (1998) Ischaemic damage of brain microvessels: inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry 65:1–9
Fukazawa S, Waki R, Hidaka A et al (1997) Angiographic A-V shunt during interventional thrombolysis for acute cerebral embolism. A new predictive sign for hemorrhagic complication. Interv Neuroradiol 3(Suppl 2):75–78
Yokogami K, Nakano S, Ohta H et al (1996) Prediction of hemorrhagic complications after thrombolytic therapy for middle cerebral artery occlusion: value of pre- and post-therapeutic computed tomographic findings and angiographic occlusive site. Neurosurgery 39:1102–1107
Jang YM, Lee DH, Kim HS et al (2006) The fate of high-density lesions on the non-contrast CT obtained immediately after intra-arterial thrombolysis in ischemic stroke patients. Korean J Radiol 7:221–228
Kawase T, Mizukami M, Araki G (1981) Mechanisms of contrast enhancement in cerebral infarction: computerized tomography, regional cerebral blood flow, fluorescein angiography, and pathological study. Adv Neurol 30:149–158
Tijssen MP, Hofman PA, Stadler AA et al (2014) The role of dual energy CT in differentiating between brain haemorrhage and contrast medium after mechanical revascularisation in acute ischaemic stroke. Eur Radiol 24:834–840
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the Institutional Review Board and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Payabvash, S., Qureshi, M.H., Khan, S.M. et al. Differentiating intraparenchymal hemorrhage from contrast extravasation on post-procedural noncontrast CT scan in acute ischemic stroke patients undergoing endovascular treatment. Neuroradiology 56, 737–744 (2014). https://doi.org/10.1007/s00234-014-1381-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1381-8