Abstract
Purpose of Review
The review provides an update on the diagnosis, pathogenesis, and treatment of cutaneous lupus erythematosus (CLE).
Recent Findings
Diagnostic challenges exist in better defining CLE as an independent disease distinct from systemic lupus erythematosus with cutaneous features and further classifying CLE based on clinical, histological, and laboratory features. Recent mechanistic studies revealed more genetic variations, environmental triggers, and immunologic dysfunctions that are associated with CLE. Drug induction specifically has emerged as one of the most important triggers for CLE. Treatment options include topical agents and systemic therapies, including newer biologics such as belimumab, rituximab, ustekinumab, anifrolumab, and BIIB059 that have shown good clinical efficacy in trials.
Summary
CLE is a group of complex and heterogenous diseases. Future studies are warranted to better define CLE within the spectrum of lupus erythematosus. Better insight into the pathogenesis of CLE could facilitate the design of more targeted therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lupus erythematosus (LE) encompasses a diverse group of autoimmune diseases characterized by a spectrum of clinical, histological, and immunological findings. Cutaneous manifestations may occur as a single separate entity of the disease (cutaneous lupus erythematosus, CLE) or in association with systemic involvement of multiple organs, such as the heart, lung, and kidney (systemic lupus erythematosus, SLE) [1]. As a result, LE can be extremely debilitating, resulting in significant medical morbidity and psychological stress in many patients. This review focuses on recent insights in the pathogenesis and treatment of CLE.
Diagnosis and Classification of CLE
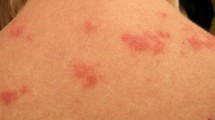
The spectrum of cutaneous findings in CLE is broad and heterogenous. Therefore, CLE is an ill-defined set of disorders categorized together based on common features and similar responses to treatment. Currently, CLE can be further divided into several subtypes based on constellations of clinical features, duration of the cutaneous lesions, histological features, and laboratory abnormalities [1]. These are: (1) acute cutaneous lupus erythematosus (ACLE) (Fig. 1); (2) subacute cutaneous lupus erythematosus (SCLE) (Fig. 2); (3) chronic cutaneous lupus erythematosus (CCLE), which includes discoid lupus erythematosus (DLE) (Fig. 3), lupus erythematosus panniculitis (LEP), and chilblain lupus erythematosus (CHLE); and (4) intermittent cutaneous lupus erythematosus (ICLE), which includes lupus erythematosus tumidus (LET)—although this division is not universally accepted [2]. Detailed clinical, histologic, and immunologic features of different CLE subtypes are summarized in Table 1.
Many diagnostic challenges exist in better defining CLE as an independent disease versus as a subtype of SLE with cutaneous features. Currently, the diagnosis of CLE relies on the criteria for the classification of SLE established by the American College of Rheumatology (ACR). The ACR guidelines require 4 out of 11 criteria to be met for a diagnosis of SLE. However, four criteria included in the guidelines are related to cutaneous findings—malar rash, discoid lesions, oral ulcers, and photosensitivity [3]. Therefore, many argue that these diagnostic criteria skew diagnosis and inadequately distinguish CLE from SLE [4]. In addition, the ACR criteria are currently in transition as the European League Against Rheumatism (EULAR) and ACR proposed an updated classification criteria system for SLE in 2019 [5••]. Consequently, others have proposed a need for a more uniform definition for CLE with international consensus on diagnostic and classification criteria. In 2012, the Systemic Lupus International Collaborating Clinics Classification Criteria (SLICC) proposed revised dermatologic criteria for ACLE, CCLE, oral ulcers, and non-scarring alopecia when diagnosing SLE [6]. Recently, another formal evaluation process was initiated for employing the Delphi method to better define DLE, one of the most common and recognizable subtypes of CLE [7]. Further epidemiologic and mechanistic studies in this area are warranted.
Clinical presentation can be variable, but classically SCLE presents as arcuate, erythematous plaques with variable scale which can sometimes be mistaken for urticarial lesions. DLE typically presents as plaques with dyspigmentation, erythema, scale, and sometimes ulceration, which frequently result in scarring. Additionally, pruritus frequently accompanies CLE and is most commonly reported by ACLE patients [8].
Once diagnosed, disease severity can be quantified using the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) [9]. The rating system consists of two scores that measure both activity and damage of the disease based on the degree of erythema, scale, mucous membrane lesions, alopecia, dyspigmentation, and scarring of the lesional skin. Scored on a 0–10 scale from both patients’ and physicians’ perspectives, it has correlated well with the clinical aspects of CLE. Additionally, the CLASI has been validated and proven to be useful in therapeutic trials [10].
Epidemiology
Epidemiologically, CLE was estimated to occur two to three times more frequently than SLE by Tebbe and Orphanos [11]. However, in the USA, few population-based studies have estimated the prevalence of CLE. In a study with a predominantly Caucasian population in Minnesota, prevalence rates of CLE were found to be 73.24 per 100,000 with an annual incidence rate of 4.3 per 100,000 [12]. Though not demographically representative of the USA as a whole, the study reported an incidence rate of 3.56 per 100,000 for DLE, which is similar to an incidence rate of 3.7 per 100,000 for DLE in a more racially diverse population in the southeastern USA reported in 2019 [13]. As with other autoimmune diseases, CLE is also consistently more prevalent in females than males. The Georgia Lupus Registry study showed a female:male ratio of 3.1:1 for DLE, and a nationwide cohort study in Denmark showed a female:male ratio of 4:1 for CLE [13, 14]. In addition, there appears to be racial differences among CLE patients, with African Americans having a 5.4-fold higher risk for DLE than Caucasians [13].
