Abstract
The BTEX compounds (benzene, toluene, ethylbenzene, and xylenes) and formaldehyde (FA) have harmful impacts on human health and are also important precursors of tropospheric ozone and secondary organic aerosols (SOA). Thus, the objective of this study was to perform a human health risk assessment considering the lifetime carcinogenic (LCR) and non-carcinogenic (as hazard quotient (HQ)) risks for 3 different age groups associated with exposure to BTEX and FA by inhalation using a probabilistic approach with Monte Carlo simulation, as well as to evaluate the contributions of these compounds to ozone formation potential (OFP) and SOA formation potential (SOAFP), at seven sites in the city of Salvador, Bahia, Brazil, during the dry and rainy periods. The HQ values associated with BTEX and FA compounds were below the limit set by the USEPA (HQ = 1) for all groups in both periods. The LCR values for benzene and FA at the 95th percentile considering 3 evaluated groups were 2.49 × 10−6, 3.56 × 10−6, 9.16 × 10−6 and 1.83 × 10−5, 2.53 × 10−5, 6.55 × 10−5 in the dry period and 2.83 × 10−6, 3.94 × 10−6, 1.01 × 10−5 and 7.97 × 10−6, 1.02 × 10−5, 2.40 × 10−5 in the rainy period, respectively, being all values above the acceptable limit by the USEPA (1.0 × 10−6). For all 3 groups of the population, the LCR values for benzene and FA were higher during the rainy period and dry period, respectively, following the same pattern as the concentrations. FA, xylenes, and toluene accounted for up to 97.0% of total OFP, whereas toluene, benzene, and xylenes contributed up to 88.5% of total SOAFP. The results obtained showed the need to adopt measures to reduce BTEX and FA emissions in order to minimize the impacts on health of the exposed population and on air quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution is one of the main problems in large urban centers. The rapid growth of urbanization with the increase in the circulating vehicular fleet and industrial activities in urban areas is related to the demand for fuel consumption, which often associated with unfavorable weather conditions and contribute to the degradation of air quality (Cruz et al. 2019; Masih et al. 2017). Previous studies have shown that many adverse effects on human health, such as cardiovascular and respiratory diseases, some types of cancer, and mortality risks, are associated with air pollution (Bourdrel et al. 2017; Kim et al. 2018; Lefler et al. 2019; Lelieveld et al. 2015). According to the World Health Organization (WHO), about 99% of the world’s population lives in areas where concentrations of air pollutants exceed their air quality guidelines, and 7 million people die each year from ambient air pollution and household air pollution (WHO 2022).
Volatile organic compounds (VOCs) comprise an important group of air pollutants (alkanes, alkenes, alcohols, ketones, aldehydes, carboxylic acids, and aromatics) emitted into the atmosphere by biogenic sources and mainly by anthropogenic sources such as7 million people die each year from ambient air pollution and household air pollution. vehicle fleet, gas stations, oil refining and storage, solvent evaporation, waste biodecomposition in landfills and composting sites, as well as industrial emissions (Cruz et al. 2017, 2020a; Shinohara et al. 2019; Tohid et al. 2019). Among these emission sources, vehicular fleet is the most important in urban centers (Hanif et al. 2021).
Among the VOCs that have attracted great interest for air quality assessment are the BTEX compounds (benzene, toluene, ethylbenzene, and the ortho-, meta-, and para-xylene isomers), as they are the most prevalent VOCs in urban and industrial areas (Shuai et al. 2018). Another prominent species is formaldehyde (FA), the most abundant carbonyl compound in the atmosphere (Santana et al. 2017). These compounds are considered by the United States Environmental Protection Agency (USEPA) as air toxics and have been included among the 187 hazardous air pollutants (HAP), since they are known or suspected to cause cancer and other serious health effects (USEPA 2021). According to the International Agency for Research on Cancer (IARC), benzene and formaldehyde are classified as carcinogenic to humans (Group 1), ethylbenzene as a probable carcinogen to humans (Group 2B), while toluene and xylenes are included in Group 3, non-carcinogenic to humans (IARC 2018).
Benzene can cause neurological, endocrine, reproductive disorders and some hematological diseases such as aplastic anemia, acute myeloid leukemia, and myelodysplastic syndrome. In addition, it is also associated with myocardial infarction, nasopharyngeal cancer, and respiratory diseases (Bolden et al. 2015; Snyder 2012). The World Health Organization has estimated that exposure of populations to benzene concentrations of 1.7 μg m−3 can cause 1 case of leukemia per 100,000 populations (WHO 2000). Toluene can affect the reproductive and central nervous systems causing premature birth, birth defects, and dementia (Filley et al. 2004; WHO 2000). Ethylbenzene and xylenes can lead to harmful effects on the immune, respiratory, and neurological systems (Bolden et al. 2015). Formaldehyde is associated with the likelihood of nasopharyngeal cancer in humans, in addition to effects such as eye and respiratory tract irritation, fatigue, migraine, and nausea (NTP 2011).
BTEX and FA compounds are pollutants of interest not only because of their adverse effects on human health, but also because of their importance in atmospheric chemistry. These compounds are involved in photochemical reactions in the troposphere, leading to the formation of photochemical oxidants such as ozone (O3) and peroxyacetyl nitrate (PAN), as well as secondary organic aerosols (SOA) (Bauri et al. 2016; Garg and Gupta 2019; Hajizadeh et al. 2018). Ozone is phytotoxic, can negatively impact human health, and is related to the formation of photochemical smog, a major air pollution problem in urban areas (Bauri et al. 2016). SOA are responsible for human health damage, contribute to the increase in the amount of atmospheric particulate matter, decreased visibility, and act as cloud condensation nuclei (CCN) causing changes in the Earth’s radiation balance (Kalbande et al. 2022; Nault et al. 2021; Sartelet et al. 2018).
Health risk assessment (HRA) is a tool used to determine the impacts caused by exposure to pollutants such as BTEX and FA on human health and can help in defining public policies to address environmental problems (Du et al. 2014; Masih et al. 2016). The HRA can be estimated by both deterministic and probabilistic methods (Rajasekhar et al. 2020). Deterministic methods use point estimates, where a single numerical value is chosen for each variable in the risk equation, representing a central tendency or upper bound estimate of variability (USEPA 2001). Probabilistic methods, on the other hand, have been used in some studies to estimate the variability and uncertainties associated with HRA, using random variable distributions instead of fixed values (Cruz et al. 2022; Dehghani et al. 2018; Mojarrad et al. 2020; Rajasekhar et al. 2020; Rostami et al. 2021).
In recent years, several studies have been conducted to determine BTEX and FA concentrations in the air of Brazilian cities (Alvim et al. 2017; Carvalho et al. 2020; Coelho et al. 2021; Cruz et al. 2020b; Dantas et al. 2022; Galvão et al. 2016; Nogueira et al. 2017; Paralovo et al. 2016, 2019; Santana 2019; Santana et al. 2017; Santiago et al. 2021; Siqueira et al. 2017). However, studies focusing on HRA based on deterministic methods, contribution to ozone formation potential (OFP), and SOA formation potential (SOAFP) associated with exposure to BTEX and FA compounds are still scarce in these cities. Furthermore, to the best of our knowledge, no study has been found to date in Brazil on the assessment of health risks using probabilistic methods associated with BTEX and FA compounds.
The current Brazilian environmental legislation establishing national outdoor air quality standards (CONAMA 2018) does not define standards for BTEX and FA compounds, which consequently contributes to a lack of information regarding HRA, OFP, and SOAFP associated with BTEX and FA compounds in Brazilian cities, thereby hindering regulatory actions aimed at protecting human health and the environment. Thus, the objective of this study was to evaluate the human health risks considering the lifetime carcinogenic (LCR) and non-carcinogenic (expressed as hazard quotient (HQ)) risks associated with exposure to BTEX and FA compounds by inhalation, through a probabilistic approach using Monte Carlo simulation, and considering three groups of the resident population with different age ranges. Additionally, the contribution of these compounds to the OFP and SOAFP was also evaluated at 7 sites in the city of Salvador, state of Bahia, Brazil, during two different periods of the year (rainy and dry).
Material and methods
Study area and data collection
The city of Salvador (12°58′16″ S and 38°30′39″ W), capital of the state of Bahia, in northeastern Brazil, has about 3 million inhabitants in a peninsular area of 361 km2 and an estimated vehicular fleet of 1,058,322 vehicles until September 2022 (DETRAN 2022), representing an increase of more 60% compared to the year 2010 (648,323 vehicles). This city has a humid tropical climate, and the topographic distribution is characterized by low altitudes associated with little thermal variation, allowing the penetration of trade winds (SE, E, and NE) into the city, which favors a good dispersion of air pollutants. This town receives little influence from industrial emissions and constitutes an urban center mainly impacted by vehicular emissions.
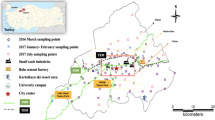
The data of BTEX and FA concentrations at different sites in the city of Salvador used in this study to assess human health risks associated with exposure to BTEX and FA compounds and their contribution to OFP and SOAFP were previously reported by the authors of this study (Santana et al. 2017; Cruz et al. 2020b). BTEX and FA compounds were sampled at 7 selected sites considering traffic density (Fig. 1) during two distinct periods (dry and rainy). For sampling BTEX compounds were used Radiello® passive diffusive samplers patented by Fondazione Salvatore Maugeri (Padova, Italy). The samplers consist of a stainless steel cylinder (100 mesh and 5.8 mm diameter) containing activated charcoal as adsorbent inserted in a cylindrical diffusive body (Cruz et al. 2020b), while for FA, sampling was employed a passive sampler based on gas molecular diffusion through static air layer containing fiberglass filters impregnated with 30 mmol L−1 2,4-dinitrophenylhydrazine (DNPH) + 1 mol L−1 glycerol solution developed by Santana et al. (2017).
