Abstract
The chemical composition of the atmospheric bulk deposition is a good indicator of atmospheric pollution and air quality. Bulk deposition is a collective term for wet deposition in the forms of rain, snowfall, fog, hail, or ice crystals and dry deposition of atmospheric chemical components mainly under the gravitational settling. The objective of the study was to quantitatively determine rainwater quality parameters, using standard procedures, in selected areas of Sri Lanka. Analysis of weekly sampling of bulk deposition in three sampling sites in Divulapitiya, Kandy, and University of Peradeniya (UOP) of Sri Lanka performed for a period of 24 weeks from 08th of February 2022 to 19th of July 2022 indicated that the three sites had 16.7%, 8.7%, and 8.3% dry-only depositions, respectively; with rainfall levels of 30.2 ± 37.4 mm, 30.6 ± 32.6 mm, and 33.7 ± 39.8 mm; and volume-weighted mean (VWM) pH values of 6.23, 6.29, and 6.47, respectively. Acidic deposition events below pH 5.60 level were not recorded from any site. Chloride (Cl−) was determined to be the predominant anion, and the VWM of anions varied in the order of NO3− < SO42− < Cl− in all three sites. Among trace metals investigated, Fe, Zn, and Al were predominant. Moreover, a very strong positive correlation for conductivity, total dissolved solids, and salinity among each parameter, was observed in the Pearson correlation analysis for all sites. Divulapitiya area showed low air pollution levels with respect to chemical and physical parameters determined in the study as compared to Kandy and UOP areas. Possible causes for the results would be vehicular, constructional, and industrial emissions, and natural geographical factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution, a global environmental issue, is caused by the release of air pollutants into the atmosphere by natural and anthropogenic sources (Kampa and Castanas 2008; Le Roux et al. 2016; Chathuranga et al. 2020). Anthropogenic activities are the major source of air pollutants which adversely affect the biosphere, and cause risks to human health (Kampa and Castanas 2008). Despite its source, chemical components present in the atmosphere are scavenged back to the earth’s surface by vertical transportation methods, namely, wet deposition, dry deposition, surface sink absorption, and cloud water deposition (Lovett and Kinsman 1990; Weiner and Matthews 2003; Kajino and Aikawa 2015; Araujo et al. 2015). Wet deposition and dry deposition are collectively termed as atmospheric bulk deposition (Chathuranga et al. 2020). Wet deposition is the main scavenging mode of atmospheric chemical components to the earth’s surface (Wang and Han 2011; Kajino and Aikawa 2015), and it can occur in the forms of rain, snowfall, fog drops, hail, or ice crystals. These forms are collectively known as hydrometeors (Seinfeld and Pandis 2016; Holopainen et al. 2020).
Rain or precipitation is the most effective scavenging pathway of atmospheric chemical components and pollutants (Migliavacca et al. 2005). Cloud type, precipitation type, solubility of gaseous species in rain, and air mass trajectory are some important factors that affect scavenging efficiency. Furthermore, the nature, size, and shape of precipitation media, and hydrometeors involved are important in the process of wet deposition (Gonçalves et al. 2007). The composition of wet deposition depends on pollutant transport, emission levels, elevation, sizes of raindrops, and the method of scavenging process (Migliavacca et al. 2005). Consequently, the composition of rainwater could be changed due to geographical and anthropogenic factors (Rathnayaka et al. 2020). In this regard, atmospheric quality in selected areas has been determined by both direct and indirect means as well as at different altitudes (Gautam et al. 2021; Bisht et al. 2022; Gupta et al. 2022a, b).
Atmospheric particles and pollutants become nuclei for the formation of rain droplets and initiate precipitation events by releasing the raindrops out of the cloud, called in-cloud scavenging or rainout. The nuclei activation process occurs under supersaturated conditions inside the clouds (Kajino and Aikawa 2015). Cloud droplets or ice crystals, when grown up to a precipitation size, are removed from the clouds scavenging the atmospheric chemical compounds and aerosols (Holopainen et al. 2020). Below-cloud scavenging or washout occurs below the clouds while rain or other hydrometeor falls through the atmosphere dissolving soluble gases and entraining atmospheric components in droplets (Weiner and Matthews 2003). Water-soluble gases, such as NH3, SO2, and NO2 dissolve efficiently in the washout and contribute to SO42−, NO3−, and NH4+ deposition, while aerosols show slow washout rates (Kajino and Aikawa 2015).
Dry deposition is the direct deposition of gases and dry particles onto the earth’s surface or the vegetation in the absence of precipitation (Lovett and Kinsman 1990; Wesely 2000; Seinfeld and Pandis 2016). The turbulence of the atmosphere, the chemical nature of the depositing species, and the surface factors affect dry deposition. Particle size, particle density, and shape of the particles are important characteristics of depositing species. Complex mechanisms, such as Brownian diffusion, turbulent diffusion, sedimentation, interception, and inertial forces, affect the dry deposition process (Liyandeniya et al. 2020c).