Pathogenesis
Though the skin manifestations of CLE have been extensively studied and reported in the past decade, the pathophysiology of CLE remains incompletely understood. However, current data suggest that the initiation and persistence of CLE involves a complex interplay between the skin and the innate and adaptive immune systems, genetic risk factors, and environmental triggers, including UV exposure and drug induction [3, 15, 16].
Previous reports have highlighted important associations between several genes and an increased risk of CLE, including human leukocyte antigen (HLA) subtypes, tumor necrosis factor-alpha (TNF-α), and complement promoter variants. In addition, single nucleotide polymorphisms in interferon regulatory factor 5 (IRF5), cytotoxic T-lymphocyte-associated protein 4 (CTLA4), integrin alpha M (ITGAM), and tyrosine kinase 2 (TYK2) have also been reported [17,18,19,20,21]. A recent large genome-wide association study comparing 183 CLE cases to 1288 healthy controls identified polymorphisms in casein kinase 2 and ribonuclease P protein subunit p21 (RPP21) [22]. In addition, genome-wide DNA methylation studies have reported many differentially methylated regions associated with a malar rash and discoid rash in the CD4+ T cells of patients with SLE. These regions were found to be mainly associated with cell proliferation, apoptosis, and antigen processing and presentation [23].
UV radiation remains one of the most important triggers for CLE, with a large percentage of CLE patients exhibiting photosensitivity. Furukawa et al. and Toberer et al. proposed that UV radiation alters keratinocyte morphology and function, promotes the expression of autoantigens on cell membranes, and triggers cell apoptosis [24, 25]. In addition, several inflammatory cytokines and chemokines, including TNF-α, IL-18, and type I interferons (IFNs), are upregulated upon UV exposure and have been found to be involved in the pathogenesis of CLE [26,27,28,29]. Increased expression of genes regulated by IFN was found in both the lesional epidermis and dermis of CLE patients. Type I IFNs also directly promote the infiltration of T helper 1 (Th1) cells, further accelerating cutaneous inflammation [30]. Lastly, upregulation of these cytokines and chemokines is intimately associated with the production of autoantibodies that are frequently deposited at the dermal–epidermal junction and may result in an antibody-dependent cell-mediated cytotoxicity. Whether these autoantibodies contribute to the pathogenesis of CLE remains unclear. However, these autoantibodies may offer prognostic value: studies found an association between anti-Smith (Sm) antibody and photosensitivity and discoid rash, between anti-U1RNP antibody and malar rash and Raynaud’s, between anti-SSA/Ro antibody with malar rash and oral ulcers [31], and between anti-SSA/Ro with photosensitivity in CLE patients, among others [32].
Drug induction has also emerged to be one of the most important triggers for CLE with many reports citing newly developed biologics, immunotherapeutic, and chemotherapeutic agents that can induce onset of this disease [33•]. TNF-α inhibitors have previously been reported to trigger drug-induced SCLE (DI-SCLE), the most commonly described form of drug-induced CLE (DI-CLE). In a recent report, TNF-α inhibitors were found to have the second highest odds ratio following terbinafine in inducing SCLE [34]. Recent case studies also highlighted the association between SCLE and several immunomodulatory drugs targeting programmed death 1 (PD-1), programmed death ligand 1 (PD-L1), cytotoxic T-lymphocyte associated protein-4 (CTLA-4), IL-17, and IL-12/23 [35,36,37,38,39,40]. As these agents become increasingly popular, physicians should consider the potential dermatologic adverse effects. With an ever growing list of more than forty medications that can trigger this condition, understanding the underlying mechanisms for DI-CLE is more important than ever. Despite this, DI-CLE remains poorly understood. Previous reports have proposed several mechanisms, including genetic susceptibility, drug biotransformation, and epigenetic alterations in immune cells [33•, 41]. Interestingly, neutrophil extracellular trap (NET) formation was recently discovered to be causative of drug-induced lupus, suggesting that dysregulation of innate immune system cell clearance could play a critical role in the pathogenesis of DI-CLE or DI-SLE [42]. However, most of these mechanistic studies primarily focused on drug-induced SLE (DI-SLE). Whether the same pathogenic mechanisms are applicable in DI-CLE remains unclear.
Treatments
Numerous therapies exist to treat patients with CLE, each with varying degrees of efficacy and supporting evidence. These range from topical agents—including corticosteroids and calcineurin inhibitors—to systemic therapies, including antimalarials, immunosuppressants, retinoids, thalidomide/lenalidomide, and biologics. Presently, there is no medication specifically approved for the treatment of CLE, as most of the therapeutic options used in CLE patients, with the exception of thalidomide, are also used in SLE. Our discussion of the treatment of SLE will primarily focus on newly emerging agents and the evidence of their efficacy.
