Abstract
Rainwater quality is of utmost importance as it can be used to predict atmospheric quality which of course governs, to some extent, the health of the ecosystem. In this regard, the research reported here was to study chemical characteristics of precipitation in Kelaniya, Gampaha District, one of the most populated and most urbanized districts in Sri Lanka. This study was conducted during the period from 2012 to 2014, and wet precipitation was analyzed for pH, conductivity, Na+, NH4+, K+, Mg2+, Ca2+, F−, Cl−, NO3−, SO42−, Pb, Cu, Mn, Al, Zn, and Fe for a total of seventy-eight (78) samples. Volume-weighted average concentrations of the major ionic species present in precipitation samples were in the order of Na+ > Ca2+ > Cl− > SO42− > NH4+ > Mg2+ > NO3− > K+ > F−. Neutralization of the acidic species would take place due to the presence of NH4+ and CaCO3. When considering marine contribution, SO42−/Na+, Ca2+/Na+, and Mg2+/Na+ ratios are higher than the reference value suggesting that contribution of sources be possible other than marine. Among metallic constituents, the concentration of Al is the highest while that of Mn is the lowest. Significant correlation is observed among certain ions and elements (Ca2+, SO42−, Mg2+, Al, Cu, Fe, Zn, and H+) which indicates their source of origin and chemical interaction. This study reveals that chemical composition of rain water depends on both anthropogenic activities and natural phenomena.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The chemical composition of rain water has become a major concern in the cities with high pollution level (Liu et al. 2018). Urbanization is a process which involves economic and industrial development, and consequently, concentration of population into mega cities would be an end result (Singh et al. 2018; Czerwińska and Wielgosiński 2019). In addition, investigation of chemical composition of the atmosphere in a certain area is very important as it would lead to take air pollution control measures (Martins et al. 2019). As dissolution of pollutants would change the chemistry of rainwater, and therefore, determination of the chemical composition of rainwater with respect to pollutants present is an indirect measure of the extent of pollution (Alkhashman 2005; Huang et al. 2008).
Among various sources of atmospheric pollution, the prominent source of aerosols is the dust formed in arid regions, followed by soil and marine erosion, and anthropogenic sources (Romero Orué et al. 2019; Carvalho et al. 2014; Rao et al. 2016; Deusdará et al. 2017; Lin et al. 2019; Ouyang et al. 2019). Wet deposition which occurs through rain events is defined as the process by which atmospheric compounds attached to and dissolved in cloud and precipitation droplets followed by their delivery to the earth’s surface by rain, hail, or snow. Thus, dry deposition is predominantly associated with the transfer of particulate matter, total phosphorous, Ca2+, Mg2+, and K+, whereas wet deposition dominates the atmospheric delivery of Na+ and nitrogen-containing species (Akkoyunlu and Tayanc 2003; Staelens et al. 2005; Cao et al. 2009; Huston et al. 2009).
In coastal areas, the Na+/Cl− ratio and the contents of SO42−, Mg2+, and Ca2+ in the atmosphere are useful in evaluating the influence of both anthropogenic and natural sources (Park et al. 2015; Bhaskar and Rao 2017). Chemical characteristics of rainwater at a Southeastern site of Brazil have identified the presence of Mg2+, K+, Ca+2, and HCO3− in the atmosphere due to soil contribution; Na+ and Cl− to sea-salt contribution; and NO3−, SO4−2, and NH4+ to anthropogenic sources. Some studies conducted in India indicate that atmospheric particulates, which contain significant amounts of basic cations, and rich in metal particulate matter, could affect physicochemical properties and microbial interactions in soil. The metals deposited may enter living body through dietary intake and subsequently cause adverse health effects (Chakraborty et al. 2016; Rao et al. 2016; Bhaskar and Rao 2017; Bisht et al. 2017; Chakraborty and Gupta 2018; Singh et al. 2018). Influence of pollutant sources on rainwater chemistry is investigated at an industrial coastal site in Iran and has revealed that nearly all NO3− and most Ca2+, SO42−, and Mg2+ have been originated from non-marine and local sources (Rastegari et al. 2019). Further, rainwater chemistry, investigated at a semirural site in Ya’an, Sichuan basin in China has revealed that pH values ranged from 3.25 to 6.86, with an annual volume-weighted mean (VWM) of 4.38, with a high acid rain frequency of 74%. Such a severe acidification of the total events has shown pH < 4.0, which was attributed to the deficiency of Ca2+, a basic cation which would have neutralized acidity to some extent. This rainy pattern in this area is contributed by significant anthropogenic pollution. The annual VWM of total ionic concentration was determined to be 477.19 μeq/l, where NH4+ was the most abundant ionic species, followed by SO42−, NO3−, Ca2+, Cl−, Na+, K+, Mg2+, and F− in descending order (Li et al. 2016).