Acidic deposition, which occurs as both wet and dry depositions, has created environmental, socio-economical, and health issues. Rainwater having a pH < 5.6 is considered acid rain which is lower than the natural acidity level of rainwater (Ileperuma 2015). Sulfur dioxide (SO2) and oxides of nitrogen (NOx) mainly contribute to acidic precipitation. Transboundary air pollution would transport acidic atmospheric components to longer distances thereby affecting even in nearby countries (Chathuranga et al. 2020; Du et al. 2020). Soil acidification, heavy metal leaching, forest dieback, reduction of yields, health issues for humans and animals, and damaging manmade structures are some undesirable effects of acid depositions (Larssen et al. 1999; Singh and Agrawal 2007; Duan et al. 2016).
Bulk deposition can be used as an indicator of atmospheric pollution. Many countries have adapted rainwater chemical composition studies to measure the levels of atmospheric pollution (Liyandeniya et al. 2020c). Passive bulk deposition sampling is cost-effective for a developing country like Sri Lanka where it lacks data on atmospheric pollution (Weerasundara et al. 2018). Nevertheless, in Sri Lanka, only a few bulk deposition studies have been conducted, and most of them have been focused on Gampaha and Kandy districts. Four climate seasons can be identified in Sri Lanka throughout the year (Domroes & Ranatunge 1993), namely, first inter-monsoon (FIM)—March to mid-May; South-west monsoon (SWM)—mid-May to September; second inter-monsoon (SIM)—October to November; and North-east monsoon (NEM)—December to February (Weather and Climate Data 2022).
According to studies reported, air pollution levels in Kandy are often 4–5 times higher than those in Colombo. Owning a unique topography situated within a valley surrounded by mountain ranges, the Kandy area shows less dispersion of air pollutants with higher accumulation. The emission from traffic during crowded hours increases the effect due to the limited road system within the main city (Ileperuma 2015). The air pollution of the Western coastal region is dispersed effectively to the sea reducing the concentrations. Gampaha district also experiences a higher level of vehicular emission. Studies have suggested that the Colombo to Gampaha bus route as the highest polluted route compared to others. Katunayake industrial zone, Sapugaskanda power station, and oil refinery are some major sources of pollution in Gampaha district (Liyandeniya et al. 2020b).
The chemical and trace metal composition of bulk deposition was characterized by several studies focusing on Peradeniya and Kandy city (Abeyratne & Ileperuma 2006; Weerasundara et al. 2017, 2018; Liyandeniya et al. 2020a, c; Rathnayaka et al. 2020; Madhushani et al. 2023). Some isolated studies have characterized the effects of trace metals and microorganisms in bulk deposition sampling (Weerasundara et al. 2017). In Gampaha district, few bulk deposition studies have been undertaken in Kelaniya and Gampaha (Chathuranga et al. 2020; Liyandeniya et al. 2020b). Some other studies have used different methods to collect bulk deposition such as moss biomonitoring methods in Sri Lanka (Jayalath et al. 2021).
It is important to continue and maintain a database on the composition of bulk deposition in these regions, which it is most susceptible to atmospheric pollution in Sri Lanka. The data continuously gathered on atmospheric pollution is important for decision-making processes on human health and socio-economical aspects. Moreover, data collected over a long period of time can be used to formulate atmospheric pollution models which will be of immense value to understand the present trends and predict future scenarios.
It is hypothesized that the rainwater quality is affected by many anthropogenic factors, including the extent of urbanization, state of traffic conditions, and generation of particulate matter due to industrialization. In this respect, the objective of the study was to quantify rainwater quality parameters of bulk deposition, using standard procedures, collected over a period of 6 months from February 2022 to July 2022 at selected sites in Kandy and Gampaha districts of Sri Lanka. Such a cost-effective study is of great significance in an attempt to monitor and compare atmospheric quality of regions having different geographical and urbanization patterns. Correlation among different pollution parameters was investigated through statistical means, followed by prediction of point and nonpoint sources of atmospheric pollution of the above areas. One sampling site representing semi-urban environment in Gampaha district, and two sites representing urban environment in Kandy district were specified for the study. The site in Gampaha is less susceptible to vehicular traffic congestion, and further, small-scale industries and paddy fields are abundant in the area. More importantly, no previous studies have been reported to assess the level of air pollution in the area, which contributes to the novelty of the study.
Methodology
Site selection
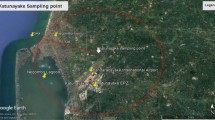
Three sampling sites were used in the study based on their urbanization and level of exposure to the emissions including Gampaha and Kandy districts of Sri Lanka. Divulapitiya sampling site which is located 18.9 km away from the Western coast of Sri Lanka is an agricultural sub-urban area near the Colombo-Kurunegala main road. The GPS coordinates of Divulapitiya sampling site are 7.231258°, 80.024364°. Kandy and University of Peradeniya (UOP) sampling sites are located in the GPS coordinates of 7.290712°, 80.598328° and 7.259326°, 80.633093°, respectively, in central highlands in Kandy district of Sri Lanka (Fig. 1). The area is surrounded by Hanthana mountain range with a unique geographical setup of terrain. Kandy sampling site is situated in the central provincial, educational department premises in the S.W.R.D. Bandaranayaka Mawatha of Kandy city.