Topical Agents
Topical corticosteroids are the accepted first-line therapy for patients with CLE, although there has been limited published evidence in recent years supporting their efficacy. Nonetheless, many patients with localized disease are successfully managed with photoprotection and a high potency or super-high potency topical corticosteroid. Patients with more extensive disease can be bridged with topical corticosteroids while initiating slower acting systemic therapies such as antimalarials or immunosuppressants [3, 43•, 44].
Topical calcineurin inhibitors (TCIs) were also extensively studied in recent years for their steroid-sparing effects, especially in sensitive areas with thinner skin, and found to have equivalent benefit to some topical corticosteroid therapies. Wang et al. compared tacrolimus 0.03% ointment with triamcinolone acetonide 0.1% cream in 31 patients with biopsy-proven DLE and found no difference in rate of healing, reduction in erosion, erythema, or reticulation in both groups with treatment [45]. Pothinamthong et al. showed good clinical response in CLE patients with a combined regimen of twice daily tacrolimus 0.1% and once daily clobetasol 0.05% ointments [46]. Another TCI, pimecrolimus 1% cream, also showed similar clinical efficacy in patients with moderate-to-severe facial DLE when compared to betamethasone valerate 0.1% cream [47]. There are conflicting data on whether TCIs are effective in all subtypes of CLE. One study reported that tacrolimus 0.1% ointment was more effective in patients with DLE, LET, and ACLE than patients with SCLE, whereas another report demonstrated equal clinical efficacy in patients with DLE, SCLE, and SLE with malar rash [48, 49].
Antimalarials
Antimalarials are recommended as the first-line and long-term systemic therapy for patients with severe or widespread CLE, particularly those with a high risk of scarring and/or developing systemic disease [50, 51]. The mechanism of this therapeutic class is not fully understood, but the latest evidence implicates modulation of autoantigen presentation, blockages and inhibition of inflammatory cytokines, inhibition of toll-like receptor (TLR) signaling, and prostaglandin effects as central to its benefit to CLE patients [52, 53]. Its family members include hydroxychloroquine (HCQ), chloroquine, and quinacrine, with HCQ being the most commonly studied and used agent. In a multicenter, randomized, double-blinded randomized clinical trial (RCT), Yokogawa et al. found that both HCQ and placebo resulted in significant reduction in symptom severity measured by CLASI in patients with active CLE, although the HCQ group reduced CLASI scores by − 4.6 on average compared to − 3.2 in the placebo group [54]. In addition, two observational studies published in 2018 also consistently reported > 50% response rates on average for patients with mixed CLE, with the most common adverse effect being retinopathy [55, 56]. Interestingly, Chasset et al. reported that the response to HCQ can decrease over time, but patients generally had > 50% response to a second antimalarial agent after switching from HCQ [55]. There is significant evidence that blood levels of hydroxychloroquine correlate with therapeutic response, suggesting that adherence and absorption may play a role in response [57]. Lastly, Ugarte et al. reported a statistically significant improvement in CLASI scores at all time points with a 91% complete/partial response rate when treated with HCQ and quinacrine combination therapy for 46 SLE patients with refractory skin and/or joint disease [58]. Overall, the side effects of antimalarials are tolerable in most patients, but it is recommended by the American Academy of Ophthalmology that their dosage not exceed 5 mg/kg of real body weight per day to minimize the risk of retinopathy and that they be followed closely for development of this complication [59].
Immunosuppressants
Methotrexate (MTX) and mycophenolate mofetil (MMF) are immunosuppressants with reported efficacy in the treatment of CLE or SLE with active cutaneous involvement. MTX has been successfully used as a second-line treatment in refractory SCLE and DLE [1]. A retrospective study of 43 patients with refractory CLE treated with 7.5–25 mg/week MTX found a 98% response rate in DLE patients, especially those with localized disease [60]. More recently, an RCT published in 2012 showed that MTX has equivalent therapeutic efficacy as chloroquine, and both led to significant improvement in skin disease at 24 weeks [61]. In refractory CLE, MMF has also been shown to be effective when combined with HCQ and/or systemic corticosteroids. Gammon et al. reported a 62% complete/near complete recovery rate in these patients treated with MMF in addition to standard therapy with a mean treatment time to initial response of 2.76 months [62, 63]. Additionally, mycophenolate acid, an enteric-coated form of MMF, reduced CLASI score by 73% as a monotherapy in patients with SCLE who are resistant to standard therapy [64]. However, with the emergence of newer and more targeted biologics, fewer studies have focused on the clinical efficacy and safety profile of these immunosuppressants in more recent years. Known side effects of methotrexate are GI intolerance, bone marrow suppression, pulmonary fibrosis, and hepatitis, whereas MMF can cause significant GI distress and bone marrow suppression and may increase the risk of some cancers [65, 66]. Regardless, the S2k guidelines recommend MTX up to 20 mg per week as a second-line therapy and MMF as a third-line therapy in refractory CLE patients, preferably in addition to antimalarials [51].