Analysis and quality control
The BTEX compounds were extracted using chemical desorption with carbon disulfide (CS2) in ultrasonic bath for 10 min and determined by gas chromatography with flame ionization detection. The analysis conditions were as follows: capillary column HP – 5 (30 m, 0.32 mm, 0.25 μm), helium as carrier gas at a flow rate of 1.5 mL min−1, and nitrogen as make-up gas, injector with division of flow (split) in the ratio 1:20 at 250 °C, and temperature program (40 °C for 3 min, increased to 140 °C at a rate of 8 °C min−1, increased to 220 °C at a rate of 20 °C min−1, and then held for 4.5 min).
Calibration curves for BTEX compounds based on the external standardization method were constructed from dilution in methanol of a standard solution (UST HC BTEX MIX 2000 μg mL−1, Sigma-Aldrich). These curves were composed of seven points, with a linear dynamic range between 0.7 and 20 μg mL−1. The regression equation for each calibration curve was calculated using the linear regression method, and values of R2 > 0.9990 were obtained demonstrating excellent linearity for all analytes. The values of limit of detection (LOD) and limit of quantification (LOQ) were determined by statistical analysis of the data, and the values ranged from 0.21 to 0.40 μg mL−1 and 0.77 to 1.40 μg mL−1, respectively, with relative standard deviations (RSD) less than 5%.
The FA fixed as hydrazone in the filters of the passive samplers was extracted with acetonitrile (ACN) for further quantification by high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection. The analysis conditions were as follows: separation column Zorbax ODS 5 um, 4.6 × 250 mm, the mobile phase 60% ACN/H2O, and flow rate of 1.5 mL min−1. The calibration curves were constructed from dilution in acetonitrile of 1.0 μg mL−1 Aldehyde/Ketone-DNPH (Sigma-Aldrich) standard solution, with a linear dynamic range between 5.0 and 90 μg L−1. Calibration results demonstrated excellent linearity (R2 > 0.9995), and the values of LOD and LOQ were 1.62 and 3.78 μg L−1, respectively.
Health risk assessment
Health risk assessment (HRA) is used to estimate the potential adverse human health effects of toxic compounds (Garg and Gupta 2019). The standard HRA method developed by USEPA assesses human health effects resulting from prolonged exposure by inhalation, ingestion, or dermal absorption to toxic substances in different environmental matrices (USEPA 2009, 2016). This method based on a toxicological approach is the most widely used and also the most comprehensive (USEPA 2016), as it considers many factors which can affect exposure, such as duration and frequency of exposure, inhalation rate, body weight, average life span of the target population, and pollutant concentration (Cruz et al. 2020a).
However, uncertainties are associated with HRA due to the use of point values for estimating risks, as values assigned to exposure variables (such as inhalation rates between individuals), and daily variations in pollutant concentrations depending on location. Therefore, probabilistic risk assessment (PRA) uses models to represent the likelihood of different risk levels in a population (i.e., variability) or to characterize uncertainty in the risk estimates (Cruz et al. 2022; Du et al. 2014; USEPA 2001). The most widely used technique for PRA is Monte Carlo simulation (MCS), in which probability distributions are assigned to each parameter in the input model of the risk equation. Then, for each parameter, random values are chosen within the ranges determined for each type of distribution to achieve the output model. This process can be repeated thousands of times to obtain the output distribution (USEPA 2001). Thus, PRA has been used in some studies to determine carcinogenic and non-carcinogenic risks associated with exposure to BTEX and FA compounds (Baghani et al. 2019; Du et al. 2014; Ghaffari et al. 2021; Golkhorshidi et al. 2019; Rajasekhar et al. 2020; Yaghmaien et al. 2019).
In the present study, PRA was used to estimate the health risks of three population groups in different age ranges (0–5, 6–19, and 20–70 years, corresponding to G1, G2, and G3, respectively) in urban areas of the city of Salvador (state of Bahia, Brazil) due to exposure to BTEX and FA compounds by inhalation. The indicators used include the carcinogenic risk estimated as lifetime cancer risk (LCR), which is defined as the highest probability of cancer incidence by a continuous lifetime exposure to a specific compound, and the non-carcinogenic risk (expressed as hazard quotient (HQ)), according to the USEPA method (USEPA 2016). Crystal Ball software (version 11.1.2.4) was used to calculate the probability distributions of the risks, and a total of 10,000 repetitions were carried out to ensure the reliability of the Monte Carlo simulation (MCS) results, thereby obtaining a statistical data set comprising average, standard deviation, minimum, maximum, and percentiles. Thus, the MCS technique was performed to obtain the uncertainties and variability of each input parameter associated with the risk estimates and sensitivity analysis.
Table 1 shows the parameters and distribution models used in this study to perform Monte Carlo simulations to quantify the uncertainties of the parameters used in the HRA associated with the LCR and HQ values for BTEX and FA compounds, considering the three groups with different age ranges (G1, G2, and G3).
Lifetime cancer risk (LCR)
The LCR values associated with inhalation exposure of the population of the city of Salvador to benzene and formaldehyde, both carcinogens, were calculated considering two sampling periods (dry and rainy) and three different age groups (G1, G2, and G3) according to Eqs. 1 and 2:
where C is the contaminant concentration in ambient air (mg m−3), IR is the human inhalation rate (m3 day−1), ET is the daily exposure time (h day−1), ED is the exposure duration (year), EF is the exposure frequency (day year−1), BW is the body weight (kg), AT is the average lifetime (AT = 70 year × 365 day year−1 × 24 h day−1), CDI is chronic daily intake (mg kg−1 day−1), and SF is the carcinogenicity factor or slope factor, an estimate of the probability of an individual developing cancer as a result of exposure of life to a particular level of a potential carcinogenic compound. Values of SF for benzene and FA are 0.027 and 0.045 mg−1 kg day, respectively, according to the Integrated Risk Information System (IRIS) (USEPA 2022a).
In accordance with the limits determined by the USEPA, LCR values ≥ 1.0 × 10−4 indicate that carcinogenic risks are considerable, whereas LCR values ≤ 1.0 × 10−6 are assumed to be acceptable. In cases where values are in the range of 1.0 × 10−6 < LCR < 1.0 × 10−4, carcinogenic risks are probable (USEPA 2016). The WHO considers LCR values in the range of 1.0 × 10−5–1.0 × 10−6 as acceptable (WHO 2021a).
Hazard quotient (HQ)
Non-carcinogenic risks, expressed as hazard quotient (HQ), associated with exposure to all BTEX compounds as well as formaldehyde were calculated according to Eqs. 1 and 3.
For the non-carcinogenic risk assessment, AT = ED year × 365 day year−1 × 24 h day−1 (USEPA 2009). The RfD is the reference dose, which is compound-specific, one level below that at which adverse health effects are unlikely to occur during a lifetime (USEPA 2013). Values of RfD for the BTEX and FA compounds were obtained from the IRIS database (USEPA 2022b).
According to the hazard quotient estimation, a value of HQ ≤ 1 is considered an acceptable level, i.e., exposure to a chemical compound is unlikely to cause adverse human health effects, while a value of HQ > 1 is deemed likely to result in adverse human health effects (USEPA 2016).
Ozone formation potential (OFP)
Tropospheric O3 is a secondary pollutant formed through complex, nonlinear reactions between VOCs and nitrogen oxides (NOx = NO + NO2) in the presence of sunlight (Atkinson 2000; Wang et al. 2017). The O3 formation can be determined by the properties of the VOCs studied, meteorological conditions, and values of the concentration ratio of the precursors (VOCs/NOx), as they compete for hydroxyl radical (OH•). For high values of the VOCs/NOx ratio, the OH• will preferentially react with VOCs, while for low values of the ratio, this radical will predominantly react with NOx, thus decreasing the available amount of these oxides, and, consequently, ozone production will be limited by the amount and reactivity of VOCs (Iqbal et al. 2014; Seinfeld and Pandis 2016).
Scales representing VOCs reactivity were developed based on the concept of incremental reactivity (IR), which provides the amount of O3 formed per unit of VOC that is introduced into a representative atmospheric system (Carter 1994). Since the IR values are dependent on the environmental conditions where they are evaluated, i.e., these values vary significantly depending on the VOCs/NOx ratio at the site under study, the IR values obtained from simulations under specific VOCs/NOx ratio conditions are used.
The maximum incremental reactivity (MIR) scale is used for scenarios where NOx concentrations are high and VOCs/NOx ratio values are low. Therefore, VOCs have the greatest influence on ozone formation, and NOx concentrations have an inhibiting effect, characteristic conditions in large urban centers (Carter 1994). MIR is calculated as the amount of ozone formed when VOC is added to initiate the reaction with NOx and has been widely used for air pollution control purposes (Garg and Gupta 2019).
The presence of VOCs in the atmosphere plays an important role in the formation of tropospheric ozone through its degradation reactions (Atkinson 2000). Studies on the behavior of VOCs in the atmosphere use the calculation of the OFP in order to estimate the contributions of these compounds to tropospheric ozone formation in different areas, including urban areas (Mozaffar et al. 2020; Zhan et al. 2021).
In this study, contributions of the BTEX and FA compounds to the OFP at seven evaluated sites in the city of Salvador during the dry and rainy periods were estimated considering the concentrations in the atmosphere and the reactivity values of each compound in agreement with the MIR scale (Carter 2010), according to Eq. 4. This estimate is based on a scenario in which the production of O3 is calculated under maximum formation condition, allowing verification of which of the compounds are contributing more significantly to ozone formation at the studied sites.
where \({\mathrm{C}}_{\mathrm{i}}\) is the \({\mathrm{VOC}}_{\mathrm{i}}\) concentration in the atmosphere (µg m−3) and \({\mathrm{MIR}}_{\mathrm{i}}\) is the maximum incremental reactivity of \({\mathrm{VOC}}_{\mathrm{i}}\).