Sample collection
Sample collectors were placed in each sampling point avoiding obstacles, such as tree branches and rooftops above the collector, which was made up of a metal stand supporting a 5 L high-density poly-ethylene (HDPE) container placed 1.5 m above the ground level as shown in Fig. 2. A plastic funnel with a 20 cm diameter was fixed to the mouth of the container and sealed with polypropylene-based cello-tape into the mouth of the container. The funnel mouth was covered with a tight cotton wool plug to avoid contaminants such as insects and debris, falling into the container. Ultra-pure water (ASTM type I) was used to clean all containers and the funnel. The volume of the container used for the collection of rainwater was estimated based on existing rainfall data such that there would be no overflow at the end of the sampling period.
Bulk deposition samples were collected on a weekly basis from each station for a period of 6 months, from the 1st week of February until the 3rd week of July 2022. Although volume-weighted weekly averaged estimation on the atmospheric quality could be predicted, no attempt was made to determine the continuous variation of the magnitude of each parameter as the weekly averaged values would provide sufficient information. For wet deposition scenarios, the volume of rainwater collected was measured on-site, and a sufficient volume was preserved in pre-cleaned polyethylene terephthalate (PET) bottles for analysis. For dry-only deposition scenarios, the funnel was washed with ultra-pure water while the funnel was fixed to the container, and the filtrate was collected and preserved for analysis.
Each preserved sample was divided into two aliquots: the first aliquot was filtered using 0.45 µm alpha-cellulose filter papers and used for anion analysis and trace metal analysis separately. The sample for trace metal analysis was preserved using a few drops of analytical grade conc. HNO3 (≥ 69% W/W) until pH reached below 2. The second aliquot was stored without filtration for the determination of total hardness and water quality parameters. All sample bottles were refrigerated under 4 °C.
Sample analysis
The pH level was determined using an ORION 420A pH meter on the same day of sample collection at ambient temperature. Conductivity, salinity, and total dissolved solids (TDS) were determined using a Thermo Scientific – EuTech Con 450 multi-parameter conductivity meter. Total hardness determination was done following the ethylenediaminetetraacetic acid (EDTA) titration method using Eriochrome Black T as the indicator. The solution of EDTA was standardized using a standard Ca2+ solution, and results were reported as mg CaCO3 L−1 (Baird et al. 2017).
Thermo Scientific Dionex ICS-900 ion chromatographic system was used to determine the anion composition of the bulk deposition samples. Dionex ionpack AS23 (4 × 250 mm) anion column was used with 1.0 M H2SO4 as regenerant and 4.5 mM Na2CO3 and 8.08 mM NaHCO3 eluent concentrate to determine concentrations of Cl−, NO3− and SO42− ions.
Determination of trace metals (Pb, Cu, Mn, Al, Zn, Fe, etc.) was conducted using inductively coupled plasma mass spectrometry (ICP-MS) (Thermo Fisher iCAP™ Q ICP-MS).
Data analysis
Rainfall, water quality parameters, and concentrations of selected anions, trace metals, and main group elements were tabulated for a period of 6 months. Basic descriptive statistical parameters and volume-weighted mean (VWM) values were calculated, and Pearson correlation analysis was performed to identify significant correlations between the parameters concerned using Minitab V.18.0 software.
Results and discussion
Rainfall
Rain is the most significant wet deposition type in Sri Lanka, and it mainly depends on the monsoonal rains. Among them, SWM and NEM result in the highest rainfall in the country where SWM leads to higher rainfall in the southwestern parts, and NEM accounts for the north-eastern parts. The extent and the variation of rainfall within the three sampling sites investigated were monitored as the rainfall and atmospheric pollution would be correlated. The total sampling period of 6 months represented as week numbers includes three of the four climate seasons of Sri Lanka. There were dry weeks in all three sampling sites, and more specifically, 16.7%, 8.7%, and 8.3% dry-only deposition weeks were encountered in Divulapitiya, Kandy, and UOP sites, respectively. The rainfall variation of the three sampling stations is shown in Fig. 3.
Weeks 1 – 4 belong to the NEM period and in the month of February which is the end of the monsoon period that starts in December. Lower rainfall values were observed during the initial period of sampling as NEM has less effect on Gampaha and Kandy districts. There is a significant effect of FIM, which covers the period of weeks 5 – 15, on the entire island leading to higher rainfall. This fact is supported by rainfall measurements in all three sampling stations observed as shown in Fig. 3. The last part of the period of weeks 16 – 24, which belongs to the SWM, brings high rainfall to the Southwestern coasts and central hills of the island. Consequently, an increase in rainfall in Divulapitiya which is in the Western province, and a significant increase in rainfall in Kandy and UOP sites were observed during the FIM.
