Abstract
This study presented the research work carried out for the investigation of chemical composition of bulk precipitation in two geographically and economically distinct areas, namely Gampaha and Kandy Districts. This study was conducted from 2013 to 2014 at three sampling stations in each District. The bulk precipitation was analyzed for pH, conductivity, Na+, NH4+, K+, Mg2+, Ca2+, F−, Cl−, NO3−, SO42−, Pb, Cu, Mn, Al, Zn and Fe for a total of 375 samples. Only 8% events of acidic precipitation were recorded in Gampaha District when compared with 3% in Kandy District. The volume weighted average concentrations of the major ionic species present in precipitation samples were in the order of Na+ > Ca2+ > Cl− > NH4+ > SO42− > Mg2+ > NO3− > K+ > F− in the Gampaha stations, while the order was NH4+ > Ca2+ > Na+ > Cl− > SO42− > Mg2+ > NO3− > K+ > F− in Kandy District. Neutralization of acidity of precipitation is much more related to CaCO3 than NH3, and high content of Ca2+ ions present in both Districts strongly supports this fact. When considering marine contribution, SO42−/Na+, Ca2+/Na+ and Mg2+/Na+ ratios are higher than the reference value suggesting contribution of sources other than marine. Depositions of both Districts show that the concentration of Al is the highest while that of Mn is the lowest. Principal component analysis suggests that influencing human activities on chemical composition of rain water depends on thermal power plant, oil refinery, heavy traffic and waste incineration in the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric precipitation is probably the most effective scavenging factor of removing various inorganic and organic pollutants, including gaseous species and particulate matter, from the atmosphere (Alkhashman 2005; Migliavacca et al. 2005; Huang et al. 2008a, b; Carvalho et al. 2014; Vieira-Filho et al. 2015; Rao et al. 2016; Deusdará et al. 2017). Rapid development of industrial and agricultural sectors consumes large quantities of fossil fuels and synthetic fertilizers, releasing various pollutants to the atmosphere (Yu et al. 2017). Dissolution of these pollutants would subsequently change the chemistry of rainwater, and therefore, the determination of the chemical composition of pollutants present in rainwater is an indirect measure of the extent of pollution (Alkhashman 2005; Huang et al. 2008a, b). It can also be used to predict current and future distribution of atmospheric pollutants. In this context, monitoring rainwater quality has become vital (Granat et al. 1996).
Chemical composition of rainwater varies depending on geographical location of the sampling site owing to consequences of different pollution sources (Jain et al. 2019; Szép et al. 2019). Due to the local emission followed by long-range transportation of atmospheric pollutants, known as trans-boundary pollution, air pollution has become a severe regional atmospheric environmental problem (Pu et al. 2017). As air pollutants can be transported over long distances from the sources of their origin, they can be transported to Sri Lanka from neighboring countries, where levels of air pollution are high (Ileperuma 2015; Park et al. 2015). Study of chemistry of rainwater has been focused on in many countries as a measure of atmospheric pollution (Akkoyunlu 2003; Croisé et al. 2005; Staelens et al. 2005; Herrera et al. 2009; Paternoster et al. 2014; Yu et al. 2017). One study in China reveals that acid precipitation (pH < 5.6) has affected 30% of the country resulting in serious consequences in economically developed regions in the East, the South, and central parts of China (Zeng and Hopke 1989). Another study has shown that the annual mean pH of rain was 4.49 with the frequency of acid rain occurrence of 71% in 2005 in Shanghai, China (Huang et al. 2008a, b). A different study in Guangzhou in China shows acidic pH values as low as 3.52 with volume-weighted mean equivalent concentration of components in the order of SO42− > Ca+2 > Cl− > NH4+ > Na+ > NO3− > K+ > Mg2+ > F− (Huang et al. 2008a, b). A recent study on characteristics of ions in rainfall and PM2.5 warns that Beijing has been heavily affected by mobile sources and anthropogenic pollution. However, PM2.5 can be most effectively removed from the atmosphere by rainfall (Gao et al. 2019). A study conducted in Mahshahr, Iran, through comparison of species concentration ratios relative to those for pure seawater and earth’s crust reveals that nearly all NO3− and most Ca2+, SO42− and Mg2+ have been originated from non-marine and local sources. Compared with other areas around the world, some heavy metals (particularly Zn) have displayed higher concentrations in rainwater (Rastegari Mehr et al. 2019).