Secondary organic aerosols formation potential (SOAFP)
SOA formed from VOCs are associated with changes in climate due to their ability to absorb and scatter radiation in the atmosphere. The formation of low volatile compounds that make up SOA is governed by a complex series of reactions of many organic species (Niu et al. 2016). The uncertainties in the reactions mechanisms create difficulties in accurately modeling SOA formation in the atmosphere, so that its experimental characterization and theoretical description present a substantial challenge (Kroll and Seinfeld 2008; Shi et al. 2020). The indirect estimation of SOA is a common practice (Li et al. 2020).
Derwent et al. (2010) estimated the propensity of SOA formation relative to toluene for 113 organic compounds, and their work served as the basis for calculating SOA formation potential (SOAFP) in several studies. The calculation of SOAFP, associated with the VOC concentration, according to Eq. 5, has been used in some previous studies (Huang and Hsieh 2020; Niu et al. 2016; Zhan et al. 2021).
The SOAP (Eq. 6) represents the SOA formation potential for the VOCs and is obtained by the ratio of the increment in SOA mass concentration for these compounds to the increment in SOA with toluene multiplied by 100. Values of SOAP for benzene, toluene, ethylbenzene, m,p-xylene, o-xylene, and formaldehyde were 92, 100, 111.6, 78.7, 95.5, and 0.7, respectively (Derwent et al. 2010).
Alternatively, Zhang et al. (2017) proposed the FAC (fractional aerosol coefficient) method (Eq. 7) in calculating SOAFP, which was also used in this study.
where Ci (μg m−3) is the concentration of each VOC and FAC is defined as the ratio between the concentration of SOA formed from a volatile organic compound and its initial concentration, where the FAC of toluene is estimated to be 5.4% (Zhang et al. 2017).
Results and discussion
Concentrations of the BTEX and formaldehyde compounds in the city of Salvador
Figure 2 presents the distributions of the concentrations of BTEX and formaldehyde in urban areas of the city of Salvador in the rainy and dry periods. The average concentrations of benzene, toluene, ethylbenzene, and xylenes at different sites in the city of Salvador were 2.05 ± 0.82, 3.18 ± 0.97, 1.00 ± 0.42, and 1.72 ± 0.74 µg m−3 during the rainy period and 1.55 ± 0.99, 2.47 ± 1.49, 0.64 ± 0.25, and 1.24 ± 0.54 µg m−3 during the dry period, respectively. The formaldehyde concentrations ranged from 2.80 ± 0.21 to 4.80 ± 0.25 µg m−3 in the rainy period, while in the dry period, the concentrations varied from 4.35 ± 0.34 to 18.2 ± 1.2 µg m−3. As can be seen from Fig. 2, toluene is the compound with the highest concentrations among all BTEX compounds in both periods, followed by concentrations of benzene, xylenes, and ethylbenzene. In other studies, toluene was also the most abundant compound in the atmosphere of urban areas (Garg and Gupta 2019; Masih et al. 2016; Ramadan et al. 2019; Yousefian et al. 2020; Zhang et al. 2012).
Boxplot diagram for the distribution of BTEX and formaldehyde concentrations (μg m−3) in the air in the city of Salvador, Brazil, during dry and rainy periods. The upper and lower bars show the maximum and minimum values. Lines inside the boxes show the median values, and the “x” represents the average values
In Brazil, commercial gasoline (type C) is used to supply a large part of the vehicle fleet. According to the National Agency of Petroleum, Natural Gas, and Biofuels (ANP), gasoline type C is the second most sold oil derivative in the country, behind only diesel oil (ANP 2021). Gasoline type C (commonly known as gasohol) is a blend of gasoline type A with 25–27% v/v of anhydrous ethyl alcohol used as anti-knock additive, containing 35% v/v of aromatic hydrocarbons (ANP 2013) and an average percentage of BTEX of 1.0%, 3.0%, 1.3%, and 5.3%, respectively (Silva et al. 2009). An important source of BTEX in Brazilian urban centers comes from the burning of gasohol by light vehicles, which consist mainly of flex-fuel vehicles using gasohol or hydrated ethanol (Cruz et al. 2020b). In addition, there is also the contribution of heavy vehicles (trucks, buses) that use diesel with 10% biodiesel addition, which emit aromatic compounds such as benzene and toluene into the atmosphere (Ferreira et al. 2008). These facts, together with the process of photochemical degradation in the atmosphere that occurs more slowly for toluene and benzene, when compared to ethylbenzene and xylenes (Finlayson-Pitts and Pitts Jr 2000) justify the higher concentrations of toluene and benzene in the atmosphere of Salvador.
In Brazilian cities, although the addition of 10% biodiesel in the diesel has contributed to improvements in the quality of fuels, and consequent decrease in the concentrations of some air pollutants, regarding carbonyl compounds such as FA, this addition generated an increase in the levels these compounds (Guarieiro et al. 2011; Rodrigues et al. 2012; Santana et al. 2017). Additionally, the use of oxygenated fuels, such as hydrous ethyl alcohol and gasohol, contributes to the increase of the formaldehyde emissions to the atmosphere of these cities (Cruz et al. 2020a).
Seasonal variations were observed in this study for all BTEX compounds, where concentrations were higher during the rainy period compared to the dry period. This variation in concentration levels occurs mainly due to the photochemical degradation process of these compounds in the atmosphere, which is more intense in warmer seasons when solar intensity is higher, leading to a decrease of BTEX compounds in the atmosphere of the city of Salvador in the dry period. Moreover, in the rainy period, calm and stable atmospheric conditions prevail, thus hindering the dispersion and dilution of pollutants in the atmosphere. Additionally, since BTEX compounds are non-polar organic compounds and are not subject to the washout effect, there is a tendency to increase the concentrations of these compounds in the rainy season. On the other hand, in the dry period, atmospheric instability prevails contributing to the dispersion and dilution of the BTEX compounds in the atmosphere, thereby favoring a decrease of their concentrations in the atmosphere during this period of the year (Omokungbe et al. 2020; Paralovo et al. 2019). For FA, the concentrations were higher in the dry period, certainly influenced by direct emissions from the vehicular fleet, combined with the reduction in precipitation rates and increased photochemical oxidation of hydrocarbons due to greater insolation, because the dry period coincides with summer in the city of Salvador (Santana et al. 2017).
With regard to legislations and recommendations, the current Brazilian environmental legislation (CONAMA 2018) that establishes national outdoor air quality standards sets no standards for BTEX and FA compounds; therefore, it was necessary to use international legislation or guidelines to evaluate the data used in this study. For benzene, which is classified as a carcinogen for humans by the IARC, the concentrations found in the studied areas were below the annual mean value of 5.0 μg m−3, established in the European Air Quality Directive 2008/50/EC (EU—Directive 2008), as a limit value for the concentration of benzene in air, aiming to protect human health. However, according to the Ambient Air Quality Criteria (AAQCs) established by the Ontario Ministry of the Environment for the protection of human health (OME 2012), in all the samples analyzed, the concentrations were above the annual limit (0.45 μg m−3), whereas 30% of the samples showed values above the limit set for 24 h (2.30 μg m−3). Furthermore, in 57% of the samples analyzed, the concentrations were above the World Health Organization guideline (WHO 2000), which established that benzene concentrations of 1.7 μg·m−3 can cause 1 case of leukemia per 100,000 inhabitants, thus revealing a risk to human health for the population living in the city of Salvador in the studied sites. For formaldehyde, which is also carcinogenic and may cause nasopharyngeal cancer in humans, a total of 78% of the reported levels exceeded the limit for long exposure indicated by the Texas Commission on Environmental Quality (TCEQ 2016) of 3.3 μg m−3.
Probabilistic health risk assessment
Figure 3 summarizes the statistical data concerning the LCR values for benzene and formaldehyde obtained through the probabilistic risk assessment considering the groups with different age ranges in the city of Salvador during the rainy and dry periods. All the LCR values obtained for benzene were higher during the rainy period than in the dry period. On the other hand, the LCR values obtained for formaldehyde were higher during the dry period when compared to those obtained in the rainy period, following the same pattern as the concentrations obtained for these compounds in the evaluated periods (Fig. 2).
Boxplot diagram for the LCR values for benzene and formaldehyde considering groups G1, G2, and G3 in the city of Salvador, Brazil, during the rainy and dry periods. The upper and lower bars show the 5th and 95th percentiles. Lines at the top and bottom of the box correspond to the 20th and 80th percentiles, lines inside the boxes show the median values, and the “x” represents the average values
Table 2 presents the LCR values (average, minimum, and maximum) and the percentiles (10th–90th) obtained by MCS for benzene and formaldehyde in the seven sites evaluated in the city of Salvador, considering the different groups of exposed population with distinct age ranges (G1, G2, G3). It was possible to observe that the average LCR values for all groups, except for benzene considering G1 in the dry period, were above the acceptable limit set by the USEPA (1.0 × 10−6).
The maximum LCR values found for benzene were about 16, 22, 40 and 13, 17, 33 times above the USEPA acceptable level of 1 × 10−6 during the rainy and dry periods, respectively, for groups G1, G2, and G3, thus revealing a significant cancer risk for the population of Salvador due to inhalation benzene exposure in all age groups evaluated. Also, according to the highest limit considered by the WHO (1.0 × 10−5), the obtained values for groups G1, G2, and G3 were about 1.5, 2.2, 4.0 and 1.3, 1.7, 3.3 times above the acceptable limit during the rainy and dry periods, respectively, also confirming that even with a more restrictive limit, there is a risk of cancer associated with benzene exposure for the population of this city.