Basic rainwater quality parameters: pH, conductivity, salinity, TDS, and total hardness
Table 1 shows the variation of the volume-weighted mean (VWM) values of important rainwater quality parameters measured and concentrations determined. All parameters were reported in triplicate, and the respective VWM values were determined to minimize the effect of varying deposition volumes.
The pH of deposition is contributed by anthropogenic or natural activities. For instance, burning of fossil fuels during transportation or industrial activities releases sulfuric acid and nitric acid into the atmosphere (Payus et al. 2020). Furthermore, the sources of acidity in rural areas would be biogenic volatile organic carbon emissions from forests, vegetation, and livestock manure. On the other hand, Ca2+ is the dominant cation species, and HCO3− is the major anion species contributing to rising the pH of deposition (Zhang 2002). Consequently, the pH of deposition would be a valuable parameter for the prediction of rainwater quality. The pH variation of the three sites is shown in Fig. 4.
The UOP site, in general, showed the highest VWM pH level followed by Kandy and Divulapitiya. Furthermore, none of the sites showed deposition events with pH < 5.6 to be qualified as acidic depositions. The pH values of bulk depositions represent the balance of atmospheric anions and cations in a particular area (Zong-Jie et al. 2017). Although the overall pH range of all three sites was in the range of 5.77–8.60, the number of occurrences (weeks) having a pH greater than 7.80 was found to be only two out of 24 weeks, both of which belonged to the UOP site. Occurrences of high pH (> 8.00) of deposition have been reported at the UOP site within the period 2019–2021 although such high pH values have not been recorded in the Kandy site within the same period. The VWM pH of the depositions of the UOP site excluding these two points was 6.47. Moreover, the lowest pH values of the three sites were 5.77, 5.89, and 5.96 for Divulapitiya, Kandy, and UOP, respectively, most of which were observed in the month of May which belongs to the FIM season. Figure 5 shows the histogram of the pH ranges reported in the three sites.
Divulapitiya sampling site is situated in an agricultural area which has more probability to observe the influence of biogenic organic material originating from vegetation and agricultural lands. The less effect of neutralizing basic compounds, less exposure to dust, and low air pollution contribution from emissions, might be the reason for the comparatively low average pH level in Divulapitiya.
Kandy and UOP sites being situated in Kandy districts are more vulnerable to air pollution which is mostly associated with acidic pollutants and shows more basic average pH levels. Even with a higher pollution level in the Kandy district, observing higher pH values is contradictory to what could be expected, which could be postulated by considering the possibility of the presence of basic substances in the atmosphere. Soil-borne dust particles affect the pH value of rain by releasing substances that represent the soil type of the relevant area. Basic components in the atmosphere are mostly geogenic which can be transferred into the atmosphere, entrained inside the dust particles, with the effect of wind turbulence due to road transportation, construction activities, and other anthropogenic activities.
Calcium content would be an indicator of the extent of neutralizing constituents present in the environment (Glavas and Moschonas 2002). Kandy district mainly contains reddish brown latosolic soils and immature brown loam soil types. The former is rich in calcium content compared to other elements (Chathurika et al. 2015). A higher amount of calcium content was present in the dust of the Kandy area according to a previous study supporting this argument (Samaradiwakara and Pitawala 2021). There is a high possibility that this geogenic Ca ends up in the atmosphere thereby increasing the pH levels of wet deposition of Kandy district.
Unlike pH measurements which are contributed by acidic or basic atmospheric pollutants, electrical conductivity is an indicator of positive and negative ions present in the deposition. Therefore, it would indicate the extent of pollution due to the load of ionic substances entrained in the atmosphere (Mohamed et al. 2019). They are emitted due to different processes, such as vehicular emissions, biomass and fossil fuel burning, construction activities, household emissions, wind erosion, and sea spray. Since all three sites are sufficiently far away from the ocean, sea spray is an insignificant factor. The electrical conductivity variation of the three sites is shown in Fig. 6.
According to the data, Divulapitiya site shows an increasing trend in conductivity in SWM which can be explained by the availability of monsoon rain to the Western region of Sri Lanka during the SWM. During the NEM, Kandy and UOP sites have shown higher values than that of Divulapitiya site while FIM shows no clear comparable pattern between three sites.
Salinity, which is a measure of the content of salts in soil or water, is important in depositions because the accumulation of salt from rainfall over time will make the salinity of soil increase beyond the desirable levels. Figure 7 shows the temporal variation of the salinity of three sampling sites.
TDS also has some relevance to electrical conductivity and salinity. With a higher content of particulate matter in rainwater, higher TDS values can be expected (Mohamed et al. 2019). In relation to other basic rainwater quality parameters, such as conductivity and salinity, the UOP site showed the highest VWM values, and Kandy site showed the lowest VWM values. The TDS and total hardness variation for three sites for 24 weeks is shown in Fig. 8 and Fig. 9 respectively.