Sri Lanka, being an island, has both positive and negative impacts with regard to atmospheric pollution (Ileperuma 2015). Kelaniya, located close to the busiest and economically strong Western Province of the country, has much intense industrial activities including power plants, oil refineries, and many industrial zones in the vicinity. However, Kelaniya being situated somewhat close to the coast would be beneficial to diffuse some pollutants out.
Few isolated studies have been reported in Sri Lanka with regard to rain water composition. Among them, heavy metals and microorganisms present in atmospheric deposition have been characterized in Kandy, Sri Lanka, together with health implications (Weerasundara et al. 2018). It has been reported that Al and Fe are at uncontaminated levels, and other metals investigated, Cr, Mn, Ni, Cu, Zn, Cd, and Pb, are in the range of uncontaminated to contaminated levels. However, these metals have the potential to increase their levels in the long term (Weerasundara et al. 2017). It has also been implicated that traffic and land use influence atmospheric metal concentrations (Liyandeniya et al. 2020). Further, comparative studies of atmospheric deposition in areas having different geography, industrial activities, and anthropogenic activities are rare in the Sri Lankan context.
Due to anthropogenic activities, the environment has become polluted to an extent of being irreparable with respect to some aspects. Although the total environment cannot be compartmentalized, it has become the practice to investigate pollution aspects of water, air, and soil separately. Among these, water pollution studies and its remedial measures have taken a lead, probably due to ease of following water quality monitoring protocols. On the other hand, atmospheric monitoring and modeling would be of great importance as the general public should be aware of the air they breathe although not much attention has been given in this aspect.
It is important that rain water quality guidelines should be met for rain water to be used in harvesting (Mosley 2005). Nevertheless, rapid industrialization and urbanization of surrounding area lead to air pollution thus changing the chemical composition of rain water, and further, regular rain water quality measurement programs are not much practiced. This project thus aims to fill this void by investigating composition of rain water collected in Kelaniya, Sri Lanka, for selected chemical parameters which will lead to establish baseline data.
Material and methods
Study area
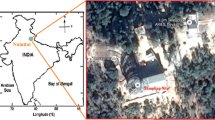
The sampling site is at the University of Kelaniya (GPS coordinates—latitude 6.973701, longitude 79.915256) located in Gampaha District of the Western Province of Sri Lanka (Fig. 1) where high industrialization and urbanization are prevalent; the University of Kelaniya is located by the Kandy-Colombo (A1) highway where high traffic congestion is present in many parts of the daytime. Additionally, the only oil refinery of the country, Bandaranayake International Airport, and a number of thermal power plants are located in close proximity to the sampling site.
Sampling apparatus
Wet deposition samples were collected using a manual collector [high density polyethylene (HDPE) container with a funnel], which was placed 120 cm above the ground level (Fig. 2). Diameter of the funnel was 20.6 cm. Containers and collectors were pre-washed with ASTM type 1 deionized water before being used. Sampling was done after each precipitation event starting from October 2012 to July 2014. Collectors were fully opened to the air during the total collection time period.
The volume of each wet deposition sample collected was measured to determine the rainfall. Prior to reinstallation of sample containers and funnels, they were washed thoroughly with ASTM type I deionized water. Conductivity and pH of wet samples were measured soon after sampling, and thereafter, the samples were filtered using 0.45-μm nylon filters and divided into two parts; one part was stored in pre-cleaned polyethylene bottles and refrigerated at 4 °C prior to ion analysis. The other part was preserved by acidification to bring pH below 2 by adding analytical grade conc. HNO3 (BDH VWR), refrigerated at 4 °C prior to metal analysis and stored in pre-cleaned polyethylene bottles. Acidification is needed to all metallic species to soluble form.