Concerning formaldehyde, the maximum LCR values of groups G1, G2, and G3 found were about 40, 50, 71 and 62, 85, 227 times above the acceptable limit established by the USEPA (1.0 × 10−6) during the rainy and dry periods, respectively, whereas according to the highest limit set by the WHO (1.0 × 10−5), the same risk values were around 4.0, 5.0, 7.1 and 6.2, 8.5, 22.7 times above the acceptable limit during the rainy and dry periods, respectively, for groups G1, G2, and G3.
In order to calculate these maximum values in the risk models, their respective maximum values were used for all input variables. Thus, the maximum risk value corresponds to the acute chronic exposure condition, which is less likely to occur, and therefore the maximum risks may be overestimated (Rajasekhar et al. 2020). According to the risk assessment guidelines (USEPA 2001), the value referring to the 95th percentile has been adopted as the upper limit estimate of potential risk in several previous studies (Cruz et al. 2022; Miri et al. 2016; Mohammadi et al. 2020; Mojarrad et al. 2020; Tehrani et al. 2020; Zhou et al. 2011) and was also used in this study.
Figures 4 and 5 represent the probability distributions of LCR for benzene and formaldehyde, respectively, considering the exposed population groups with different age ranges, within a confidence interval of 5 to 95% in the seven sites evaluated in the city of Salvador during the rainy and dry periods. The LCR values for benzene and FA referring to the 95th percentile were 2.83 × 10−6, 3.94 × 10−6, 1.01 × 10−5 and 7.97 × 10−6, 1.02 × 10−5, 2.37 × 10−5 during the rainy period and 2.49 × 10−6, 3.56 × 10−6, 9.16 × 10−6 and 1.83 × 10−5, 2.53 × 10−5, 6.55 × 10−5 during the dry period, for groups G1, G2, and G3, respectively. Hence, all these values are above the acceptable limit (1.0 × 10−6) and in the range of probable risk limit (1.0 × 10−6–1.0 × 10−4) set by the USEPA. Furthermore, the LCR values obtained for benzene were 2.5 to 10 times above the acceptable limit, and those for FA were 8 to 66 times above this limit. Based on the results obtained, it was observed that risks are high considering the different age groups in both periods and, therefore, indicating all the evaluated groups of the exposed population are susceptible to carcinogenic risks throughout the year.
The group G3 exhibited the highest LCR values in all cases at the 95th percentile. Moreover, considering G3, even at the 5th percentile (adopted as the lowest estimate of potential risk), all LCR values for benzene and formaldehyde were also above the USEPA acceptable limit in both periods, showing that the adult-exposed population group in the city of Salvador is more susceptible to developing cancer, due to the longer exposure time. For benzene, considering the G3 group in the rainy season, the 95th percentile LCR value was also above the highest limit considered by the WHO (1.0 × 10−5). For formaldehyde, except for G1 in the rainy season, all 95th percentile LCR values were above this limit.
Some recent studies performed in different locations have used probabilistic risk assessment (PRA) to estimate at the 95th percentile LCR value associated with inhalation benzene exposure in urban centers. Miri et al. (2016) obtained the LCR (95th) value of 5.92 × 10−7 in regions with high vehicular traffic, in the city of Tehran (Iran). Mohammadi et al. (2020) estimated LCR (95th) values of 2.29 × 10−5 during winter and 2.0 × 10−5 during summer in Urmia City (Azerbaijan). A similar study was carried out by Alahabadi et al. (2021) in Mashhad, the second largest metropolis in Iran, in which LCR (95th) values of 3.73 × 10−6 during winter and 4.97 × 10−6 during summer were found.
Other studies also conducted recently using PRA have reported 95th percentile LCR values associated with FA exposure in urban areas. Delikhoon et al. (2018) estimated LCR (95th) values between 9.61 × 10−5 and 1.12 × 10−4 in summer and 7.25 × 10−5–1.24 × 10−5 for winter according to the age group studied in an urban area in Iran, showing that all these values exceeded the limits set by the USEPA and WHO. Kanjanasiranont et al. (2017) found LCR value (95th) equal to 2.52 × 10−4 in urban atmosphere of Bangkok (Thailand). According to Du et al. (2014), FA was associated with the highest median cancer risk estimates (> 1 × 10−5) evaluated in China. Values of LCR referring to the 95th percentile of 2.48 × 10−6 and 2.01 × 10−5, respectively, in health centers and industrial environments in Iran were reported by Soltanpour et al. (2022).
The LCR values predicted from the deterministic method (base case) were found in higher positions than the average values obtained by the probabilistic approach, as shown in the probability distributions (Figs. 4 and 5), evidencing that the deterministic model, which adopts that all variables are known constants and does not consider the uncertainties, leads to risk values above those closest to reality.
In order to determine the proportion of the population free from the carcinogenic risks associated with exposure to benzene and formaldehyde, the percentile corresponding to the lower risk limit established by the USEPA (1 × 10−6) was predicted for each group assessed in the two periods (rainy and dry). For benzene, the percentages obtained for G1, G2, and G3 were 65.20%, 27.27%, and 0.25% during the rainy period and 74.56%, 46.39%, and 2.65% during the dry period, respectively. This indicates, e.g., that the G3 group has 99.75% (100%—0.25%) and 97.35% (100%—2.65%) probability that the LCR value exceeds the limit recommended by the USEPA in the rainy and dry periods, respectively. These values confirm that the oldest age group (G3) had the highest percentage of the population susceptible to carcinogenic risks in both periods.
Regarding formaldehyde, the USEPA-established limit of 1 × 10−6 as acceptable was found in the 13.82% (G1) and 0.11% (G2) percentiles during the rainy period and 4.84% (G1) and 0.09% (G2) during the dry period, indicating that the highest percentage of the population is exposed to risk values exceeding the acceptable limit. On the other hand, the G3 group showed minimum values above the limit established as acceptable by the USEPA in both the rainy and dry periods. This means that for 100% of the population, the LCR values obtained in all percentiles were above the acceptable limit, showing that the G3 group was the most susceptible to cancer risk compared to the other groups evaluated.
The HQ values (P5%, mean, and P95%) for BTEX and FA compounds obtained by the probabilistic model in this study (Table 3) were below the USEPA acceptable level (HQ ≤ 1) in the dry and rainy periods, thus indicating that non-cancer risks to the health of the population due to inhalation exposure to these compounds were not observed considering the groups evaluated (G1, G2, and G3) in the city of Salvador in both periods. Kumari et al. (2021) used the deterministic method to perform the HRA associated with exposure to BTEX and FA compounds in Agra, India. HQ values for BTEX and FA were also within USEPA acceptable limit. Golkhorshidi et al. (2019) carried out an assessment of the human health risks associated with exposure to BTEX compounds by inhalation using the probabilistic method and considering three groups of the population with different age ranges at four bus terminals in Tehran, Iran, during two seasons (winter and summer). The non-carcinogenic risks found for BTEX were less than 1 (HQ < 1) in both seasons for different age groups, showing that these compounds have no adverse human health effect in the four bus terminals.
In this study, the evaluation of the total non-cancer risks (HI) considering BTEX compounds was performed by summing the HQ values. Values of HI for all groups in the two periods studied were also < 1, which indicates that combined exposure to BTEX compounds does not cause adverse health effects either. Similar results were found in previously performed studies (Garg and Gupta 2019; Hajizadeh et al. 2018).
A sensitivity analysis was also carried out in this study to determine the contributions of the input variables in estimating the LCR values for benzene and formaldehyde compounds. The results obtained are shown in Fig. 6 considering the three population groups with different age groups in the city of Salvador (G1, G2, and G3).
Results of the sensitivity analysis of LCR considering the population groups with different age ranges revealed that the concentration (C) was the most significant contributing parameter to LCR values for benzene and FA, accounting for 35.1%, 59.8%, and 80.8% and 27.6%, 50.6%, and 73.4% for groups G1, G2, and G3, respectively. As the contributions of the concentrations to sensitivity were positive values, there was a positive correlation between concentrations and LCR values. These results indicate that a decrease in the concentrations of benzene and FA contributes to the reduction of carcinogenic risks. There was a negative correlation between body weight (BW) and LCR values. In general, the variables that contributed least to LCR values were IR, ED, ET, AT, and EF.
Ozone formation potential (OFP) of BTEX and FA compounds
Table 4 shows the values of ozone formation potential associated with BTEX and formaldehyde compounds obtained from the MIR scale in the city of Salvador during the rainy and dry periods. The contribution to ozone formation potential showed an opposite tendency when comparing BTEX and FA compounds, due to the seasonal variation of their respective concentrations. BTEX had a higher contribution to O3 formation during the rainy season, while FA contributed more significantly during the dry season. The results obtained indicated OFP average values in the order formaldehyde > xylenes > toluene > ethylbenzene > benzene for the two periods studied in urban areas of Salvador. Among the BTEX compounds, xylenes are the most reactive compounds, thereby justifying their higher OFP values, while benzene was the compound that showed the lowest contribution to OFP, since it is the most stable species in the atmosphere and therefore a lower reactivity. The pattern xylenes > toluene > ethylbenzene > benzene concerning the contribution of BTEX compounds to OFP obtained in this study was also found in previous studies (Hajizadeh et al. 2018; Mojarrad et al. 2020).