The range of conductivity, salinity, and TDS of all samples in Divulapitiya were 10.4–401.9 µS cm−1, 0.015–0.191 ppt, and 5.2–198.4 ppm, respectively. The corresponding values in Kandy and UOP sites collectively were 7.8–137.5 µS cm−1, 0.013–0.069 ppt, and 4.0–68.6 ppm, respectively. The total hardness, another rainwater quality parameter, showed the highest VWM values in Divulapitiya followed by Kandy and UOP. More specifically, the range of the total hardness of Divulapitiya was 1.6–42.0 ppm and 2.53–27.5 ppm for Kandy and UOP collectively. As the hardness of soft water is less than 17 ppm and that of slightly hard water is 17–60 ppm, rainwater collected at all three sites was either soft or slightly hard. However, dissolved CO2 from the atmosphere is able to dissolve soluble minerals in the rocks, increasing the hardness of water sources nearby located.
When considered collectively, Kandy and UOP sites show higher levels for water quality parameters except for the total hardness levels. The samples with low rainfall volumes showed higher pH levels and a trend of giving higher values for conductivity, salinity, and TDS similar to the trend which was observed in other studies in different countries also (Zong-Jie et al. 2017).
Anion concentrations
VWM concentrations of anions varied as NO3− < SO42− < Cl− for all three sites within the 10-week period of investigation. Similar trends were reported in studies conducted in 2012–2014 (Liyandeniya et al. 2020a, c). Specifically, Divulapitiya site showed a range of 3.95–33.83 μeq L−1, 9.17–279.89 μeq L−1, and 23.46–491.41 μeq L−1 for NO3−, SO42−, and Cl− concentrations, respectively. When considered collectively, Kandy and UOP sites showed a range of 0.0932–5.2315 ppm, 0.4977–39.7739 ppm, and 0.6108–62.7091 ppm for concentrations of respective anions.
Divulapitiya Kandy and UOP sites are located 20.4 km, 87.7 km, and 83.7 km away from the seashore towards the Western shore of Sri Lanka. Since all sites are not much closer to the ocean, the origin of the ions from sea spray may not be prominent. Since Divulapitiya area is a semi-urban area, the sources of the anions should be of natural origins such as wind erosion, weathering, and degradation of natural components, livestock farming, and other agricultural activities. In Kandy district, there should be a significant contribution of vehicular emissions, wind erosion and dust component to the anion sources than in Divulapitiya. Sulphates can have the possibility to originate from the CaSO4 bearing rocks while nitrates and chlorides could be originated from natural and anthropogenic emission sources.
Trace metals
The trend in the VWM concentrations of trace metals of all three sites was similar; Divulapitiya and Kandy varied as Pb < Mn < Cu < Al < Zn < Fe, and UOP site showed the variation as Pb < Mn < Cu < Zn < Al < Fe. It is clear that Fe was the predominant trace metal in all three sites. Descriptive statistics for trace metal compositions of the three sites are shown in Tables 2, 3, and 4. Possible sources of these trace metals are found to be anthropogenic activities that include construction, industrial, and vehicular emissions in Kandy district.
The elements, Fe and Al, have a high probability to originate from natural and geogenic sources (Weerasundara et al. 2017). Construction activities, physical weathering of soil, and industries can be the probable anthropogenic sources of high Fe concentrations. The brass industry, metal workshops, and industries are the possible sources of Zn. Al can be emitted from terrigenous sources with dust and industrial emissions.
Zn, Cu, and Mn could originate from the metallic wearing of the vehicular engine parts and brake pads. Moreover, Zn which is used as a vulcanization agent in tires can be emitted to higher extents the high wearing rates of tires, due to extensive road systems and winding roads with higher slopes (Gupta et al. 2022a; Samaradiwakara & Pitawala 2021).
The main sources of the Pb found in the depositions can be derived from road dust. Pb can be released from lubricant oils, paint types containing lead chromate of traffic signs on road surfaces, and entrain in dust particles which ultimately can end up in the rain or as dry depositions (Gupta et al. 2022a, b).
The weeks with higher rainfall levels throughout the week and weeks with dry-only depositions show comparatively low levels of trace metal concentrations, while the weeks with few millimeters of rainfall show higher levels of trace metal concentrations.
VWM values for Pb concentration were 1.21 ppb, 1.15 ppb, and 1.19 ppb respectively for three sites which did not exceed the minimum permissible levels of Pb according to Sri Lankan standards and US-EPA standards. The concentration of different trace metals in Divulapitiya, Kandy, and UOP sites are given in Figs. 10, 11, 12, 13, 14, and 15.
Owing to the dynamic nature of anthropogenic processes, the type and the levels of pollutants reached to the atmosphere would continuously vary. In the Sri Lankan context, the population is concentrated in isolated main cities, such as Kandy. The increase in population is connected to various other anthropogenic activities, such as building construction and hotel industry, leading to increased pollution levels. This is clear according to published reports with respect to metallic contents and conductivity in precipitations of Kandy and Peradeniya areas (Table 5). Unexpectedly, low levels of the above pollutants recorded during this study in the year 2022 can be attributed to post-Covid era because Sri Lanka had not completely become normal during the sampling period.