Thalidomide and Lenalidomide
Thalidomide and lenalidomide are thought effective in treating refractory CLE due to their inhibitory effects on the production of inflammatory cytokines and their ability to prevent keratinocyte apoptosis induced by UVB light [43•]. There are currently no RCTs investigating the clinical efficacy of these agents. However, several non-controlled trials all report consistently high rates of response (98–100%) to thalidomide with complete clearance rates ranging from 54.5 to 85% in patients with various subtypes of CLE [67,68,69,70]. Recent observational studies and small case series have also found high rates of response [71,72,73]. However, therapeutic efficacy of thalidomide was limited by the high risk of relapse upon cessation of treatment, as well as sensory neuropathy in up to 50–70% of patients [67, 69,70,71,72]. Additionally, because of the potential for sedation, patients should be counseled to use with caution when driving or performing tasks that require alertness [74]. Several non-controlled trials and observational studies also have assessed response of CLE patients to lenalidomide since 2012 and reported encouraging responses to lenalidomide with lower rates of neuropathy [75,76,77,78,79]. In addition, subgroup analysis seemed to show that lenalidomide is more effective in DLE and SCLE compared to LEP [76, 79]. Overall, recent reports have shown exciting results for the use of thalidomide and lenalidomide in CLE patients, but peripheral neuropathy and thromboembolic events related to thalidomide and cytopenia related to lenalidomide may limit their use.
Biologics
Over the past decade, many biologics have been studied and more are currently under investigation in clinical trials. Out of all the available biologics, in SLE most focused on belimumab and rituximab [43•]. Newer biologics under investigation for SLE/CLE include ustekinumab, a monoclonal antibody (mAb) targeting IL-12/23 [80]; anifrolumab, a mAb against the type I interferon receptor [81]; BIIB059, a mAb targeting dendritic cell antigen 2 [82]; AMG811, an anti-interferon gamma (IFN-γ) antibody [83]; and sirukumab, an anti-IL6 antibody [84].
Belimumab is a mAb that reduces B lymphocyte survival by interfering with the binding of soluble human B lymphocyte stimulator to its B-cell receptors [43•]. In a randomized, double-blinded, placebo-controlled trial of 836 SLE patients (735 with mucocutaneous manifestations), 61.4% of patients on belimumab had significant improvement, compared to 48.4% of those on placebo, measured by the SLE Responder Index 4 (SRI4), a method that was developed to capture improvement of SLE disease activity for use in clinical trials [85]. Another non-controlled trial involving 67 patients with mixed CLE/SLE also reported significant reduction in CLASI activity score compared to baseline at 6, 12, and 24 months after treatment initiation [86]. Lastly, a small series of 62 patients with refractory SLE showed improvement measured by SLEDAI-2 K and CLASI scores with the addition of belimumab to standard therapy [87]. Nevertheless, none of the studies specifically focused on CLE, and further studies focusing on CLE are needed.
Rituximab is a chimeric monoclonal antibody specific for human antigen CD20, a B lymphocyte specific membrane-bound glycoprotein [43•]. Three recent observational studies investigated the use of rituximab, with two 1000 mg doses administered 2 weeks apart and accompanied by intravenous cyclophosphamide or methylprednisone [88,89,90]. While two studies reported promising response rates that were greater than 70%, one showed a mucocutaneous response rate of only 35% [88,89,90]. In this same study, patients with DLE did not respond to rituximab [89]. Overall, patients with ACLE appeared to be most responsive to rituximab, while very little evidence supported the use of rituximab in DLE or SCLE [88, 90].
The potential therapeutic effects of ustekinumab were highlighted in one RCT that compared ustekinumab to placebo in cutaneous manifestations of SLE, as measured by the CLASI scoring system. Response rate, defined as 50% or more reduction in CLASI score, was found to be significantly greater in the ustekinumab group, whereas there was no significant difference in adverse effects found between the two groups [80]. A similar effect was also found when Furie et al. examined anifrolumab administered intravenously at 300 mg or 1000 mg every 4 weeks for 48 weeks for patients with moderate-to-severe SLE. Anifrolumab 300 mg, combined with standard therapy, was found to have a significantly higher frequency of ≥ 50% improvement in CLASI scores when compared to standard therapy alone [81]. BIIB059 treatments for SLE patients with active skin disease also demonstrated higher rate of improvement in CLASI scores than placebo, which was associated with a reduction of interferon (IFN) pathway marker activation on biopsies [82].
However, AMG811, another interferon-pathway targeting agent, was evaluated in patients with DLE with or without SLE, and it did not show any difference in response between treatment and placebo groups as measured by the CLASI activity score [83]. Another RCT involving sirukumab also showed mixed results, while SELENA-SLEDAI scores were reduced in the treatment group, no decrease in CLASI scores was observed [84]. Overall, the newer biologics show promising results for patients suffering from moderate-to-severe CLE or SLE with cutaneous involvement.
Management for DI-CLE
Though the pathogenesis of DI-CLE is incompletely understood, there is a general consensus that the crucial first step is cessation of any causative agent [33, 41]. Most patients will have resolution of symptoms in a matter of weeks. While discontinuation of the offending drug is usually sufficient for resolution of the disease, some patients do require further treatments, depending on the extent of clinical symptoms. Localized disease can often be successfully managed with potent topical corticosteroids or TCIs, but systemic or widespread disease activity can sometimes require antimalarials such as HCQ or a short course of systemic corticosteroids, which should be used with caution. Immunosuppressants and biologics that are effective in treating idiopathic non-drug induced CLE are normally not required for DI-CLE patients [91].