Formaldehyde, xylenes, and toluene were the largest contributors to OFP in both periods, with significant contribution of FA. The formaldehyde contributed 54.6% to total OFP during the rainy period and 77.3% in the dry period, whereas xylenes and toluene contributed equally 19.4% each in the rainy period and 9.9% and 9.8%, respectively, in the dry period (Table 4). These compounds together accounted for 93.4% and 97.0% of the ozone formed from photochemical reactions in the atmosphere of Salvador during the rainy and dry periods, respectively. The total OFP values obtained in this study were above the acceptable limit of 100 µg m−3 established by the World Health Organization (WHO 2021b) for the dry period in the sites evaluated in the city of Salvador.
The OFP results for BTEX compounds obtained in this study were lower compared to the values obtained in other parts of the world (Iran, Malaysia, India) (Garg and Gupta 2019; Mojarrad et al. 2020; Zulkifli et al. 2022). On the other hand, the values found of OFP for FA were similar to the values found in Taiyuan, China (Liu et al. 2021). Certainly, these differences are related to the concentrations of these compounds obtained in each study, which may be related to several factors such as different fuels used in each country, size and age of the vehicle fleet, sampling period (rainy or dry), presence of industries at the sampling site, as well as the meteorological conditions (Cerón-Bretón et al. 2015).
Studies conducted in the southeast region of Brazil, in Rio de Janeiro by da Silva et al. (2018) and in São Paulo by Alvim et al. (2011), showed that the OFP values obtained due to the contributions of benzene, toluene, ethylbenzene, and xylenes were 2.09, 17.47, 5.13, and 22.28 µg m−3 and BTEX and FA were 2.51, 42.8, 11.5, 50.6, and 55.1 µg m−3, respectively. According to ANP (2021), the southeast region has the largest vehicle fleet of the country, which contributes to the higher levels of these compounds and values OFP in this region if compared to the values obtained in this study. Moreover, although the solar radiation intensity values have been high in the city of Salvador throughout the year, which contributes to the formation of tropospheric ozone, this city has a privileged geographical location and receives air masses from the Atlantic Ocean most of the time, which makes it possible to affirm that the processes of removal of BTEX and FA compounds in the troposphere predominated over ozone formation.
SOA formation potential (SOAFP) of BTEX and FA compounds
Volatile organic compounds are precursors of SOA in urban atmosphere; prominent among these is the group of aromatic compounds, which is responsible for most of the SOA formation in the atmosphere in urban areas (Niu et al. 2016). Each VOC plays a specific role in SOA formation, according to the concentration and their respective formation potentials.
The average SOAFP values at the sites evaluated in this work during the rainy and dry periods are presented in Table 5. Toluene, benzene, and xylenes were the species that contributed the most to SOA formation in the two periods studied, and together they contributed up to 88.5% to SOAFP, being also the compounds showing the highest concentration values at the sites evaluated. However, FA was the species that exhibited the lowest contribution (Table 5). The contribution trend of the VOCs studied to SOAFP was toluene > benzene > xylenes > ethylbenzene > formaldehyde. Toluene was the compound that was the largest contributor to SOA formation in this study, as well as in others conducted previously (Coelho et al. 2021; Huang and Hsieh 2020; Mozaffar et al. 2020), probably because toluene is one of the most abundant aromatic hydrocarbons in the atmosphere, in addition to its high SOAP value.
Yuan et al. (2013) reported that aromatic compounds accounted for more than 90% of total SOA formation, and among these, BTEX compounds were the most prominent in SOA production under conditions of low and high NOx concentrations. Studies carried out elsewhere in the world have showed that among the BTEX compounds, toluene is the major contributor to SOA formation in urban atmosphere (Huang and Hsieh 2020; Li et al. 2020; Niu et al. 2016; Mozaffar et al. 2020), as observed in this study. Li et al. (2020), in a study conducted in the city of Beijing (China), obtained higher SOAFP values for BTEX compounds than those obtained in this work, presenting values for benzene, toluene, ethylbenzene, and xylenes of 125, 240, 80, and 184 µg m−3, respectively.
Only one study has been conducted so far in Brazil to assess the contribution of BTEX compounds to SOAFP (Coelho et al. 2021). These authors, at two sites in the São Paulo metropolitan area (southeastern Brazil), obtained average SOAFP values associated with BTEX compounds of 12, 40, 14, 12, and 5 µg m−3 in the industrial area and 8, 28, 12, 11, and 4 µg m−3 in the urban area, respectively. These values are consistent, but above the SOAFP values obtained in this study, which can be explained by the characteristics of the sites evaluated; the first is an industrial area related to petrochemical activities, involving use of large amounts of fossil fuels, while the second area is associated with intense vehicle traffic, as it represents one of the main avenues in the region, connecting several cities and therefore having a high flow of vehicles.
Conclusions
In this study, a health risk assessment was conducted for three population groups with different ages associated with exposure to BTEX and FA compounds, using a probabilistic approach with Monte Carlo simulation, for the first time in the city of Salvador. The contributions of these compounds to ozone formation potential (OFP) and SOA formation potential (SOAFP) were also determined in two different periods (rainy and dry).
Regarding human health risk assessment, the HQ values associated with BTEX and FA compounds were below the limit set by the USEPA (HQ = 1) for all exposed population groups in the dry and rainy periods. However, the 95th percentile LCR values obtained for benzene considering different age groups (G1, G2, and G3) were 2.5 to 10 times above the acceptable limit by the USEPA (1.0 × 10−6) and 8 to 66 times above for formaldehyde, thus confirming that for all population groups investigated in this study, the carcinogenic risks are high.
Formaldehyde, xylenes, and toluene were the compounds that most contributed to the OFP values, accounting for up to 97.0% of the tropospheric ozone formed considering the photochemical reactions of BTEX and FA in the atmosphere of the city of Salvador. In addition, ΣOFP exceeded the acceptable limit of 100 µg m−3 set by the WHO in the dry period, being strongly influenced by the contribution of FA. The emission trends observed for the VOCs studied were formaldehyde > xylenes > toluene > ethylbenzene > benzene for OFP, and toluene > benzene > xylenes > ethylbenzene > formaldehyde for SOAFP.
The results obtained in this study showed the need to adopt measures to decrease the emissions of BTEX and FA compounds into the atmosphere of the city of Salvador, as well as a revision of the Brazilian legislation to introduce air quality standards for these compounds, in order to reduce the impacts on the health of the exposed population and air quality. Moreover, these results can contribute to a better understanding of the contribution of these compounds to the formation of secondary pollutants (O3 and SOA).
Thus, this study can also contribute to stimulate governments about the need to implement public policies to achieve the Sustainable Development Goals (SDGs) contained in the United Nations Agenda 2030, linked to goal 11, particularly in its target 11.6 which states the need for measures to reduce the negative environmental impact per capita of cities, with special attention to air quality, municipal waste management, and others.
Data availability
Data are available from the corresponding author on reasonable request.
References
Alahabadi A, Fazeli I, Rakhshani MH, Najafi ML, Alidadi H, Miri M (2021) Spatial distribution and health risk of exposure to BTEX in urban area: a comparison study of different land-use types and traffic volumes. Environ Geochem Health 43:2871–2885. https://doi.org/10.1007/s10653-020-00799-6
Alvim DS, Gatti LV, Santos MH, Yamazaki A (2011) Estudos dos compostos orgânicos voláteis precursores de ozônio na cidade de São Paulo. Eng San Amb 16:189–196. https://doi.org/10.1590/S1413-41522011000200013
Alvim DS, Gatti LV, Corrêa SM, Chiqueto JB, Rossatti CS, Santos MH, Yamazaki A, Orlando JP, Santos GM (2017) Main ozone-forming VOCs in the city of Sao Paulo: observations, modelling and impacts. Air Qual Atmos Health 10:421–435. https://doi.org/10.1007/s11869-016-0429-9