Statistical analysis
The Pearson correlation analysis is important in atmospheric research to understand relationships between different parameters. Pearson correlation coefficient (PCC) gives the type of relationship between two parameters according to the criteria shown below:
-
0.00–0.19 “Very weak”
-
0.20–0.39 “Weak”
-
0.40–0.59 “Moderate”
-
0.60–0.79 “Strong”
-
0.80–1.00 “Very strong”
PCC is always recorded with the p value which gives the statistical significance of the relationship. When p < 0.05, the relationship is statistically significant.
For the water quality parameters, a very strong significant positive correlation higher than 0.900 can be observed between conductivity, salinity, and TDS among each other parameter for all three sites as shown in Tables 6, 7, and 8 respectively.
All three sites showed a very strong significant positive correlation for [SO42−] – [Cl−] (Table 10), while the Kandy site showed a very strong significant negative correlation for rainfall—[SO42−] and rainfall—[Cl−] (Table 9).
All sites showed a strong significant positive correlation for Fe and Cu and a strong to very strong significant positive correlation for Cu and Zn as shown in Tables 10, 11, 12, and 13. A previous study conducted on the particulate matter of Kandy urban area also reported similar highly significant (> 0.6) correlations between the same trace metals (Samaradiwakara and Pitawala 2021). It also explains that the composition of the bulk deposition is highly affected by the contribution of the dust particle fraction in the Kandy district.
Conclusion
Among the three sites at which the rainwater quality was monitored, in terms of both physical and chemical parameters, for a period of 6 months, the Divulapitiya area was found to be the most unpolluted. This is attributed to low traffic and low industrialization. On the other hand, the Kandy site, being the central capital of Sri Lanka, experiences high traffic congestion and industrial activities leading to high levels of rainwater quality parameters, for instance, conductivity and total dissolved solids (TDS), as determined throughout the study period. The extent of atmospheric pollution at the UOP site and the Kandy site, in general, are on the high side. This is due to the fact that although the UOP site experiences less traffic levels, the atmospheric quality is affected by fumes generated from the laboratories of the UOP and the nearby livestock farm. Moreover, the water quality parameters of rainwater are correlated to the volume of the wet precipitation, and low levels of rainfall lead to elevated levels of all parameters, as witnessed by pH levels. Availability of dust particles in higher amounts during dry weather conditions, which are brought to the atmosphere due to wind erosion, and moreover, isolated rainfall events scavenge a larger amount of pollutants at once. When rain events occur throughout long periods, pollutants added to the atmosphere are constantly scavenged back to the earth’s surface with rain. In fact, calcium-containing dust in the atmosphere is responsible for the alkaline rain pH levels, due to neutralization of acidic pollutants. This effect is however less during heavy rainfall as larger volumes lead to less neutralizing effect.
Data availability
The data generated during and/or analyzed during the current study are available from the corresponding author, N. Priyantha, upon request.
References
Abeyratne V, Ileperuma O (2006) Air pollution monitoring in the city of Kandy: possible transboundary effects. J Natl Sci Found 34(3):137. https://doi.org/10.4038/jnsfsr.v34i3.3644
Araujo TG, Souza MF, Mello WZ, Silva DM (2015) Bulk atmospheric deposition of major ions and dissolved organic nitrogen in the lower course of a Tropical River basin, Southern Bahia, Brazil. Journal of the Brazilian Chemical Society 26(8):1692–1701. https://doi.org/10.5935/0103-5053.20150143
Baird R, Eaton A, Rice E, Bridgewater L (2017) Standard methods for the examination of water and wastewater, 23rd edn, pp. 166–167
Bisht DS, Srivastava AK, Singh V, Tiwari S, Gautam AS, Gautam S, Santosh M, Kumar S (2022) High-altitude air pollutants monitored from rainwater chemistry in the Central Himalaya. Water Air Soil Pollut 233(9). https://doi.org/10.1007/s11270-022-05855-8
Chathuranga R, Liyandeniya A, Dharmapriya T, Deeyamulla M, Priyantha N (2020). Risk assessment and source apportionment of wet bulk deposition in three typical sites of Gampaha District, Sri Lanka. SN Appl Sci 2(8). https://doi.org/10.1007/s42452-020-3007-6