Geographically, Sri Lanka is situated very close to Indian mainland, and thus, atmospheric air quality of India could greatly influence the Sri Lankan air quality. One study revealed that the mean pH of rain water was 6.00 ranging from 5.05 to 7.57 in Hyderabad in India (Venkateshwarlu and Satyanarayana 2014). The same study reveals that the order of chemical composition follows the order of Ca2+ > SO4− > NH4+ > NO3− > Na+ > Cl− > HCO3− > F− > Mg2+ > Br− >K+. A study conducted in a coastal location in the eastern part of India elaborates that oceanic and continental sources supply Mg and Sr to the site, whereas Ca and Ba are pre-dominantly supplied through continental sources (Tripathy et al. 2019).
In contrast to global direction towards study of chemical composition of rainwater, only few studies have been reported in Sri Lanka (Dissanayake and Weerasooriya 1985; Aberatne and Ileperuma 2006; Ileperuma 2015; Tennakoon et al. 2006). Some of these studies revealed of occurrence of acid rain during some precipitation events. One study shows that the mean monthly pH of rainwater was in the range from 5.67 to 6.35 (Tennakoon et al. 2006). Another study shows that mean rainwater pH values of 5.15 and 5.60 have been determined for the Wet and Dry Zones of Sri Lanka, respectively (Dissanayake and Weerasooriya 1985). With the expansion of economic activities in Sri Lanka, particularly urbanization and industrialization, levels of atmospheric pollution would be on the rise. An alarming situation with regard to the deterioration of atmospheric quality in Sri Lanka is the fact that the number of automobiles has been doubled over the decade of 1992–2012 (Ileperuma 2015). Further, the establishment of 900 MW coal power plant at Norochcholei as well as diesel thermal power plants near Colombo has led to increase in fossil fuel burning releasing sulfur dioxide and other pollutants (Ileperuma 2015). Having identified the gap in the existing findings with regard to atmospheric pollution, the purpose of this study was to determine the chemical composition of bulk precipitation in six sampling locations representing Kandy and Gampaha Districts in Sri Lanka, and to identify possible sources of such constituents present in rainwater, which contribute to atmospheric pollution.
Materials and methods
Study area
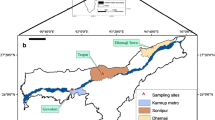
Six sampling sites were located in Gampaha and Kandy Districts of Sri Lanka where tropical climate is prevalent (Fig. 1). High rainfall occurs in both Districts by monsoon, and much rainfall is received during the South-Western monsoon period from May to September. Gampaha District is located in the Western Province where three sampling stations, namely University of Kelaniya, Sapugaskanda and Sedawaththa, were set-up. High industrialization and urbanization are prevalent in these three sampling areas; University of Kelaniya is located by the Kandy-Colombo (A1) highway where high traffic congestion is present in many parts of the daytime. The area of the study in Sapugaskanda sampling site shows more anthropogenic activities. The only oil refinery in the country is operated in close proximity. Additionally, Sapugaskanda power station and many industries have been established nearby. On the other hand, Kelanithissa power station is situated near the Sedawaththa sampling site, very close to the municipal boundary of Colombo, the Sri Lankan Capital.
The three remaining sampling sites, namely University of Peradeniya, Kandy and Polgolla, were set-up in Kandy District of the Central Province of Sri Lanka. The mean altitude of these locations is 400 m above sea level and covered by mountains. This area of study shows limited anthropogenic activities. The sampling point of the University of Peradeniya is located about 1 km from the Kandy-Colombo (A1) highway where high traffic congestion is present in many parts of the daytime. Kandy city is located in a basin surrounded by mountains and it is in the vicinity to vehicular sources due to high traffic congestion present during peak hours of daytime. The Polgolla site is located in the banks of Polgolloa dam. This site is located approximately 7 km from Kandy, and neither industries nor heavy traffic is encountered in the vicinity.
Sampling, sample preservation and analysis
Collectors (Polyethylene container and funnel) were placed 120 cm above the ground level (Fig. 2). Diameter of the funnel was 20.6 cm. Containers and the collectors were pre-washed with ASTM Type 1 deionized water before placing in the sampler. Sampling was done on weekly basis starting from May 2013 to November 2014 at six different sampling locations (Migliavacca et al. 2005; Uchiyama et al. 2017).
After measuring the volume of each sample, they were filtered using 0.45-μm nylon filters and divided into two parts; one part was stored in pre-cleaned polyethylene bottles and refrigerated at 4 °C for analysis of anions and common cations (Rao et al. 2016). The other part was stored in pre-cleaned polyethylene bottles followed by acidification to about pH < 2 by adding analytical grade HNO3 (BDH VWR) and refrigerated at 4 °C for trace metal analysis. Conductivity and pH of rainwater samples were measured soon after sampling using Sens ION+ EC5 portable conductivity meter and Orion 3 Star pH meter, respectively.