ANP (2013) Resolution ANP nº 40, 2013. https://atosoficiais.com.br/anp/resolucao-n-40-2013?origin=instituicao&q=40/2013. Accessed 16 Sep 2022
ANP (2021) Anuário estatístico brasileiro do petróleo, gás natural e biocombustíveis, 2021. https://www.gov.br/anp/pt-br/centrais-de-conteudo/publicacoes/anuarioestatistico/arquivos-anuario-estatistico-2021/anuario-2021.pdf. Accessed 16 Sep 2022
Atkinson R (2000) Atmospheric chemistry of VOCs and NOx. Atmos Environ 34:2063–2101. https://doi.org/10.1016/S1352-2310(99)00460-4
Baghani AN, Sorooshian A, Heydari M, Sheikhi R, Golbaz S, Ashournejad Q, Kermani M, Golkhorshidi F, Barkhordari A, Jafari AJ, Delikhoon M, Shahsavani A (2019) A case study of BTEX characteristics and health effects by major point sources of pollution during winter in Iran. Environ Pollut 247:607–617. https://doi.org/10.1016/j.envpol.2019.01.070
Bauri N, Bauri P, Kumar K, Jain VK (2016) Evaluation of seasonal variations in abundance of BTXE hydrocarbons and their ozone forming potential in ambient urban atmosphere of Dehradun (India). Air Qual Atmos Health 9:95–106. https://doi.org/10.1007/s11869-015-0313-z
Bolden AL, Kwiatkowski CF, Colborn T (2015) New look at BTEX: are ambient levels a problem? Environ Sci Technol 49:5261–5276. https://doi.org/10.1021/es505316f
Bourdrel T, Bind M-A, Béjot Y, Morel O, Argacha J (2017) Cardiovascular effects of air pollution. Arch Cardiovasc Dis 110:634–642. https://doi.org/10.1016/j.acvd.2017.05.003
Carter WPL (1994) Development of ozone reactivity scales for volatile organic compounds. Air Waste 44:881–899. https://doi.org/10.1080/1073161X.1994.10467290
Carter WPL (2010) Development of the SAPRC-07 chemical mechanism. Atmos Environ 44:5324–5335. https://doi.org/10.1016/j.atmosenv.2010.01.026
Carvalho J, Fortes J, Corrêa S, Martins E (2020) Impactos dos BTEX em áreas urbanas da cidade do Rio de Janeiro. Quim Nova 7:870–877. https://doi.org/10.21577/0100-4042.20170562
Cerón-Bretón JG, Cerón-Bretón RM, Kahl JDW, Ramírez-Lara E, Guarnaccia C, Aguilar-Ucán CA, Montalvo-Romero Anguebes-Franseschi F, López-Chuken U (2015) Diurnal and seasonal variation of BTEX in the air of Monterrey, Mexico: preliminary study of sources and photochemical ozone pollution. Air Qual Atmos Health 8:469–482. https://doi.org/10.1007/s11869-014-0296-1
Coelho MS, Dominutti PA, Boian C, Santos TC, Nogueira T, Oliveira CAV, Fornaro A (2021) Non-methane hydrocarbons in the vicinity of a petrochemical complex in the Metropolitan Area of São Paulo, Brazil. Air Qual Atmos Health 14:967–984. https://doi.org/10.1007/s11869-021-00992-1
CONAMA (2018) Resolution 491, of November 19, 2018. Provides for air quality standards. https://pesquisa.in.gov.br/imprensa/jsp/visualiza/index.jsp?data=21/11/2018&jornal=515&pagina=15. Accessed 16 Sep2022
Cruz LPS, Alve LP, Santos AVS, Esteves MB, Gomes IVS, Nunes LSS (2017) Assessment of BTEX concentrations in air ambient of gas stations using passive sampling and the health risks for workers. J Environ Prot 08:12–25. https://doi.org/10.4236/jep.2017.81002
Cruz LPS, Mota ER, Campos VP, Santana FO, Luz SR, Santos FS (2019) Inorganic and organic acids in the atmosphere of the urban area of the city of Salvador, Brazil. J Braz Chem Soc 30:904–914. https://doi.org/10.21577/0103-5053.20180227
Cruz LPS, Luz S, Campos V, Santana FO, Alves RS (2020a) Determination and risk assessment of formaldehyde and acetaldehyde in the ambient air of gas stations in Salvador, Bahia, Brazil. J Braz Chem Soc 06:1137–1148. https://doi.org/10.21577/0103-5053.20190278
Cruz LPS, Santos DF, dos Santos IF, Gomes IVS, Santos AVS, Souza KSPP (2020b) Exploratory analysis of the atmospheric levels of BTEX, criteria air pollutants and meteorological parameters in a tropical urban area in Northeastern Brazil. Microchem J 152:104265–104274. https://doi.org/10.1016/j.microc.2019.104265
Cruz LPS, Alves RS, da Rocha FOC, Moreira MS, Júnior AS (2022) Atmospheric levels, multivariate statistical study, and health risk assessment of odorous compounds (H2S and NH3) in areas near polluted urban rivers in the city of Salvador, in Northeastern Brazil. Air Qual Atmos Health 15:159–176. https://doi.org/10.1007/s11869-021-01095-7
da Silva CM, da Silva LL, Corrêa SM, Arbilla G (2018) A minimum set of ozone precursor volatile organic compounds in an urban environment. Atmos Pollut Res 9:369–378. https://doi.org/10.1016/j.apr.2017.11.002
Dantas G, Gorne I, da Silva CM, Arbilla G (2022) Benzene, toluene, ethylbenzene and xylene (BTEX) concentrations in urban areas impacted by chemical and petrochemical industrial emissions. Bull Environ Contam and Toxicol 108:204–211. https://doi.org/10.1007/s00128-021-03336-y
Dehghani M, Fazlzadeh M, Sorooshian A, Tabatabaee HR, Miri M, Baghani AN, Delikhoon M, Mahvi AH, Rashid M (2018) Characteristics and health effects of BTEX in a hot spot for urban pollution. Ecotoxicol Environ Saf 155:133–143. https://doi.org/10.1016/j.ecoenv.2018.02.065
Delikhoon M, Fazlzadeh M, Sorooshian A, Tabatabaee HR, Miri M, Baghani AN, Delikhoon M, Mahvi AH, Rashidi M (2018) Characteristics and health effects of formaldehyde and acetaldehyde in an urban area in Iran. Environ Pollut 242:938–951. https://doi.org/10.1016/j.envpol.2018.07.037
Derwent RG, Jenkin ME, Utembe SR, Shallcross E, Murrells TP, Passant NR (2010) Secondary organic aerosol formation from a large number of reactive man-made organic compounds. Sci Total Environ 408:3374–3381. https://doi.org/10.1016/j.scitotenv.2010.04.013
DETRAN (2022) Bahia State Department of Transit - Vehicular Fleet. https://www.detran.ba.gov.br/upload/frota/frota-4367829. Accessed 16 Sep 2022
Du Z, Mo J, Zhang Y (2014) Risk assessment of population inhalation exposure to volatile organic compounds and carbonyls in urban China. Environ Int 73:33–45. https://doi.org/10.1016/j.envint.2014.06.014
EU - Directive (2008) Directive 2008/50/EC of on ambient air quality and cleaner air for Europe. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0050&from=en. Accessed 16 Aug 2022
Ferreira SL, dos Santos AM, Souza GR, Polito WL, Módolo DL (2008) Análise por cromatografia gasosa de BTEX nas emissões de motor de combustão interna alimentado com diesel e mistura diesel-biodiesel (B10). Quim Nova 31:539–545. https://doi.org/10.1590/S0100-40422008000300015
Filley CM, Halliday W, Kleinschmidt-Demasters BK (2004) The effects of toluene on the central nervous system. J Neuropathol Exp Neurol 63:1–12. https://doi.org/10.1093/jnen/63.1.1
Finlayson-Pitts BJ, Pitts JN Jr (2000) Chemistry of the upper and lower atmosphere: theory, experiments and applications. Academic Press, San Diego, USA
Galvão ES, Santos JM, Reis Junior NC, Stuetz RM (2016) Volatile organic compounds speciation and their influence on ozone formation potential in an industrialized urban area in Brazil. Environ Technol 37:2133–2148. https://doi.org/10.1080/09593330.2016.1142001
Garg A, Gupta NC (2019) A comprehensive study on spatio-temporal distribution, health risk assessment and ozone formation potential of BTEX emissions in ambient air of Delhi, India. Sci Total Environ 659:1090–1099. https://doi.org/10.1016/j.scitotenv.2018.12.426
Ghaffari HR, Kamari Z, Fazlzadeh M, Heidari M (2021) Level of air BTEX in urban, rural and industrial regions of Bandar Abbas, Iran; indoor-outdoor relationships and probabilistic health risk assessment. Environ Res 200:111745. https://doi.org/10.1016/j.envres.2021.111745
Golkhorshidi F, Sorooshian A, Jafari AJ, Baghani AB, Kermani M, Kalantary RR, Ashournejad Q, Delikhoon M (2019) On the nature and health impacts of BTEX in a populated middle eastern city: Tehran, Iran. Atmos Pollut Res 10:921–930. https://doi.org/10.1016/j.apr.2018.12.020
Guarieiro LLN, Vasconcellos PC, Solci MC (2011) Air pollutants from the burning of fossil fuels and biofuels: a brief review. Rev Virtual Quim 3:434–445. https://doi.org/10.5935/1984-6835.20110047
Hajizadeh Y, Mokhtari M, Faraji M, Mohammadi A, Nemati S, Ghanbari R, Abdolahnejad A, Fard RF, Nikoonahad A, Jafari N, Miri M (2018) Trends of BTEX in the central urban area of Iran: a preliminary study of photochemical ozone pollution and health risk assessment. Atmos Pollut Res 9:220–229. https://doi.org/10.1016/j.apr.2017.09.005
Hanif NM, LimiHawari NSS, Othman M, Hamid HHA, Ahamad F, Uning R, Ooi MCG, Wahab MIA, Sahani M, Latif MT (2021) Ambient volatile organic compounds in tropical environments: potential sources, composition and impacts – a review. Chemosphere 285:131355. https://doi.org/10.1016/j.chemosphere.2021.131355
Huang YS, Hsieh CC (2020) VOC characteristics and sources at nine photochemical assessment monitoring stations in western Taiwan. Atmos Environ 240:117741. https://doi.org/10.1016/j.atmosenv.2020.117741
IARC (2018) Agents classified by the IARC monographs volumes 1–123. https://monographs.iarc.fr/wp-content/uploads/2018/09/ClassificationsAlphaOrder.pdf. Accessed 16 Aug 2022