Conclusion and Perspectives
CLE is a group of heterogenous diseases with a wide range of dermatologic symptoms. To date, the proper diagnostic criteria and classification system for CLE are still being debated. In addition, the underlying pathogenesis of CLE remains incompletely understood. Drug induction has emerged to be one of the important clinical considerations when managing patients with new onset CLE. Photoprotection and topical corticosteroids remain the gold standard for patients with localized disease. For more severe and systemic diseases, antimalarials are considered the first-line treatment with a good level of supporting evidence. More research is underway to investigate novel agents that target the immune cells and inflammatory pathways that are involved in the pathogenesis of CLE. Future studies are warranted to better define CLE within the spectrum of LE, and a better insight into the pathogenesis of CLE will also be instrumental in designing more targeted therapies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hejazi EZ, Werth VP. Cutaneous lupus erythematosus: an update on pathogenesis, diagnosis and treatment. Am J Clin Dermatol. 2016;17:135–46.
Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391–404.
Stannard JN, Kahlenberg JM. Cutaneous lupus erythematosus: updates on pathogenesis and associations with systemic lupus. Curr Opin Rheumatol. 2016;28:453–9.
Biazar C, Sigges J, Patsinakidis N, Ruland V, Amler S, Bonsmann G, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444–54.
•• Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. European League against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151–9. Comment: These are the latest guidelines for classification criteria.
Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86.
Elman SA, Joyce C, Nyberg F, Furukawa F, Goodfield M, Hasegawa M, et al. Development of classification criteria for discoid lupus erythematosus: results of a Delphi exercise. J Am Acad Dermatol. 2017;77:261–7.
Samotij D, Szczech J, Kushner CJ, Mowla MR, Danczak-Pazdrowska A, Antiga E, et al. Prevalence of pruritus in cutaneous lupus erythematosus: brief report of a multicenter, multinational cross-sectional study. Biomed Res Int. 2018;2018:3491798.
Albrecht J, Taylor L, Berlin JA, Dulay S, Ang G, Fakharzadeh S, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005;125:889–94.
Jolly M, Kazmi N, Mikolaitis RA, Sequeira W, Block JA. Validation of the Cutaneous Lupus Disease Area and Severity Index (CLASI) using physician- and patient-assessed health outcome measures. J Am Acad Dermatol. 2013;68:618–23.
Tebbe B, Orfanos C. Epidemiology and socioeconomic impact of skin disease in lupus erythematosus. Lupus. 1997;6:96–104.
Durosaro O, Davis MD, Reed KB, Rohlinger AL. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249–53.
Drenkard C, Parker S, Aspey LD, Gordon C, Helmick CG, Bao G, et al. Racial disparities in the incidence of primary chronic cutaneous lupus erythematosus in the Southeastern US: the Georgia Lupus Registry. Arthritis Care Res (Hoboken). 2019;71:95–103.
Petersen MP, Moller S, Bygum A, Voss A, Bliddal M. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424–30.
Achtman JC, Werth VP. Pathophysiology of cutaneous lupus erythematosus. Arthritis Res Ther. 2015;17:182.
Wenzel J. Cutaneous lupus erythematosus: new insights into pathogenesis and therapeutic strategies. Nat Rev Rheumatol. 2019;15:519–32.
Osmola A, Namysl J, Jagodzinski PP, Prokop J. Genetic background of cutaneous forms of lupus erythematosus: update on current evidence. J Appl Genet. 2004;45:77–86.
Fischer GF, Pickl WF, Fae I, Anegg B, Milota S, Volc-Platzer B. Association between chronic cutaneous lupus erythematosus and HLA class II alleles. Hum Immunol. 1994;41:280–4.
Pickering MC, Fischer S, Lewis MR, Walport MJ, Botto M, Cook HT. Ultraviolet-radiation-induced keratinocyte apoptosis in C1q-deficient mice. J Invest Dermatol. 2001;117:52–8.
Werth VP, Zhang W, Dortzbach K, Sullivan K. Association of a promoter polymorphism of tumor necrosis factor-alpha with subacute cutaneous lupus erythematosus and distinct photoregulation of transcription. J Invest Dermatol. 2000;115:726–30.
Jarvinen TM, Hellquist A, Koskenmies S, Einarsdottir E, Koskinen LL, Jeskanen L, et al. Tyrosine kinase 2 and interferon regulatory factor 5 polymorphisms are associated with discoid and subacute cutaneous lupus erythematosus. Exp Dermatol. 2010;19:123–31.
Kunz M, Konig IR, Schillert A, Kruppa J, Ziegler A, Grallert H, et al. Genome-wide association study identifies new susceptibility loci for cutaneous lupus erythematosus. Exp Dermatol. 2015;24:510–5.
Renauer P, Coit P, Jeffries MA, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, et al. DNA methylation patterns in naive CD4+ T cells identify epigenetic susceptibility loci for malar rash and discoid rash in systemic lupus erythematosus. Lupus Sci Med. 2015;2:e000101.