Selected common cations (Na+, NH4+, K+, Mg2+, Ca2+) and anions (Cl−, NO3−, SO42−) were quantified using Thermo Dionex ICS 900 ion chromatograph with chemical suppression mode. For anion analysis, Dionex ionpack AS23 (4 × 250 mm) column was used, while Dionex ionpack CS12A (4 × 250 mm) column was used for cation analysis. The detection limits of F−, Cl−, NO3− and SO42− ions were found to be 1.6, 1.1, 1.0 and 2.0 μeq l−1, respectively, and those of Na+, NH4+, K+, Mg2+ and Ca2+ were 2.2, 2.8, 1.5, 6.5 and 6.0 μeq l−1, respectively.
Trace metals (Al, Fe, Mn, Zn, Cu, Pb) were determined using Graphite Furnace Atomic Absorption Spectrometer (GFAAS) (Thermo electron M5 GF95 Graphite Furnace System) with deuterium background correction. Detection limits for elements were within the range of 0.1–2.0 μg l−1.
Quality of the analytical procedure was maintained using analytical grade chemicals. All chemicals used for samples collection, preservation, and analysis were analytical grade with certifications. All preparation and final washing done with freshly prepared deionized water were qualified to be ASTM Type I water. Standards were prepared using certified standard materials. Trace metals standards (Al, Fe, Mn, Zn, Cu, Pb) were Sigma-Aldrich and standards used for ion chromatography were Thermo Dionex. Anionic eluent required for anion determination was prepared by diluting IC grade 0.45 M Na2CO3 and 0.08 M NaHCO3 (Thermo Dionex) while 1.0 M H2SO4 (Thermo Dionex) was used to regenerate anionic suppressor. Cationic eluent required for anion determination was prepared by diluting IC grade methanesulfonic acid (Thermo Dionex) while tetrabutylammonium hydroxide solution (Thermo Dionex) was used to regenerate cationic suppressor.
Quality of the analysis was achieved by running known standard with the same matrix as analyte (both ions and elements) between 20 sample-intervals to check reproducibility of analytical results. Recovery of the check standard was determined to be within 97–105% range.
Weather data were measured using Watchdog 2700 automated weather stations located at two stations.
Statistical analysis
Spearman’s correlation coefficient determined in this research is a statistical measure of the strength of a monotonic relationship between paired data, and its interpretation is similar to that of Pearsons. Further, the principal component analysis (PCA) was applied to the variables chosen from the Spearman correlation coefficient. The PCA was applied to the determination of the sources of respective pollutants whether anthropogenic or natural. Minitab 16 statistical software was used for statistical treatment of data.
Results and discussion
Rainfall data
Figure 3 shows rainfall of both sites; Gampaha and Kandy determined at 25 °C using the bulk volume collected. Both locations received rain during South-Western monsoon that prevails during May to September, and moderate drought season prevails in the months from October to March. During April, the first inter-monsoon rain prevails, and rain is received during this time period for both locations. During the period of study, Kandy received comparatively less amount of rain during the South-West monsoon. This might have increased dry particulate matter in the atmosphere, and deposited with rain as well as with dry deposition.
Investigation of pH variation of depositions
Figure 4 shows the frequency histogram of pH for samples of atmospheric precipitation in Gampaha and Kandy Districts as average values of the three sites in each District. The pH of all precipitation samples was determined at 25 °C in triplicate. Few acid precipitation events were recorded in Gampaha District as compared with Kandy sampling points. Detailed analysis of histograms indicates that only 8% events of acidic precipitation in which pH less than 5.60 were recorded in the Gampaha sampling stations when compared with 3% of the Kandy stations. Both stations had more than 40% precipitation events having pH values between 6.00 and 7.00. The average pH for the Gampaha sites was 6.52, while that of the Kandy stations was 6.62. The overall volume weighted average (VWA) pH values for the two types of sampling stations were 5.49 and 5.64 for Gampaha and Kandy stations, respectively. Further, the pH range was between 4.69 and 8.50 for the Gampaha stations and between 5.14 and 7.38 for the Kandy stations. It should be stated that individual sampling stations showed no significant variation in pH values. The most acidic pH values were recorded in Sedawaththa and Sapugaskanda stations. On the contrary, only two acid precipitation events were recorded in Kandy District at the sampling station located in the Kandy City Center (KCC). This would probably be due to heavy traffic congestion and emission of NOx and SOx which are considered as precursors for atmospheric HNO3 and H2SO4.