Chathurika JAS, Indraratne SP, Dandeniya WS (2015) Site specific fertilizer recommendations for maize (Zea mays L.) grown in reddish brown earth and reddish brown Latasolic soils. Trop Agric Res 25(3):287. https://doi.org/10.4038/tar.v25i3.8039
Domroes M, Ranatunge E (1993) A statistical approach towards a regionalization of daily rainfall in Sri Lanka. Int J Climatol 13(7):741–754. https://doi.org/10.1002/joc.3370130704
Du X, Jin X, Zucker N, Kennedy R, Urpelainen J (2020) Transboundary air pollution from coal-fired power generation. Journal of Environmental Management 270:110862. https://doi.org/10.1016/j.jenvman.2020.110862
Duan L, Yu Q, Zhang Q, Wang Z, Pan Y, Larssen T et al (2016) Acid deposition in Asia: emissions, deposition, and ecosystem effects. Atmos Environ 146:55–69. https://doi.org/10.1016/j.atmosenv.2016.07.018
Gautam S, Sammuel C, Bhardwaj A, Shams Esfandabadi Z, Santosh M, Gautam AS, Joshi A, Justin A, John Wessley GJ, James EJ (2021) Vertical profiling of atmospheric air pollutants in rural India: a case study on particulate matter (PM10/PM2.5/PM1), carbon dioxide, and formaldehyde. Measurement 185:110061. https://doi.org/10.1016/j.measurement.2021.110061
Glavas S, Moschonas N (2002) Origin of observed acidic–alkaline rains in a wet-only precipitation study in a Mediterranean coastal site, Patras, Greece. Atmos Environ 36(19):3089–3099. https://doi.org/10.1016/s1352-2310(02)00262-5
Gonçalves F, Morinobu W, Andrade M, Fornaro A (2007) In-cloud and below-cloud scavenging analysis of sulfate in the metropolitan area of São Paulo, Brasil. Rev Bras Meteorol 22(1):94–104. https://doi.org/10.1590/s0102-77862007000100010
Gupta V, Bisht L, Arya AK, Singh AP, Gautam S (2022a) Spatially resolved distribution, sources, exposure levels, and health risks of heavy metals in <63 μm size-fractionated road dust from Lucknow City, North India. Int J Environ Res Public Health 19(19):12898. https://doi.org/10.3390/ijerph191912898
Gupta V, Bisht L, Deep A, Gautam S (2022b) Spatial distribution, pollution levels, and risk assessment of potentially toxic metals in road dust from major tourist city, Dehradun, Uttarakhand India. Stoch Environ Res Risk Assess 36(10):3517–3533. https://doi.org/10.1007/s00477-022-02207-0
Holopainen E, Kokkola H, Laakso A, Kühn T (2020) In-cloud scavenging scheme for sectional aerosol modules – implementation in the framework of the sectional aerosol module for large scale applications version 2.0 (SALSA2.0) global aerosol module. Geosci Model Dev 13(12):6215–6235. https://doi.org/10.5194/gmd-13-6215-2020
Ileperuma O (2015) Model assessment of acid deposition potential by SOx in Sri Lanka. J Natl Sci Found 43(3):281. https://doi.org/10.4038/jnsfsr.v43i3.7956
Jayalath KG, Deeyamulla MP, De Silva RCL (2021) A comparison of heavy metal deposition in two metropolitan areas in Western Province of Sri Lanka using the moss biomonitoring method. Res J Chem Environ 25:2
Kajino M, Aikawa M (2015) A model validation study of the washout/rainout contribution of sulfate and nitrate in wet deposition compared with precipitation chemistry data in Japan. Atmos Environ 117:124–134. https://doi.org/10.1016/j.atmosenv.2015.06.042
Kampa M, Castanas E (2008) Human health effects of air pollution. Environ Pollut 151(2):362–367. https://doi.org/10.1016/j.envpol.2007.06.012
Larssen T, Seip HM, Semb A, Mulder J, Muniz IP, Vogt RD, Eilertsen O (1999) Acid deposition and its effects in China: an overview. Environ Sci Policy 2(1):9–24
Liyandeniya A, Deeyamulla M, Priyantha N (2020b) Source apportionment of rainwater chemical composition in wet precipitation at Kelaniya in Sri Lanka. Air Qual Atmos Health 13(12):1497–1504. https://doi.org/10.1007/s11869-020-00903-w
Liyandeniya A, Deeyamulla M, Priyantha N (2020a) Atmospheric chemical composition of bulk deposition at two geographically distinct locations in Sri Lanka. Environ Monit Assess 192(7). https://doi.org/10.1007/s10661-020-08412-0
Liyandeniya A, Deeyamulla M, Abeysundara S, Priyantha N (2020c) Chemical characteristics of wet precipitation at Peradeniya in Sri Lanka. Environ Monit Assess 193(1). https://doi.org/10.1007/s10661-020-08772-7
Lovett G, Kinsman J (1990) Atmospheric pollutant deposition to high-elevation ecosystems. Atmos Environ A Gen Top 24(11):2767–2786. https://doi.org/10.1016/0960-1686(90)90164-i
Madhushani LS, Dharmapriya TN, Dharaka BDP, Deeyamulla MP, Priyantha N (2023) Chemical composition of rainwater at three sites in Kandy/Peradeniya, Sri Lanka, and its effect on air pollution. Energy Nexus 100243. https://doi.org/10.1016/j.nexus.2023.100243
Migliavacca D, Teixeira E, Wiegand F, Machado A, Sanchez J (2005) Atmospheric precipitation and chemical composition of an urban site, Guaıba hydrographic basin, Brazil. Atmos Environ 39(10):1829–1844. https://doi.org/10.1016/j.atmosenv.2004.12.005