Furukawa F, Itoh T, Wakita H, Yagi H, Tokura Y, Norris DA, et al. Keratinocytes from patients with lupus erythematosus show enhanced cytotoxicity to ultraviolet radiation and to antibody-mediated cytotoxicity. Clin Exp Immunol. 1999;118:164–70.
Toberer F, Sykora J, Gottel D, Hartschuh W, Werchau S, Enk A, et al. Apoptotic signal molecules in skin biopsies of cutaneous lupus erythematosus: analysis using tissue microarray. Exp Dermatol. 2013;22:656–9.
Bashir MM, Sharma MR, Werth VP. UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription. J Invest Dermatol. 2009;129:994–1001.
Kahlenberg JM, Thacker SG, Berthier CC, Cohen CD, Kretzler M, Kaplan MJ. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol. 2011;187:6143–56.
Wang D, Drenker M, Eiz-Vesper B, Werfel T, Wittmann M. Evidence for a pathogenetic role of interleukin-18 in cutaneous lupus erythematosus. Arthritis Rheum. 2008;58:3205–15.
Nakamura K, Jinnin M, Kudo H, Inoue K, Nakayama W, Honda N, et al. The role of PSMB9 upregulated by interferon signature in the pathophysiology of cutaneous lesions of dermatomyositis and systemic lupus erythematosus. Br J Dermatol. 2016;174:1030–41.
Wenzel J, Worenkamper E, Freutel S, Henze S, Haller O, Bieber T, et al. Enhanced type I interferon signalling promotes Th1-biased inflammation in cutaneous lupus erythematosus. J Pathol. 2005;205:435–42.
Fredi M, Cavazzana I, Quinzanini M, Taraborelli M, Cartella S, Tincani A, et al. Rare autoantibodies to cellular antigens in systemic lupus erythematosus. Lupus. 2014;23:672–7.
Ioannides D, Golden BD, Buyon JP, Bystryn J-C. Expression of SS-A/Ro and SS-B/La antigens in skin biopsy specimens of patients with photosensitive forms of lupus erythematosus. Arch Dermatol. 2000;136:340–6.
• He Y, Sawalha AH. Drug-induced lupus erythematosus: an update on drugs and mechanisms. Curr Opin Rheumatol. 2018;30:490–7. Comment: Drug induced lupus is a common, and commonly overlooked, entity and should be recognized. Many common medications are implicated.
Gronhagen CM, Fored CM, Linder M, Granath F, Nyberg F. Subacute cutaneous lupus erythematosus and its association with drugs: a population-based matched case-control study of 234 patients in Sweden. Br J Dermatol. 2012;167:296–305.
Marano AL, Clarke JM, Morse MA, Shah A, Barrow W, Selim MA, et al. Subacute cutaneous lupus erythematosus and dermatomyositis associated with anti-programmed cell death 1 therapy. Br J Dermatol. 2019;181:580–3.
Liu RC, Sebaratnam DF, Jackett L, Kao S, Lowe PM. Subacute cutaneous lupus erythematosus induced by nivolumab. Australas J Dermatol. 2018;59:e152–4.
Michot JM, Fusellier M, Champiat S, Velter C, Baldini C, Voisin AL, et al. Drug-induced lupus erythematosus following immunotherapy with anti-programmed death-(ligand) 1. Ann Rheum Dis. 2019;78:e67.
Tarazi M, Aiempanakit K, Werth VP. Subacute cutaneous lupus erythematosus and systemic lupus erythematosus associated with abatacept. JAAD Case Rep. 2018;4:698–700.
Tierney E, Kirthi S, Ramsay B, Ahmad K. Ustekinumab-induced subacute cutaneous lupus. JAAD Case Rep. 2019;5:271–3.
Wehrmann C, Sondermann W, Korber A. Secukinumab-induced subacute-cutaneous lupus erythematosus. Hautarzt. 2018;69:64–6.
Borucki R, Werth VP. Cutaneous lupus erythematosus induced by drugs - novel insights. Expert Rev Clin Pharmacol. 2019:1–8.
Irizarry-Caro JA, Carmona-Rivera C, Schwartz DM, Khaznadar SS, Kaplan MJ, Grayson PC. Brief report: drugs implicated in systemic autoimmunity modulate neutrophil extracellular trap formation. Arthritis Rheumatol. 2018;70:468–74.
• Fairley JL, Oon S, Saracino AM, Nikpour M. Management of cutaneous manifestations of lupus erythematosus: a systematic review. Semin Arthritis Rheum. 2019; Comment: Recent extensive review of treatment options.
Company-Quiroga J, Alique-Garcia S, Romero-Mate A. Current insights into the management of discoid lupus erythematosus. Clin Cosmet Investig Dermatol. 2019;12:721–32.
Wang X, Zhang L, Luo J, Wu Z, Mei Y, Wang Y, et al. Tacrolimus 0.03% ointment in labial discoid lupus erythematosus: a randomized, controlled clinical trial. J Clin Pharmacol. 2015;55:1221–8.
Pothinamthong P, Janjumratsang P. A comparative study in efficacy and safety of 0.1% tacrolimus and 0.05% clobetasol propionate ointment in discoid lupus erythematosus by modified cutaneous lupus erythematosus disease area and severity index. J Med Assoc Thail. 2012;95:933–40.