Chemical composition
Table 1 presents VWA values, ranges obtained for pH, conductivity, inorganic ions including F−, Cl−, NO3−, SO42−, Na+, NH4+, K+, Mg2+ and Ca2+ for a total of 375 precipitation samples collected at six sampling stations in two locations. The VWA concentrations of the major ionic species present in precipitation samples were in the order of Na+ > Ca2+ > Cl− > NH4+ > SO42− > Mg2+ > NO3− > K+ > F− in the Gampaha stations, while the order was NH4+ > Ca2+ > Na+ > Cl− > SO42− > Mg2+ > NO3− > K+ > F− in the Kandy stations. Although there is a difference in the order of ions, Na+, Ca2+, Cl−, NH4+ and SO42− are found to be the dominant ions in both Districts. Similar observation has been made in India in 2017 (Bhaskar and Rao 2017; Bisht et al. 2017). It has also been reported that marine contribution was indicated by the presence of Na+ and Cl− (Li et al. 2016). The most abundant cation is Na+ in Gampaha due to the fact that the sampling sites are somewhat near the cost, where sea salt plays a role for abundant presence of not only Na+ but also Cl−. On the other hand, the most abundant cation in the sampling sites in Kandy District is NH4+, which is probably be due to agricultural activities including fertilizer application for tea cultivation as well as biomass burning in surrounding areas (Migliavacca et al. 2005; Martins et al. 2019). Other abundant cations present in the precipitation were Ca2+ and Mg2+, both of which were frequently found in dust particles present in the atmosphere. These two ions play a major role in neutralization of acidified rain. More importantly, these ions are mainly found in dry months during November to February where South-East monsoon is not occurred.
Acidification and neutralization potentials
Acidity of precipitation is mainly controlled by strong acids, such as H2SO4 and HNO3, and to lesser extent by organic acids. Neutralization of these species would take place due to the presence of NH4+ and CaCO3. Figure 5 shows the analysis of linear regression applied to the variables relevant to acidification and neutralization potential separately for the two regions, Gampaha and Kandy. The correlation coefficient in the samples of Gampaha is 0.39, which is higher than that of Kandy (0.20).
Table 2 shows the results of regression coefficient analysis for the two Districts. In the bulk precipitation of Gampaha District, approximately 44.2% of SO42− and 18.8% of NO3− were able to be explained by the two independent variables, Ca2+ and NH4+. Of 44.2% of SO42− explained, 37.7% appears as CaSO4 and only 6.5% as (NH4)2SO4. In the bulk precipitation of Kandy District, 26.5% of SO42− and 13.6% of NO3− were explained by the two independent variables, and out of this total,16.8% was found as CaSO4 and 9.7% as (NH4)2SO4. As for NO3−, it is found as Ca(NO3)2 in 11.7% and as NH4NO3 in only 1.9% of the cases in the bulk precipitation. Neutralization of acidity of precipitation is much more related to CaCO3 than to NH3, and high content of Ca2+ ions in both districts strongly supports these results.
Marine contribution
Ratio between the ion analyzed and Na+ is higher than the ratio of the two ions for sea water indicating negligible marine contribution. Both Districts show similar trends; the behavior is the same when considering the ratios for SO42−/Na+, Ca2+/Na+ and Mg2+/Na+ being higher than the reference values suggesting that the marine contribution is negligible and contribution of anthropogenic sources is high for these ions (Table 3). Furthermore, the ratios for Cl−/Na+ and K+/Na+ for both Districts being less than those of the reference values indicate strong marine influence for these ions. However, the higher ratio of Ca2+/Na+ for Kandy can be attributed to the contribution from dust material containing CaCO3 in addition to other anthropogenic activities. The presence of CaCO3 in the atmosphere is contributed by many dolomite quarries established in surrounding areas of the Kandy sampling station. This fact is further supported by higher loading of Ca2+ at a VWA of 88 μeq−l observed in the Polgolla sampling station. This station is located in close proximity to dolomite quarries when compared with other two sampling stations in Kandy District.
Quantification of chemical elements
Concentration of elements (Pb, Cu, Al, Zn, Fe and Mn) determined in rainwater, as shown in Table 4, indicates that almost all stations have Pb at concentrations lower than the MDL. This is because of lack of Pb sources in these areas and abandoning the usage of leaded gasoline which had been used many years ago. The concentrations of almost all ions except for Zn were higher in Gampaha. This is contributed by industrial activities and steel works factories located near the Sedawaththa and Sapugaskanda sampling stations. These industries contribute to loading of Cu and Fe as well. On the other hand, Al is originated mainly from earth crust with dust particles.