Mohamed AA, Kombo UM, Kombo MA (2019) Rain water characterization at urban and rural (north B) Unguja. Hydro Sci Marine Eng 1(2). https://doi.org/10.30564/hsme.v1i2.1494
Payus CM, Jikilim C, Sentian J (2020) Rainwater chemistry of acid precipitation occurrences due to long-range transboundary haze pollution and prolonged drought events during Southwest Monsoon season: climate change driven. Heliyon 6(9):e04997. https://doi.org/10.1016/j.heliyon.2020.e04997
Rathnayaka I, Dharmapriya T, Liyandeniya A, Deeyamulla M, Priyantha N (2020) Trace metal composition of bulk precipitation in selected locations of Kandy district, Sri Lanka. Water Air Soil Pollut 231(10). https://doi.org/10.1007/s11270-020-04840-3
Le Roux G, Hansson SV, Claustres A (2016) Inorganic Chemistry in the Mountain Critical Zone. Developments in Earth Surface Processes 21:131–154. https://doi.org/10.1016/b978-0-444-63787-1.00003-2
Samaradiwakara DS, Pitawala HM (2021) Extent of air pollution in Kandy area, Sri Lanka: morphological, mineralogical and chemical characterization of dust. Ceylon J Sci 50(4):475. https://doi.org/10.4038/cjs.v50i4.7946
Seinfeld J, Pandis S (2016) Atmospheric chemistry and physics, 3rd edn, p 856
Singh A, Agrawal M (2007) Acid rain and its ecological consequences. J Environ Biol 29(1):15
Wang H, Han G (2011) Chemical composition of rainwater and anthropogenic influences in Chengdu, Southwest China. Atmos Res 99(2):190–196. https://doi.org/10.1016/j.atmosres.2010.10.004
Weather and Climate Data (2022) Meteo.gov.lk. Retrieved 17 August 2022, from http://meteo.gov.lk
Weerasundara L, Amarasekara R, Magana-Arachchi D, Ziyath A, Karunaratne D, Goonetilleke A, Vithanage M (2017) Microorganisms and heavy metals associated with atmospheric deposition in a congested urban environment of a developing country: Sri Lanka. Sci Total Environ 584–585:803–812. https://doi.org/10.1016/j.scitotenv.2017.01.121
Weerasundara L, Magana-Arachchi D, Ziyath A, Goonetilleke A, Vithanage M (2018) Health risk assessment of heavy metals in atmospheric deposition in a congested city environment in a developing country: Kandy City, Sri Lanka. J Environ Manage 220:198–206. https://doi.org/10.1016/j.jenvman.2018.04.036
Weiner RF, Matthews RA (eds) (2003) Chapter 18 - Meteorology and air pollution. In: Environmental Engineering, 4th edn, pp. 351–374). essay, Butterworth-Heinemann. https://doi.org/10.1016/B978-075067294-8/50018-X
Wesely M (2000) A review of the current status of knowledge on dry deposition. Atmos Environ 34(12–14):2261–2282. https://doi.org/10.1016/s1352-2310(99)00467-7
Zhang DD (2002) Alkaline rains on the Tibetan Plateau and their implication for the original ph of natural rainfall. J Geophys Res 107(D14). https://doi.org/10.1029/2001jd001332
Zong-Jie L, Ling-Ling S, Qing T, Yong-ge L (2017) Characteristics and sources of atmospheric composition based on precipitation chemistry in the Shiyang River Basin, Northwestern China. Arab J Geosci 10(2). https://doi.org/10.1007/s12517-016-2820-0
Acknowledgements
The authors declare that this work was supported by Department of Environmental and Industrial Sciences and Department of Chemistry of University of Peradeniya, Sri Lanka, for the chemical analysis and by providing the necessary laboratory facilities with chemicals and instruments.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and designing of the study in different aspects. Initial planning of the study, supervision and guidance, data interpretation, compiling of the discussion and conclusion, and final corrections were performed by N. Priyantha. Sample collection, determination of rainwater quality parameters, their interpretation and analysis, and compiling of the initial draft of the manuscript were performed by B.D.P. Dharaka. All authors have read, commented, and approved the final manuscript of the study.
Corresponding author
Ethics declarations
Ethics approval
Not applicable—This research did not involve any human or animal subjects or biological material or their data.
Consent to participate
Not applicable—This research did not involve any human subjects.
Consent for publication
Not applicable—This research did not involve any human subjects.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dharaka, B.D.P., Priyantha, N. Atmospheric quality through analysis of dry and wet deposition at selected locations in Kandy and Gampaha districts of Sri Lanka. Air Qual Atmos Health 17, 71–87 (2024). https://doi.org/10.1007/s11869-023-01431-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-023-01431-z