Barikbin B, Givrad S, Yousefi M, Eskandari F. Pimecrolimus 1% cream versus betamethasone 17-valerate 0.1% cream in the treatment of facial discoid lupus erythematosus: a double-blind, randomized pilot study. Clin Exp Dermatol. 2009;34:776–80.
Kuhn A, Gensch K, Haust M, Schneider SW, Bonsmann G, Gaebelein-Wissing N, et al. Efficacy of tacrolimus 0.1% ointment in cutaneous lupus erythematosus: a multicenter, randomized, double-blind, vehicle-controlled trial. J Am Acad Dermatol. 2011;65:54–64 64.e51–52.
Tzung TY, Liu YS, Chang HW. Tacrolimus vs. clobetasol propionate in the treatment of facial cutaneous lupus erythematosus: a randomized, double-blind, bilateral comparison study. Br J Dermatol. 2007;156:191–2.
Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179–93.
Kuhn A, Aberer E, Bata-Csorgo Z, Caproni M, Dreher A, Frances C, et al. S2k guideline for treatment of cutaneous lupus erythematosus - guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2017;31:389–404.
Wozniacka A, Carter A, McCauliffe DP. Antimalarials in cutaneous lupus erythematosus: mechanisms of therapeutic benefit. Lupus. 2002;11:71–81.
Torigoe M, Sakata K, Ishii A, Iwata S, Nakayamada S, Tanaka Y. Hydroxychloroquine efficiently suppresses inflammatory responses of human class-switched memory B cells via Toll-like receptor 9 inhibition. Clin Immunol. 2018;195:1–7.
Yokogawa N, Eto H, Tanikawa A, Ikeda T, Yamamoto K, Takahashi T, et al. Effects of Hydroxychloroquine in patients with cutaneous lupus erythematosus: a multicenter, double-blind, randomized, parallel-group trial. Arthritis Rheum. 2017;69:791–9.
Chasset F, Arnaud L, Jachiet M, Monfort JB, Bouaziz JD, Cordoliani F, et al. Changing antimalarial agents after inefficacy or intolerance in patients with cutaneous lupus erythematosus: a multicenter observational study. J Am Acad Dermatol. 2018;78:107–114.e101.
Kishi C, Motegi S-i, Yasuda M, Ishikawa O. Therapeutic efficacy and adverse events of hydroxychloroquine administration in Japanese systemic/cutaneous lupus erythematosus patients. J Dermatol. 2018;45:1020–2.
Mok CC, Penn HJ, Chan KL, Tse SM, Langman LJ, Jannetto PJ. Hydroxychloroquine serum concentrations and flares of systemic lupus erythematosus: a longitudinal cohort analysis. Arthritis Care Res (Hoboken). 2016;68:1295–302.
Ugarte A, Porta S, Ríos R, Martinez-Zapico A, Ortego-Centeno N, Agesta N, et al. Combined mepacrine–hydroxychloroquine treatment in patients with systemic lupus erythematosus and refractory cutaneous and articular activity. Lupus. 2018;27:1718–22.
Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision). Ophthalmology. 2016;123:1386–94.
Wenzel J, Brahler S, Bauer R, Bieber T, Tuting T. Efficacy and safety of methotrexate in recalcitrant cutaneous lupus erythematosus: results of a retrospective study in 43 patients. Br J Dermatol. 2005;153:157–62.
Islam MN, Hossain M, Haq SA, Alam MN, Ten Klooster PM, Rasker JJ. Efficacy and safety of methotrexate in articular and cutaneous manifestations of systemic lupus erythematosus. Int J Rheum Dis. 2012;15:62–8.
Sadlier M, Kirby B, Lally A. Mycophenolate mofetil and hydroxychloroquine: an effective treatment for recalcitrant cutaneous lupus erythematosus. J Am Acad Dermatol. 2012;66:160–1 author reply 161-162.
Gammon B, Hansen C, Costner MI. Efficacy of mycophenolate mofetil in antimalarial-resistant cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;65:717–721.e712.
Kreuter A, Tomi NS, Weiner SM, Huger M, Altmeyer P, Gambichler T. Mycophenolate sodium for subacute cutaneous lupus erythematosus resistant to standard therapy. Br J Dermatol. 2007;156:1321–7.
Cipriani P, Ruscitti P, Carubbi F, Liakouli V, Giacomelli R. Methotrexate: an old new drug in autoimmune disease. Expert Rev Clin Immunol. 2014;10:1519–30.
Velo-Garcia A, Ntatsaki E, Isenberg D. The safety of pharmacological treatment options for lupus nephritis. Expert Opin Drug Saf. 2016;15:1041–54.
Cortés-Hernández J, Torres-Salido M, Castro-Marrero J, Vilardell-Tarres M, Ordi-Ros J. Thalidomide in the treatment of refractory cutaneous lupus erythematosus: prognostic factors of clinical outcome. Br J Dermatol. 2012;166:616–23.
Wang D, Chen H, Wang S, Zou Y, Li J, Pan J, et al. Thalidomide treatment in cutaneous lesions of systemic lupus erythematosus: a multicenter study in China. Clin Rheumatol. 2016;35:1521–7.
Kyriakis KP, Kontochristopoulos GJ, Panteleos DN. Experience with low-dose thalidomide therapy in chronic discoid lupus erythematosus. Int J Dermatol. 2000;39:218–22.