Correlation between analytes and probable sources
Spearman correlation coefficient was used at 95% confidence interval, for statistical analysis of the concentration values of major ions and chemical elements in precipitation samples and to understand their relationship among themselves as shown in Tables 5 and 6. The spearman correlation matrix was then used to input in PCA to predict possible sources of these analytes in the atmosphere. Strong correlation observed between Cl− and Na+ (0.961 for Gampaha and 0.631 for Kandy) is indicative that both these ions have been originated from same source. Moderate correlation observed between Ca2+ and Mg2+ (0.528 in Gampaha and 0.744 in Kandy) also suggests that contribution for these two ions is from the same (Mimura et al. 2016). The same argument holds for SO42− and NO3− coming from the same source because they also show moderate positive correlation (0.608 in Gampaha and 0.597 in Kandy). These acid precursors probably originated from fossil fuel burning and oil refining activities are supported by the fact that, in Kandy District, heavy traffic is observed in Kandy city and Peradeniya. The elements, Fe, Al, Cu and Zn, which show weak positive correlation, would have been originated from natural sources, such as crustal erosion, and from anthropogenic activities, such as brass industry and steel works.
These correlated species are then input to the PCA analysis summarized in Tables 7 and 8. The resulting principal components were selected such that eigenvalues > 1.0. Table 7 shows the results of PCA for major ions of bulk precipitation samples for Gamapaha and Kandy. In Gampaha District, 66.4% of variation can be explained by total variation from the first three PCs where the eigenvalue is greater than 1.0. Similar variation of 66.5% in Kandy District can also be explained by the first 3 PCs.
In Gampaha District, PC1 with 39.1 variation of the total variation showed a higher loading of SO42− and Ca2+ characterized by contribution from both natural and anthropogenic activities. Loading of SO42− is mainly due to the oil refinery and the Kelanithissa and Lakdanavi Thermal Power Stations located near the Sapugaskanda and Sedawaththa sampling stations. Loading of Ca2+ is probably originated from natural sources, such as soil dust and less commonly from construction additives. The second factor PC2 explains 15% variation from the total variation showing higher loading for NH4+ which indicates waste incineration nearby Orugodawaththa open waste dumping site and emission from combustion of fossil fuel. The third factor PC3 explains only 12% of the total variation and shows higher loading of Cl− and Mg2+ both of which are from natural sources, such as sea salts and soil dust.
In Kandy District, although industrial activities are less, heavy traffic, waste incineration, biomass burning and natural soil composition would lead to different loading of the above ions. PC1 which explains 42% of the total variation shows higher loading of SO42− and Mg2+ where SO42− loading is mainly due to anthropogenic activities, such as emission of SOx from the combustion of fossil fuel. These gases are oxidized forming H2SO4 and dissolved in rain water. Heavy traffic may lead to higher loading of these gaseous pollutants into the air. During day time, especially in morning hours, after school hours and evening, heavy traffic can be seen in Kandy city, Peradeniya, and Polgolla sub-urban areas where three sampling stations were located. On the other hand, Mg2+ is mainly from natural sources where high amounts of Mg-bearing minerals and excavation of such minerals for construction industries are encountered in surrounding areas. The second factor PC2 explains 12% of the total variation and shows higher loading of NH4+, probably due to fertilizer application and frequent biomass burning in dry season in forest areas. Waste incineration may also lead to higher loading of NH4+. The third factor explains 11% of the total variation and shows higher loading for H+ and K+. These ions are mainly originated from anthropogenic sources, such as combustion of fossil fuel burning and biomass burning. Table 8 shows the summarized results of the PCA analysis of both Districts, and the variables selected were those with a significant correlation. In Gampaha District, 54% variation can be explained from the total variation by two PCs with eigenvalues > 1. The first factor explains 33.5% variation with significant loading of Cu, Al and Fe, where Cu and Fe would be originated form steel works and related industries which can be found in surrounding areas. Being a major element, Al is mainly associated with natural sources which can be found in earth crust as alumino silicates. The second factor explains 29.1% which shows higher loading for H+ and Zn coming from anthropogenic sources. In particular, Zn loading is mainly due to burning of waste in nearby dumping sites and to lesser extent by vehicular emission. Zinc is widely used in the manufacture of many products, including paints, rubber, cosmetics, pharmaceuticals, plastics, batteries, textiles, and electrical equipment. Some of these industries located in nearby areas would thus release Zn to the environment.