Materials and instrumentation
All chemicals used for sample collection, preservation, and analysis were of analytical grade with certifications. All preparation and final washing were done with freshly prepared deionized water which complied with the standards of ASTM type I water. All standard solutions were prepared using certified standard materials. Trace metal standards (Al, Fe, Mn, Zn, Cu, and Pb) were Sigma-Aldrich, and standards used for ion chromatography were Thermo Dionex. Eluent required for anion determination was prepared by diluting IC grade 0.45 M Na2CO3 and 0.08 M NaHCO3 (Thermo Dionex) while 1.0 M sulfuric acid (Thermo Dionex) was used to regenerate the anionic suppressor. Eluent required for cation determination was prepared by diluting IC grade methanesulfonic acid (Thermo Dionex) while tetrabutylammonium hydroxide solution (TBAOH) (Thermo Dionex) was used to regenerate cationic suppressor.
Rainfall data were measured using Watchdog 2700 automated weather station located at the premises of the University of Kelaniya.
Conductivity and pH were determined using HACH Sens ION+ EC5 portable conductivity meter and Thermo Orion 3 Star pH meter, respectively. Selected cations (Na+, NH4+, K+, Mg+2, Ca+2) and anions (Cl−, NO3−, SO42−) were quantified using Thermo Dionex ICS 900 ion chromatograph with chemical suppression mode. For anion analysis, Dionex ionpack AS23 (4 × 250 mm) analytical column was used, while Dionex ionpack CS12A (4 × 250 mm) column was used for cation analysis. Each column was equipped with a separate guard column for the protection of the analytical column. The detection limits of F−, Cl−, NO3−, and SO42− ions were found to be 1.6, 1.1, 1.0, and 2.0 μeq l−1, respectively, and those of Na+, NH4+, K+, Mg2+, and Ca2+ were 2.2, 2.8, 1.5, 6.5, and 6.0 μeq l−1, respectively.
Trace metals (Al, Fe, Mn, Zn, Cu, and Pb) were determined using a graphite furnace atomic absorption spectrometer (GFAAS) equipped with an autosampler (Thermo electron M5 GF95 Graphite Furnace System) with deuterium background correction. Detection limits for elements were within the range of 0.1–2.0 μg l−1.
Quality of the analysis was achieved by running known standard with the same matrix as analyte (both ions and elements) between 20 sample intervals to check reproducibility of analytical results, and 95% confidence level was maintained for all analytes studied.
Statistical analysis
Seventy-eight (78) samples were collected on a weekly basis throughout the sampling period from the sampling station at the University of Kelaniya, and results were analyzed statistically. Spearman’s correlation coefficient, a statistical measure of the strength of a monotonic relationship between paired data, was also evaluated for the results of the rainwater samples. Interpretation of Spearman’s correlation coefficient is similar to that of Pearson’s.
Results and discussion
Investigation of pH variation of depositions
Figure 3 shows the frequency histogram of pH for samples of atmospheric precipitation collected. Only eight acid precipitation events with pH less than 5.60 were recorded during the entire sampling period from Kelaniya which accounted for 10% of total precipitation events. The average pH was 6.54 and the volume-weighted average (VWA) pH was 5.70 within the pH range between 4.38 and 8.69 at Kelaniya. This pH variation reflects the large number of anthropogenic activities in Kelaniya. The only oil refinery in the country with 500 barrels of crude oil capacity and a number of thermal power plants run on fossil fuel are located in the vicinity of the sampling points. These operations may emit acid rain precursors, such as oxides of sulfur and nitrogen. Most of the acid precipitation events were recorded during February and March where dry weather prevails. During this time period, much of the wind blows from the seaward side which prevents neutralization of pollutants in the atmosphere. On the other hand, neutralization would occur due to the presence of considerable amounts of Ca2+ and NH4+ in the atmosphere, leading to pH values of rain water higher than that would be due to acidic pollutants.