Iqbal MA, Kim K-H, Shon Z-H, Kim K, Shon Z, Sohn J, Jeon E, Kim Y, Oh J (2014) Comparison of ozone pollution levels at various sites in Seoul, a megacity in Northeast Asia. Atmos Res 138:330–345. https://doi.org/10.1016/j.atmosres.2013.12.003
Kalbande R, Yadav R, Maji S, Rathore DS, Beig G (2022) Characteristics of VOCs and their contribution to O3 and SOA formation across seasons over a metropolitan region in India. Atmos Pollut Res 13:101515. https://doi.org/10.1016/j.apr.2022.101515
Kanjanasiranont N, Prueksasit T, Morknoy D (2017) Inhalation exposure and health risk levels to BTEX and carbonyl compounds of traffic policeman working in the inner city of Bangkok, Thailand. Atmos Environ 152:111–120. https://doi.org/10.1016/j.atmosenv.2016.11.062
Kim H-B, Shim J-Y, Park B, Lee Y-J (2018) Long-term exposure to air pollutants and cancer mortality: a meta-analysis of cohort studies. Int J Environ Res Public Health 15:2608. https://doi.org/10.3390/ijerph15112608
Kroll JH, Seinfeld JH (2008) Chemistry of secondary organic aerosol: formation and evolution of low-volatility organics in the atmosphere. Atmos Environ 42:3593–3624. https://doi.org/10.1016/j.atmosenv.2008.01.003
Kumari S, Baghel N, Lakhani A, Kumari KM (2021) BTEX and formaldehyde levels at a suburban site of Agra: temporal variation, ozone formation potential and health risk assessment. Urban Clim 40:100997. https://doi.org/10.1016/j.uclim.2021.100997
Lefler JS, Higbee JD, Burnett RT, Ezzati M, Celeman NC, Mann DD, Marshall JD, Bechle M, Wang Y, Robinson AL, Popo CA (2019) Air pollution and mortality in a large, representative U.S. cohort: multiple-pollutant analyses, and spatial and temporal decompositions. Environ Health 18:101. https://doi.org/10.1186/s12940-019-0544-9
Lelieveld J, Evans JS, Fnais M, Pozzer A (2015) The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525:367–371. https://doi.org/10.1038/nature15371
Li Q, Su G, Li C, Liu P, Zhao X, Zhang C, Sun X, Mu Y, Wu M, Wang Q, Sun B (2020) An investigation into the role of VOCs in SOA and ozone production in Beijing, China. Sci Total Environ 720:137536. https://doi.org/10.1016/j.scitotenv.2020.137536
Liu Z, Cui Y, He Q, Guo L, Gao X, Feng Y, Wang Y, Wang X (2021) Seasonal variations of carbonyls and their contributions to the ozone formation in urban atmosphere of Taiyuan, China. Atmosphere 12:510. https://doi.org/10.3390/atmos12040510
Masih A, Lall AS, Taneja A, Singhvi R (2016) Inhalation exposure and related health risks of BTEX in ambient air at different microenvironments of a terai zone in north India. Atmos Environ 147:55–66. https://doi.org/10.1016/j.atmosenv.2016.09.067
Masih A, Lall AS, Taneja A, Singhvi R (2017) Exposure profiles, seasonal variation and health risk assessment of BTEX in indoor air of homes at different microenvironments of a Terai province of northern India. Chemosphere 176:8–17. https://doi.org/10.1016/j.chemosphere.2017.02.105
Miri M, Rostami Aghdam Shendi M, Ghaffari HR, Aval HE, Ahmadi E, Taban E, Gholizadeh A, Aval MY, Mohammadi A, Azari A (2016) Investigation of outdoor BTEX: concentration, variations, sources, spatial distribution, and risk assessment. Chemosphere 163:601–609. https://doi.org/10.1016/j.chemosphere.2016.07.088
Mohammadi A, Ghassoun Y, Löwner MO, Behmanesh M, Faraji M, Nemati S, Toolabi A, Abdolahnejad A, Panahi H, Heydari H, Miri M (2020) Spatial analysis and risk assessment of urban BTEX compounds in Urmia. Iran. Chemosphere 246:125769. https://doi.org/10.1016/j.chemosphere.2019.125769
Mojarrad H, Fouladi Fard R, Rezaali M, Heidari H, Izanloo H, Mohammadbeigi A, Mohammadi A, Sorooshian A (2020) Spatial trends, health risk assessment and ozone formation potential linked to BTEX. Hum Ecol Risk Assess 26:2836–2857. https://doi.org/10.1080/10807039.2019.1688640
Mozaffar A, Zhang YL, Fan M, Cao F, Lin Y (2020) Characteristics of summertime ambient VOCs and their contributions to O3 and SOA formation in a suburban area of Nanjing, China. Atmos Res 240:104923. https://doi.org/10.1016/j.atmosres.2020.104923
Nault BA, Jo DS, McDonald BC, Campuzano-Jost P, Day DA, Hu W, Schroder JC, Allan J, Blake DR, Canagaratna MR, Coe H, Coggon MM, DeCarlo PF, Diskin GS, Dunmore R, Flocke F, Fried A, Gilman JB, Gkatzelis G, Hamilton JF, Hanisco TF, Hayes PL, Henze DK, Hodzic A, Hu M, Huey G, Jobsin T, Kuster WC, Lewis A, Li M, Liao J, Nawaz MO, Pollack IB, Peischl J, Rappengluck B, Reeves CE, Richter D, Roberts JM, Ryerson TB, Shao M, Sommers JM, Walega J, Warneke C, Weibring P, Wolfe GM, Young DE, Yaun B, Zhang Q, Gouw JA, Jimenez JL (2021) Secondary organic aerosols from anthropogenic volatile organic compounds contribute substantially to air pollution mortality. Atmos Chem Phys 21:11201–11224. https://doi.org/10.5194/acp-21-11201-2021
Niu H, Mo Z, Shao M, Xir S (2016) Screening the emission sources of volatile organic compounds (VOCs) in China by multi-effects evaluation. Front Environ Sci Eng 10:01–11. https://doi.org/10.1007/s11783-016-0828-z
Nogueira T, Dominutti PA, Fornaro A, de Fatima AM (2017) Seasonal trends of formaldehyde and acetaldehyde in the megacity of São Paulo. Atmosphere 8:144–162. https://doi.org/10.3390/atmos8080144
NTP - National Toxicology Program (2011) Report on carcinogens, Twelfth Edition (2011). http://ntp.niehs.nih.gov/ntp/roc/twelfth/reviewprocess.pdf. Accessed 16 Sep 2022
OME - Ontario Ministry of the Environment (2012) Standards Development Branch Ontario Ministry of the Environment. 1–50. https://www.ontario.ca/page/ontarios-ambient-air-quality-criteria. Accessed 16 Aug 2022
Omokungbe OR, Fawole OG, Owoade OK, Popoola OAM, Jones RL, Olise FS, Ayoola MA, Abiodun PO, Toyeje AB, Olufemi AP, Sunmonu LA, Abiye OE (2020) Analysis of the variability of airborne particulate matter with prevailing meteorological conditions across a semi-urban environment using a network of low-cost air quality sensors. Heliyon 6:e04207. https://doi.org/10.1016/j.heliyon.2020.e04207
Paralovo SL, Borillo GC, Barbosa CGG, Godoi AFL, Yamamoto CI, Souza RAF, Endreoli RV, Costa PS, Almeida GP, Manzi AO, Pohlker C, Yáñez-Serrano AM, Kesselmeier J, Godoi RHM (2016) Observations of atmospheric monoaromatic hydrocarbons at urban, semi-urban and forest environments in the Amazon region. Atmos Environ 128:175–184. https://doi.org/10.1016/j.atmosenv.2015.12.053
Paralovo SL, Barbosa CGG, Carneiro IPS, Kurzlop P, Borillo GC, Schiochet MFC, Godoi AFL, Yamamoto CI, Souza RAF, Andreoli RV, Ribeiro IO, Manzi AO, Kourtchev I, Bustillos JOV, Martin ST, Godoi RHM (2019) Observations of particulate matter, NO2, SO2, O3, H2S and selected VOCs at a semi-urban environment in the Amazon region. Sci Total Environ 650:996–1006. https://doi.org/10.1016/j.scitotenv.2018.09.073
Rajasekhar B, Nambi IM, Govindarajan SK (2020) Human health risk assessment for exposure to BTEXN in an urban aquifer using deterministic and probabilistic methods: a case study of Chennai city. India. Environ Pollut 265:114814. https://doi.org/10.1016/j.envpol.2020.114814
Ramadan A, Yassin MF, Alshammari BZ (2019) Health risk assessment associated with volatile organic compounds in a parking garage. Int J Environ Sci Technol 16:2549–2564. https://doi.org/10.1007/s13762-018-1641-y
Rodrigues MC, Guarieiro LLN, Cardoso MP, Carvalho LS, Rocha GO, Andrade JB (2012) Acetaldehyde and formaldehyde concentrations from sites impacted by heavy-duty diesel vehicles and their correlation with the fuel composition: diesel and diesel/biodiesel blends. Fuel 92:258–263. https://doi.org/10.1016/j.fuel.2011.07.023
Rostami R, Fazlzadeh M, Babaei-Pouya A, Abazari M, Rastgho L, Ghasemi R, Saranjam B (2021) Exposure to BTEX concentration and the related health risk assessment in printing and copying centers. Environ Sci Pollut Res 28:31195–31206. https://doi.org/10.1007/s11356-021-12873-2
Santana FO (2019) Contaminants/atmospheric pollutants in Brazilian urban centers. Federal University of Bahia, Thesis
Santana FO, Campos VP, Cruz LPS, Luz SR (2017) Formaldehyde and acetaldehyde in the atmosphere of Salvador-Ba, Brazil, using passive sampling. Microchem J 134:78–86. https://doi.org/10.1016/j.microc.2017.04.032
Santiago ÍS, Silva TFA, Marques EV, Barreto FMS, Ferreira AG, Rocha CA, Mendonça KV, Cavalcante RM (2021) Influence of the seasonality and of urban variables in the BTEX and PM2.5 atmospheric levels and risks to human health in a tropical coastal city (Fortaleza, CE, Brazil). Environ Sci Pollut Res 28:42670–42682. https://doi.org/10.1007/s11356-021-13590-6
Sartelet K, Zhu S, Moukhtar S, André M, Gros V, Favez O, Brasseur OF, Redaelli M (2018) Emission of intermediate, semi and low volatile organic compounds from traffic and their impact on secondary organic aerosol concentrations over Greater Paris. Atmos Environ 180:126–137. https://doi.org/10.1016/j.atmosenv.2018.02.031
Seinfeld JH, Pandis SN (2016) Atmospheric chemistry and physics: from air pollution to climate change. John Wiley & Sons, New York