In Kandy District, variation explained is 63% of the total variation. Even though lack of major industrial activities and power plant emissions, waste incineration and biomass are the major sources of elemental emission to the atmosphere. First component explains 26.3% variation with higher loading of Cu and Mn emission of which Cu emission is due to anthropogenic activities, especially traditional brassware industries located in the close proximity. Furthermore, Mn can be emitted mainly due to natural factors and to lesser extent by anthropogenic sources. The second component explains 19.2% variation with higher loading of Al, probably due to natural sources, such as soil dust. On the other hand, Fe is probably due to construction materials, such as cast iron, and corrosion of such materials. The third factor explains 1% of the total variation and shows higher loading of H+ from fossil fuel burning which releases acid precursors to the atmosphere.
Conclusion
This study characterizes certain ions and selected elements for a period of 18 months starting from 2013 to 2015 in precipitation from six sampling stations covering two geographically distinct locations of Sri Lanka, namely Gampaha and Kandy Districts. Samples were harvested in 1-week intervals for a total of 375 samples. The volume weighted average (VWA) concentrations of the major ionic species present in precipitation samples were in the order of Na+ > Ca2+ > Cl− > NH4+ > SO42− > Mg2+ > NO3− > K+ > F− in the Gampaha sites, while the order is NH4+ > Ca2+ > Na+ > Cl− > SO42− > Mg2+ > NO3− > K+ > F− in Kandy District. Only 8% events of acidic precipitation in which pH was less than 5.60 were recorded in the Gampaha stations when compared with 3% of the Kandy stations. Both stations had more than 40% precipitation events having pH values between 6.00 and 7.00.
In the bulk precipitation in Gampaha District, approximately 44.2% of SO42− and 18.8% of NO3−, and in Kandy District, the corresponding values of 26.5% and 13.6% can be explained by two independent variables, Ca2+ and NH4+. Furthermore, neutralization of acidity of precipitation is much more related to CaCO3 than NH3, and higher content of Ca2+ ions in both Districts strongly supports this result. The ratios, SO42−/Na+, Ca2+/Na+ and Mg2+/Na+, are higher than the reference values suggesting that marine contribution is negligible and contribution of anthropogenic sources is predominant for these ions while the ratios of Cl−/Na+ and K+/Na+ are less than the reference values indicating strong marine influence of these ions. It is also found that Al is the most predominant element in precipitations of both Districts, while Mn is the least. Further, the concentrations of almost all ions except for Zn were higher in Gampaha stations. Investigation of correlation between major ionic species and elements (NH4+, SO42−, K+, Cu, Fe, Zn and H+) in the bulk precipitation samples identified probable anthropogenic sources, such as thermal power plant (Kelanithissa-Gampaha), oil refinery (Sapugaskanda-Gampaha), steel works (Sedawaththa and Sapugaskanda), waste dumping site (Meethtotamulla-Gampaha, Guhagoda-Kandy) and heavy traffic in both locations. This study reveals that influencing human activities on chemical composition of rain water depends on thermal power plant, oil refinery, heavy traffic and waste incineration in the study area.
References
Aberatne, V. D. & Ileperuma, O. (2006). 3644-12881-1-PB.pdf. In National Science Foundation Sri Lanka (Vol. 34, Issue 3, pp. 137–141).
Akkoyunlu, B. (2003). Analyses of wet and bulk deposition in four different regions of Istanbul, Turkey. Atmospheric Environment, 37(25), 3571–3579. https://doi.org/10.1016/S1352-2310(03)00349-2.
Alkhashman, O. (2005). Study of chemical composition in wet atmospheric precipitation in Eshidiya area, Jordan. Atmospheric Environment, 39(33), 6175–6183. https://doi.org/10.1016/j.atmosenv.2005.06.056.
Bhaskar, V. V., & Rao, P. S. P. (2017). Annual and decadal variation in chemical composition of rain water at all the ten GAW stations in India. Journal of Atmospheric Chemistry, 74(1), 23–53. https://doi.org/10.1007/s10874-016-9339-3.
Bisht, D. S., Srivastava, A. K., Joshi, H., Ram, K., Singh, N., Naja, M., Srivastava, M. K., & Tiwari, S. (2017). Chemical characterization of rainwater at a high-altitude site “Nainital” in the Central Himalayas, India. Environmental Science and Pollution Research, 24(4), 3959–3969. https://doi.org/10.1007/s11356-016-8093-z.
Carvalho, S. C. P., de Lima, J. L. M. P., & de Lima, M. I. P. (2014). Rainwater sequential sampler: assessing intra-event water composition variability. Journal of Engineering Research and Technology, 1(1), 1–7 http://journal.iugaza.edu.ps/index.php/JERT/article/view/26.