Chemical composition
Table 1 presents VWA values, ranges obtained for pH, conductivity, and inorganic ions, namely F−, Cl−, NO3−, SO42−, Na+, NH4+, K+, Mg2+, and Ca2+ along with chemical elements for a total of seventy-eight (78) samples collected. The VWA value and the range for conductivity were 25.45 μS cm−1 and 4.00–241.23 μS cm−1, respectively. The VWA concentrations of the major ionic species present in precipitation samples were in the order of Na+ > Ca2+ > Cl− > SO42− > NH4+ > Mg2+ > NO3− > K+ > F−. This order could vary with weather and intensity of anthropogenic activities. Nevertheless, Ca2+, Na+, Cl−, NH4+, and SO42− are found to be the dominant ions. Further, Na+ and Cl− are predominant because this sampling station is close to the coast. Fluoride was present in some of the samples collected, and most of the F− were recorded during dry months of January to March. Atmospheric F−, even if present at very low concentrations, is much harmful to vegetation. Principal anthropogenic sources for fluoride include aluminum smelters; industrial activities such as brick, tile, pottery and cement works; ceramic industries; and glass manufacturing. These industries are located in Kelaniya, indicating atmospheric F− pollution in the area. The most abundant cations in the sampling sites are Ca2+ and Na+, which are probably due to dust particles which contain Ca2+ and marine contribution of Na+. Further, the Ca2+ ion plays a major role along with NH4+ in neutralization of acidified rain. More importantly, these ions are mainly found in dry months during November to February where South-East monsoon is not occurred.
Concentrations of elements (Pb, Cu, Al, Zn, Fe, and Mn) determined in rainwater are shown in Table 1. Few rainfall events detected for Pb and these events were recorded in the dry season of the area where less amounts of rain fall were recorded. Low levels of Pb observed could probably be due to the lack of Pb sources in these areas and abandoning the usage of leaded gasoline which had been used many years ago. The element having the highest concentration was Al, followed by Zn, Fe, Cu, and Mn. Many anthropogenic activities can be found in the surrounding areas of the Kelaniya sampling site which may lead to atmospheric elemental constituents other than natural sources. Aluminum and its compounds are mainly originated from natural sources, such as crustal origin, while other elements are originated from anthropogenic activities, such as waste incineration, biomass burning, fossil fuel burning, mass scale civil constructions, and some industries. The elements, Mn and Cu, were moderately enriched compared with mean crustal elements indicating the presence of other sources. Apart from the above-stated contributions, Sri Lanka, being a small island in the middle of the Indian Ocean, trans-boundary pollution would play an important role for elevated levels of the above elements. Through this phenomenon, impact of pollution that originates in one country is able to cause damage in another country’s environment as a result of transportation of pollutants via air or water.
Acidification and neutralization potentials
Acidity of precipitation is mainly controlled by strong acids, such as H2SO4 and HNO3, and to lesser extent by weak organic acids. Neutralization of these species would take place due to the presence of NH4+, MgCO3, and CaCO3. As stated earlier, the pH of precipitation is strongly controlled by the presence of these acids and bases, and neutralization reactions among them. Figure 4 shows the linear regression analysis applied to the variables with acidification and those with neutralization potential. The correlation coefficient is found to be 0.56 for Kelaniya.
Table 2 shows the results of regression coefficient analysis, and approximately 99.0% of SO42− and 42.1% of NO3− were able to be explained by the two independent variables, Ca2+, NH4+, and Mg2+ at Kelaniya. Of 99.0% of SO42− explained, 55.6% appears as CaSO4, 33.6% as MgSO4, and 9.8% as (NH4)2SO4. As for NO3−, it is found as Ca(NO3)2 in 28.6% of the cases and as NH4NO3 in only 10.3% followed by Mg(NO3)2 in only 3.2% of the cases in wet precipitation. Further, neutralization of acidity of precipitation is much more related to CaCO3 than to NH3, and MgCO3. This is further supported by high content of Ca2+ ions in both locations. Sources of NH3 are mainly the application of fertilizers and biomass burning.
Marine contribution
Table 3 shows the ratio between the ions and the Na+ analyzed in the rain water samples, and the ratio of the ions and Na+ in sea water. Ratio of the respective ion and Na+ is higher than that of the ratio of the same two ions for sea water indicating negligible marine contribution. When considering the ratio between SO42−/Na+, Ca2+/Na+, K+/Na+, and Mg2+/Na+ in rain water samples collected in Kelaniya, values obtained for SO42−/Na+, Ca2+/Na+, and Mg2+/Na+ being higher than the value obtained for sea water in that location indicate that the marine contribution of those ions are negligible. However, the ratios of Cl−/Na+ and K+/Na+ are 0.39 and 0.10, respectively, which are less than the sea water ratio indicating the significant marine contribution of those elements. The marine contribution of Kelaniya is much stronger as a result of close proximity of the location to the cost.