Ordi-Ros J, Cortes F, Cucurull E, Mauri M, Bujan S, Vilardell M. Thalidomide in the treatment of cutaneous lupus refractory to conventional therapy. J Rheumatol. 2000;27:1429–33.
Cesbron E, Bessis D, Jachiet M, Lipsker D, Cordel N, Bouaziz J-D, et al. Risk of thromboembolic events in patients treated with thalidomide for cutaneous lupus erythematosus: a multicenter retrospective study. J Am Acad Dermatol. 2018;79:162–5.
Frankel HC, Sharon VR, Vleugels RA, Merola JF, Qureshi AA. Lower-dose thalidomide therapy effectively treats cutaneous lupus erythematosus but is limited by neuropathic toxicity. Inter J Dermatol. 2013;52:1407–9.
Baret I, De Haes P. Thalidomide: still an important second-line treatment in refractory cutaneous lupus erythematosus? J Dermatol Treat. 2015;26:173–7.
Tseng S, Pak G, Washenik K, Pomeranz MK, Shupack JL. Rediscovering thalidomide: a review of its mechanism of action, side effects, and potential uses. J Am Acad Dermatol. 1996;35:969–79.
Braunstein I, Goodman NG, Rosenbach M, Okawa J, Shah A, Krathen M, et al. Lenalidomide therapy in treatment-refractory cutaneous lupus erythematosus: histologic and circulating leukocyte profile and potential risk of a systemic lupus flare. J Am Acad Dermatol. 2012;66:571–82.
Cortés-Hernández J, Ávila G, Vilardell-Tarrés M, Ordi-Ros J. Efficacy and safety of lenalidomide for refractory cutaneous lupus erythematosus. Arthritis Res Ther. 2012;14:R265.
Okon L, Rosenbach M, Krathen M, Rose M, Propert K, Okawa J, et al. Lenalidomide in treatment-refractory cutaneous lupus erythematosus: efficacy and safety in a 52-week trial. J Am Acad Dermatol. 2014;70:583–4.
Fennira F, Chasset F, Soubrier M, Cordel N, Petit A, Francès C. Lenalidomide for refractory chronic and subacute cutaneous lupus erythematosus: 16 patients. J Am Acad Dermatol. 2016;74:1248–51.
Kindle SA, Wetter DA, Davis MDP, Pittelkow MR, Sciallis GF. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Inter J Dermatol. 2016;55:e431–9.
van Vollenhoven RF, Hahn BH, Tsokos GC, Wagner CL, Lipsky P, Touma Z, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. 2018;392:1330–9.
Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, et al. Investigators ftCS: Anifrolumab, an anti–interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheum. 2017;69:376–86.
Furie R, Werth VP, Merola JF, Stevenson L, Reynolds TL, Naik H, et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J Clin Invest. 2019;129:1359–71.
Werth VP, Fiorentino D, Sullivan BA, Boedigheimer MJ, Chiu K, Wang C, et al. Brief report: pharmacodynamics, safety, and clinical efficacy of AMG 811, a human anti–interferon-γ antibody, in patients with discoid lupus erythematosus. Arthritis Rheum. 2017;69:1028–34.
Szepietowski JC, Nilganuwong S, Wozniacka A, Kuhn A, Nyberg F, van Vollenhoven RF, et al. Phase I, randomized, double-blind, placebo-controlled, multiple intravenous, dose-ascending study of sirukumab in cutaneous or systemic lupus erythematosus. Arthritis Rheum. 2013;65:2661–71.
Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two–week randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2017;69:1016–27.
Iaccarino L, Bettio S, Reggia R, Zen M, Frassi M, Andreoli L, et al. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res. 2017;69:115–23.
Parodis I, Gomez A, Frodlund M, Jonsen A, Zickert A, Sjowall C, et al. Smoking reduces the efficacy of belimumab in mucocutaneous lupus. Expert Opin Biol Ther. 2018;18:911–20.
Quelhas da Costa R, Aguirre-Alastuey ME, Isenberg DA, Saracino AM. Assessment of response to B-cell depletion using rituximab in cutaneous lupus erythematosus. JAMA Dermatol. 2018;154:1432–40.
Vital EM, Wittmann M, Edward S, Md Yusof MY, MacIver H, Pease CT, et al. Brief report: responses to rituximab suggest B cell–independent inflammation in cutaneous systemic lupus erythematosus. Arthritis Rheum. 2015;67:1586–91.
Hofmann S, Leandro M, Morris S, Isenberg D. Effects of rituximab-based B-cell depletion therapy on skin manifestations of lupus erythematosus – report of 17 cases and review of the literature. Lupus. 2013;22:932–9.
Lowe GC, Henderson CL, Grau RH, Hansen CB, Sontheimer RD. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Autoimmunity
Rights and permissions
About this article
Cite this article
Petty, A.J., Floyd, L., Henderson, C. et al. Cutaneous Lupus Erythematosus: Progress and Challenges. Curr Allergy Asthma Rep 20, 12 (2020). https://doi.org/10.1007/s11882-020-00906-8
Published:
DOI: https://doi.org/10.1007/s11882-020-00906-8