Croisé, L., Ulrich, E., Duplat, P., & Jaquet, O. (2005). Two independent methods for mapping bulk deposition in France. Atmospheric Environment, 39(21), 3923–3941. https://doi.org/10.1016/j.atmosenv.2005.03.021.
Deusdará, K. R. L., Forti, M. C., Borma, L. S., Menezes, R. S. C., Lima, J. R. S., & Ometto, J. P. H. B. (2017). Rainwater chemistry and bulk atmospheric deposition in a tropical semiarid ecosystem: the Brazilian Caatinga. Journal of Atmospheric Chemistry, 74(1), 71–85. https://doi.org/10.1007/s10874-016-9341-9.
Dissanayake, C. B., & Weerasooriya, S. V. R. (1985). The environmental chemistry of rainwater in Sri Lanka. International Journal of Environmental Studies, 26(1–2), 71–86. https://doi.org/10.1080/00207238508710245.
Gao, B., Ouyang, W., Cheng, H., Xu, Y., Lin, C., & Chen, J. (2019). Interactions between rainfall and fine particulate matter investigated by simultaneous chemical composition measurements in downtown Beijing. Atmospheric Environment, 218(September), 1–9. https://doi.org/10.1016/j.atmosenv.2019.117000.
Granat, L., Suksomsankh, K., Simachaya, S., Tabucanon, M., & Rodhe, H. (1996). Regional background acidity and chemical composition of precipitation in Thailand. Atmospheric Environment, 30(10–11), 1589–1596. https://doi.org/10.1016/1352-2310(95)00455-6.
Herrera, J., Rodríguez, S., & Baéz, A. P. (2009). Chemical composition of bulk precipitation in the metropolitan area of Costa Rica, Central America. Atmospheric Research, 94(2), 151–160. https://doi.org/10.1016/j.atmosres.2009.05.004.
Huang, Y., Wang, Y., & Zhang, L. (2008a). Long-term trend of chemical composition of wet atmospheric precipitation during 1986-2006 at Shenzhen City, China. Atmospheric Environment, 42(16), 3740–3750. https://doi.org/10.1016/j.atmosenv.2007.12.063.
Huang, K., Zhuang, G., Xu, C., Wang, Y., & Tang, A. (2008b). The chemistry of the severe acidic precipitation in Shanghai, China. Atmospheric Research, 89(1–2), 149–160. https://doi.org/10.1016/j.atmosres.2008.01.006.
Ileperuma, O. (2015). Model assessment of acid deposition potential by SOx in Sri Lanka. Journal of the National Science Foundation of Sri Lanka, 43(3), 281. https://doi.org/10.4038/jnsfsr.v43i3.7956.
Jain, C. D., Madhavan, B. L., & Ratnam, M. V. (2019). Source apportionment of rainwater chemical composition to investigate the transport of lower atmospheric pollutants to the UTLS region. Environmental Pollution, 248, 166–174. https://doi.org/10.1016/j.envpol.2019.02.007.
Li, Y. C., Zhang, M., Shu, M., Ho, S. S. H., Liu, Z. F., Wang, X. X., & Zhao, X. Q. (2016). Chemical characteristics of rainwater in Sichuan basin, a case study of Ya’an. Environmental Science and Pollution Research, 23(13), 13088–13099. https://doi.org/10.1007/s11356-016-6363-4.
Martins, E. H., Nogarotto, D. C., Mortatti, J., & Pozza, S. A. (2019). Chemical composition of rainwater in an urban area of the southeast of Brazil. Atmospheric Pollution Research, 10(2), 520–530. https://doi.org/10.1016/j.apr.2018.10.003.
Migliavacca, D., Teixeira, E., Wiegand, F., Machado, A., & Sanchez, J. (2005). Atmospheric precipitation and chemical composition of an urban site, Guaba hydrographic basin, Brazil. Atmospheric Environment, 39(10), 1829–1844. https://doi.org/10.1016/j.atmosenv.2004.12.005.
Mimura, A. M. S., Almeida, J. M., Vaz, F. A. S., de Oliveira, M. A. L., Ferreira, C. C. M., & Silva, J. C. J. (2016). Chemical composition monitoring of tropical rainwater during an atypical dry year. Atmospheric Research, 169, 391–399. https://doi.org/10.1016/j.atmosres.2015.11.001.
Park, S. M., Seo, B. K., Lee, G., Kahng, S. H., & Jang, Y. (2015). Chemical composition of water soluble inorganic species in precipitation at Shihwa Basin, Korea. Atmosphere, 6(6), 732–750. https://doi.org/10.3390/atmos6060732.