Correlation between analytes and probable sources
In order to find a possible relationship among ions and elements in rain water samples collected as wet deposition samples from Kelaniya, and to predict probable sources of pollutants, correlation between ions in the rain water was calculated based on statistical analysis. According to the results of Spearman’s correlation coefficient shown in Table 4, a very strong correlation between conductivity and Na+ (0.881) observed is indicative of a positive correlation between the two parameters. It is a clear indication of high conductivity of samples collected from Kelaniya contributed mainly from Na+ ions. Further, positive correlations among conductivity and pH (0.686), Ca2+ and pH (0.439), and Mg2+ and pH (0.559) observed provide a clear indication of increasing pH with the presence of some neutralization ions, such as Ca2+ and Mg2+. According to Table 4, significant positive correlations among conductivity and ions can be observed, probably due to the fact that the presence of these ions increases the conductivity of rain water. A moderate positive correlation of 0.654 determined between SO42− and NO3− can also be explained using a similar argument that these two species come from the same source probably vehicular emission. A significant correlation between Na+ and Ca2+/Mg2+ also indicates that natural contribution of these ions. The same argument can be made for the correlation between Na+ and Cl−, that the source is marine. There were significant correlations between acidic anions (SO42−) and cations (Ca2+/Mg2+) indicating that interaction between this acidic anion and cations which would lead to neutralization. Moderate positive correlation observed between Na+ and Fe is indicative of increase in corrosion in the presence of Na+ which enhances the corrosion of steel which is widely used during construction activities. The elements, Fe, Al, Cu, and Zn, which show weak to moderate positive correlations, would have been originated from natural sources, such as crustal erosion, and from anthropogenic activities, such as brass industry, construction activities, and vehicular emissions. Among these, Fe shows moderate correlation between Cu, Al, Zn, and Mn, which are widely used in alloy manufacturing to enhance different properties. It should also be considered that many industries in Sri Lanka are located in the Districts of Gampaha and Colombo, close to the Kelaniya sampling site.
Conclusion
This study characterizes certain ions and selected elements for a period of 16 months starting from 2012 to 2014 in precipitation at Kelaniya in Gampaha District, Sri Lanka. Analysis of samples harvested soon after each precipitation event indicates that the volume-weighted average (VWA) concentrations of the major ionic species present in precipitation samples were in the order of Na+ > Ca2+ > Cl− > SO42− > NH4+ > Mg2+ > NO3− > K+ > F−. The pH of precipitation samples was ranged between 4.38 and 8.69 with less than 10% events of acidic precipitation in which pH < 5.60. In the wet precipitation in Kelaniya, approximately 68.4% of SO42− and 38.9% of NO3− were able to be explained by the two independent variables, Ca2+ and NH4+. The ratios of SO42−/Na+, Ca2+/Na+, and Mg2+/Na+ were higher than the reference values suggesting that marine contribution be negligible and contribution of anthropogenic sources be predominant for these ions. The ratios of Cl−/Na+ and K+/Na+ were less than the reference values indicating marine influence of Cl− and K+ ions. It was also found that Al is the most predominant element while Mn was the least. Investigation of correlation between major ionic species and elements (Ca2+, SO42−, Mg2+, Al, Cu, Fe, Zn, and H+) in the wet precipitation samples identified probable anthropogenic sources, heavy traffic, oil refinery activities, thermal power plant activities, biomass burning, waste incineration, and industrial activities. This study reveals that influencing human activities on chemical composition of rain water depends on both anthropogenic activities and natural phenomena.
References
Akkoyunlu BO, Tayanc M (2003) Analyses of wet and bulk deposition in four different regions of Istanbul, Turkey. Atmos Environ 37(25):3571–3579
Alkhashman O (2005) Study of chemical composition in wet atmospheric precipitation in Eshidiya area, Jordan. Atmos Environ 39(33):6175–6183
Bhaskar VV, Rao PSP (2017) Annual and decadal variation in chemical composition of rain water at all the ten GAW stations in India. J Atmos Chem 74(1):23–53
Bisht DS et al (2017) Chemical characterization of rainwater at a high-altitude site “Nainital” in the central Himalayas, India. Environ Sci Pollut Res 24(4):3959–3969
Cao YZ et al (2009) Chemical characteristics of wet precipitation at an urban site of Guangzhou, South China. Atmos Res 94(3):462–469
Carvalho SCP, de Lima JLMP, de Lima M I P (2014) Rainwater sequential sampler: assessing intra-event water composition variability. J Engi Res Technol 1(1): 1–7.