Shi Y, Xi Z, Simayi M, Li J, Xie S (2020) Scattered coal is the largest source of ambient volatile organic compounds during the heating season in Beijing. Atmos Chem Phys 20:9351–9369. https://doi.org/10.5194/acp-20-9351-2020
Shinohara N, Okazaki Y, Mizukoshi A, Wakamatsu S (2019) Exposure to benzene, toluene, ethylbenzene, xylene, formaldehyde, and acetaldehyde in and around gas stations in Japan. Chemosphere 222:923–931. https://doi.org/10.1016/j.chemosphere.2019.01.166
Shuai J, Kim S, Ryu H, Park J, Lee CK, Kim G, Ultra VU Jr, Yang W (2018) Health risk assessment of volatile organic compounds exposure near Daegu dyeing industrial complex in South Korea. BMC Public Health 18:528–541. https://doi.org/10.1186/s12889-018-5454-1
Silva FLN, dos Santos Jr JR, Neto MJM, Silva RLGNP, Flumigan DL, Oliveira JE (2009) Determinação de benzeno, tolueno, etilbenzeno e xilenos em gasolina comercializada nos postos do estado do Piauí. Quim Nova 32:56–60. https://doi.org/10.1590/S0100-40422009000100011
Siqueira CYS, Lemos MVP, Araujo BCC, Oliveira RRPE, Gil RASS, Neto FRA (2017) Atmospheric distribution of organic compounds from urban areas near Olympic games sites in Rio de Janeiro, Brazil. Microchem J 133:638–644. https://doi.org/10.1016/j.microc.2017.04.027
Snyder R (2012) Leukemia and benzene. Int J Environ Res Public Health 9:2875–2893. https://doi.org/10.3390/ijerph9082875
Soltanpour Z, Mohammadian Y, Fakhri Y (2022) The exposure to formaldehyde in industries and health care centers: a systematic review and probabilistic health risk assessment. Environ Res 204:112094. https://doi.org/10.1016/j.envres.2021.112094
Tehrani AM, Bahrami A, Leili M, Poorolajal J, Zafari D, Samadi M, Mahvi AH (2020) Investigation of seasonal variation and probabilistic risk assessment of BTEX emission in municipal solid waste transfer station. Int J Environ Anal Chem 1:1–14. https://doi.org/10.1080/03067319.2020.1814269
Texas Commission on Environmental Quality (TCEQ) (2016) Effects screening levels (ESLs). www.tceq.state.tx/toxicology/esl/list_main.html. Accessed 10 Oct 2022
Tohid L, Sabeti Z, Sarbakhsh P, Benis KZ, Shakerkhatibi M, Rasoulzadeh Y, Rahimian R, Darvishali S (2019) Spatiotemporal variation, ozone formation potential and health risk assessment of ambient air VOCs in an industrialized city in Iran. Atmos Pollut Res 10:556–563. https://doi.org/10.1016/j.apr.2018.10.007
USEPA - United States Environmental Protection Agency (2001) United States Environmental Protection Agency, Risk Assessment Guidance for Superfund: Volume III - Part A, Process for Conducting Probabilistic Risk Assessment. In: https://www.epa.gov/sites/default/files/2015-09/documents/rags3adt_complete.pdf. Accessed 16 Aug 2022
USEPA - United States Environmental Protection Agency (2009) Environmental Protection Agency. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment), EPA-540-R-070–002, Washington, DC. https://www.epa.gov/sites/production/files/201509/documents/partf_200901_final.pdf. Accessed 16 Sep 2022
USEPA - United States Environmental Protection Agency (2011) Exposure Factor Handbook 2011 Edition. https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252. Accessed 20 Sep 2022
USEPA - United States Environmental Protection Agency (2013) Terminology services, Vocabulary catalog, Vocabulary catalog list detail—Integrated Risk Information System (IRIS) Glossary, U.S. In: http://ofmpub.epa.gov/sor_internet/registry/termreg/searchandretrieve/glossariesandkeywordlists/search.do?details=&glossaryName=IRIS%20Glossary. Accessed 16 Sep 2022
USEPA - United States Environmental Protection Agency (2016) Guidelines for Human Exposure Assessment. https://www.epa.gov/sites/production/files/2016-02/documents/guidelines_for_human_exposure_assessment_peer_review_draftv2.pdf. Accessed 16 Sep 2022
USEPA - United States Environmental Protection Agency (2021) Initial List of Hazardous Air Pollutants with Modifications. https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications. Accessed 16 Sep 2022
USEPA - United States Environmental Protection Agency (2022a) Slope Factors (SF) for carcinogens. http://www.popstoolkit.com/tools/HHRA/SF_USEPA.aspx. Accessed 20 Sep 2022
USEPA - United States Environmental Protection Agency (2022b) IRIS assessments. https://iris.epa.gov/AtoZ/?list_type=alpha. Accessed 20 Sep 2022
Wang T, Xue L, Brimblecombe P, Lam YF, Li L, Li Z (2017) Ozone pollution in China: a review of concentrations, meteorological influences, chemical precursors, and effects. Sci Total Environ 575:1582–1596. https://doi.org/10.1016/j.scitotenv.2016.10.081
WHO - World Health Organization (2000) Air quality guidelines for Europe. 2nd ed. Copenhagen: WHO Regional Publications, European Series, No. 91. http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/pre2009/who-air-quality-guidelines-for-europe,-2nd-edition,-2000-cd-rom-version. Accessed 06 Oct 2022
WHO - World Health Organization (2021a) WHO human health risk assessment toolkit: chemical hazards, second edition. In: https://www.who.int/publications/i/item/9789240035720. Accessed 06 Oct 2022
WHO - World Health Organization (2021b) Air quality guidelines. Global update 2021b. Particulate matter, ozone, nitrogen dioxide and sulfur dioxide. https://www.who.int/publications/i/item/9789240034228?ua=1. Accessed 06 Oct 2022
WHO - World Health Organization (2022) Air pollution. https://www.who.int/health-topics/air-pollution#tab=tab_1. Accessed 06 Oct 2022
Yaghmaien K, Hadei M, Hopke P, Gharibzadeh S, Kermani M, Yarahmadi M, Emam B, Shahsavani A (2019) Comparative health risk assessment of BTEX exposures from landfills, composting units, and leachate treatment plants. Air Qual Atmos Health 12(4):443–451. https://doi.org/10.1007/s11869-019-00669-w
Yousefian F, Hassanvand MS, Nodehi RN, Amini H, Rastkari N, Aghaei M, Yunesian M, Yaghmaeian K (2020) The concentration of BTEX compounds and health risk assessment in municipal solid waste facilities and urban areas. Environ Res 191:110068. https://doi.org/10.1016/j.envres.2020.110068
Yuan B, Hu WW, Shao M, Wang M, Chen WT, Lu SH, Zeng LM, Hu M (2013) VOC emissions, evolutions and contributions to SOA formation at a receptor site in eastern China. Atmos Chem Phys 13:8815–8832. https://doi.org/10.5194/acp-13-8815-2013
Zhan J, Feng Z, Liu P, He X, He Z, Chen T, Wang Y, He H, Mu Y, Liu Y (2021) Ozone and SOA formation potential based on photochemical loss of VOCs during the Beijing summer. Environ Pollut 285:117444. https://doi.org/10.1016/j.envpol.2021.117444
Zhang Y, Mu Y, Liu J, Mellouki A (2012) Levels, sources and health risks of carbonyls and BTEX in the ambient air of Beijing, China. J Environ Sci 24:124–130. https://doi.org/10.1016/S1001-0742(11)60735-3
Zhang Z, Wang H, Chen D, Li Q, Thai P, Gong D, Li Y, Zhang C, Gu Y, Zhou L, Morawska L, Wang B (2017) Emission characteristics of volatile organic compounds and their secondary organic aerosol formation potentials from a petroleum refinery in Pearl River Delta, China. Sci Total Environ 584–585:1162–1174. https://doi.org/10.1016/j.scitotenv.2017.01.179
Zhou J, You Y, Bai Z, Hu Y, Zhang J, Zhang N (2011) Health risk assessment of personal inhalation exposure to volatile organic compounds in Tianjin, China. Sci Total Environ 409:452–459. https://doi.org/10.1016/j.scitotenv.2010.10.022
Zulkifli MFH, Hawari NSSL, Latif MT, Hamid HHA, Mohtar AAA, Idris WMRW, Mustaffa NLH, Juneng L (2022) Volatile organic compounds and their contribution to ground-level ozone formation in a tropical urban environment. Chemosphere 302:134852. https://doi.org/10.1016/j.chemosphere.2022.134852
Acknowledgements
The authors acknowledge the Coordination for the Improvement of Higher Education Personnel (CAPES) of Brazil and the Federal University of Bahia (UFBA) for the fellowships.
Funding
This study was partially supported by CAPES (Finance Code 001) and UFBA (registration number 15834/2019) with two research grants.
Author information
Authors and Affiliations
Contributions
Conceptualization, supervision, writing—original draft preparation, and writing—review and editing: Lícia P. S. Cruz. Writing—review and editing: Franciele O. C. da Rocha. Data collection, analysis, and writing—original draft preparation: Mateus S. Moreira. Writing—review: Vânia P. Campos. Data collection and analysis: Keliane S. P. P. Souza. All authors reviewed the manuscript and approved the version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cruz, L.P.S., da Rocha, F.O.C., Moreira, M.S. et al. Probabilistic human health risk assessment and contributions to ozone and SOA formation potentials associated with BTEX and formaldehyde emissions in a tropical city (Salvador, Bahia, Brazil). Air Qual Atmos Health 16, 765–784 (2023). https://doi.org/10.1007/s11869-023-01305-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-023-01305-4