Paternoster, M., Sinisi, R., Mancusi, C., Pilat, K., Sabia, A., & Mongelli, G. (2014). Natural versus anthropogenic influences on the chemical composition of bulk precipitation in the southern Apennines, Italy: a case study of the town of Potenza. Journal of Geochemical Exploration, 145, 242–249. https://doi.org/10.1016/j.gexplo.2014.07.003.
Pu, W., Quan, W., Ma, Z., Shi, X., Zhao, X., Zhang, L., Wang, Z., & Wang, W. (2017). Long-term trend of chemical composition of atmospheric precipitation at a regional background station in Northern China. Science of the Total Environment, 580, 1340–1350. https://doi.org/10.1016/j.scitotenv.2016.12.097.
Rao, P. S. P., Tiwari, S., Matwale, J. L., Pervez, S., Tunved, P., Safai, P. D., Srivastava, A. K., Bisht, D. S., Singh, S., & Hopke, P. K. (2016). Sources of chemical species in rainwater during monsoon and non-monsoonal periods over two mega cities in India and dominant source region of secondary aerosols. Atmospheric Environment, 146, 90–99. https://doi.org/10.1016/j.atmosenv.2016.06.069.
Rastegari Mehr, M., Keshavarzi, B., & Sorooshian, A. (2019). Influence of natural and urban emissions on rainwater chemistry at a southwestern Iran coastal site. Science of the Total Environment, 668, 1213–1221. https://doi.org/10.1016/j.scitotenv.2019.03.082.
Staelens, J., Deschrijver, A., Vanavermaet, P., Genouw, G., & Verhoest, N. (2005). A comparison of bulk and wet-only deposition at two adjacent sites in Melle (Belgium). Atmospheric Environment, 39(1), 7–15. https://doi.org/10.1016/j.atmosenv.2004.09.055.
Szép, R., Bodor, Z., Miklóssy, I., Niță, I. A., Oprea, O. A., & Keresztesi, Á. (2019). Influence of peat fires on the rainwater chemistry in intra-mountain basins with specific atmospheric circulations (Eastern Carpathians, Romania). Science of the Total Environment, 647, 275–289. https://doi.org/10.1016/j.scitotenv.2018.07.462.
Tennakoon, P. L. K., Hettiarachchi, L. S. K., & Gunaratne, G. K. A. (2006). An assessment of rainwater quality from the tea growing areas of Sri Lanka. Sri Lankan Journal of Tea Science, 71(1), 50–62.
Tripathy, G. R., Mishra, S., Danish, M., & Ram, K. (2019). Elevated barium concentrations in rain water from east-coast of India: role of regional lithology. Journal of Atmospheric Chemistry, 76(1), 59–72. https://doi.org/10.1007/s10874-019-9387-6.
Uchiyama, R., Okochi, H., Katsumi, N., & Ogata, H. (2017). The impact of air pollutants on rainwater chemistry during “urban-induced heavy rainfall” in downtown Tokyo, Japan. Journal of Geophysical Research, 122(12), 6502–6519. https://doi.org/10.1002/2017JD026803.
Venkateshwarlu, P., & Satyanarayana, J. (2014). Chemical composition of rainwater at an urban area during southwest monsoon. International Journal of Modern Chemistry and Applied Science, 1(1), 1–4.
Vieira-Filho, M. S., Lehmann, C., & Fornaro, A. (2015). Influence of local sources and topography on air quality and rainwater composition in Cubatão and São Paulo, Brazil. Atmospheric Environment, 101, 200–208. https://doi.org/10.1016/j.atmosenv.2014.11.025.
Yu, H., He, N., Wang, Q., Zhu, J., Gao, Y., Zhang, Y., Jia, Y., & Yu, G. (2017). Development of atmospheric acid deposition in China from the 1990s to the 2010s. Environmental Pollution, 231(3), 182–190. https://doi.org/10.1016/j.envpol.2017.08.014.
Zeng, Y., & Hopke, P. K. (1989). A study of the sources of acid precipitation in Ontario, Canada. Atmospheric Environment (1967), 23(7), 1499–1509. https://doi.org/10.1016/0004-6981(89)90409-5.
Funding
Authors of this research paper would like to thank financial support from the National Research Council of Sri Lanka (Grant No. NRC/11/127) for providing equipment and other expenses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liyandeniya, A., Deeyamulla, M. & Priyantha, N. Atmospheric chemical composition of bulk deposition at two geographically distinct locations in Sri Lanka. Environ Monit Assess 192, 452 (2020). https://doi.org/10.1007/s10661-020-08412-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08412-0