Chakraborty B, Gupta A (2018) Study and interpretation of chemical composition of rainwater in selected urban and rural locations in India using multivariate analysis. AIP Conference Proceedings, 1952.
Chakraborty A, Gupta T, Tripathi SN (2016) Chemical composition and characteristics of ambient aerosols and rainwater residues during Indian summer monsoon: insight from aerosol mass spectrometry. Atmos Environ 136:144–155
Czerwińska J, Wielgosiński G (2019) Changes in the pollution of Lodz voivodship rainwater as a result of changes in pollutant immissions. Acta Innov 30:31–37
Deusdará KRL et al (2017) Rainwater chemistry and bulk atmospheric deposition in a tropical semiarid ecosystem: the Brazilian Caatinga. J Atmos Chem 74(1):71–85
Huang K et al (2008) The chemistry of the severe acidic precipitation in Shanghai. China Atmos Res 89(1–2):149–160
Huston R et al (2009) Characterisation of atmospheric deposition as a source of contaminants in urban rainwater tanks. Water Res 43(6):1630–1640
Ileperuma O (2015) Model assessment of acid deposition potential by SOx in Sri Lanka. J Nat Sci Found of Sri Lanka 43(3):281
Li YC et al (2016) Chemical characteristics of rainwater in Sichuan basin, a case study of Ya’an. Environ Sci Pollut Res 23(13):13088–13099
Lin X et al (2019) Rainwater in Guangzhou, China: oxidizing properties and physicochemical characteristics. Atmos Pollut Res 10(1):303–312
Liu P et al (2018) Assessment of heavy metal characteristics and health risks associated with PM 2.5 in Xi’an, the largest city in northwestern China. Air Qual Atmos Hlth 11(9):1037–1047. https://doi.org/10.1007/s11869-018-0598-9
Liyandeniya AB, Deeyamulla MP, Priyantha N. (2020) Atmospheric chemical composition of bulk deposition at two geographically distinct locations in Sri Lanka, Environ Monit Asses. 192(7). doi: 10.1007/s10661-020-08412-0.
Martins EH et al (2019) Chemical composition of rainwater in an urban area of the southeast of Brazil. Atmos Pollut Res 10(2):520–530
Mosley L (2005) Water quality of rainwater, SOPAC Miscellaneous Reports, (February) :1–19.
Ouyang W et al (2019) Rainwater characteristics and interaction with atmospheric particle matter transportation analyzed by remote sensing around Beijing. Sci Total Environ 651:532–540
Park SM et al (2015) Chemical composition of water soluble inorganic species in precipitation at Shihwa Basin, Korea. Atmosphere 6(6):732–750
Rao PSP et al (2016) Sources of chemical species in rainwater during monsoon and non-monsoonal periods over two mega cities in India and dominant source region of secondary aerosols. Atmos Environ 146:90–99
Rastegari MM, Keshavarzi B, Sorooshian A (2019) Influence of natural and urban emissions on rainwater chemistry at a southwestern Iran coastal site. Sci Total Environ 668:1213–1221
Romero Orué M, Gaiero D, Kirschbaum A (2019) Seasonal characteristics of the chemical composition of rainwaters from Salta city, NW Argentina. Environ Earth Sci 78(1)
Singh SP et al (2018) Cations chemistry characterization of rainwater of the Varanasi district in the indo-gangetic plains India. Inter J Chem Studies 6(1):1380–1384
Staelens J et al (2005) A comparison of bulk and wet-only deposition at two adjacent sites in Melle (Belgium). Atmos Environ 39(1):7–15
Weerasundara L et al (2017) Microorganisms and heavy metals associated with atmospheric deposition in a congested urban environment of a developing country: Sri Lanka. Sci Total Environ 584–585:803–812
Weerasundara L, Magana-Arachchi DN, Ziyath AM, Goonetilleke A, Vithanage M (2018) Health risk assessment of heavy metals in atmospheric deposition in a congested city environment in a developing country: Kandy City, Sri Lanka. J Environ Manag 220:198–206
Funding
This study was financially supported by the National Research Council of Sri Lanka (Grant No. NRC/11/127).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liyandeniya, A.B., Deeyamulla, M.P. & Priyantha, N. Source apportionment of rainwater chemical composition in wet precipitation at Kelaniya in Sri Lanka. Air Qual Atmos Health 13, 1497–1504 (2020). https://doi.org/10.1007/s11869-020-00903-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-020-00903-w