Abstract
Atmospheric pollutants including ozone, nitrogen dioxide, sulfur dioxide, and BTEX (benzene, toluene, ethylbenzene, and xylenes) compounds were evaluated concerning their spatial distribution, temporal variation, and health risk factor. Bolu plateau where sampling was performed has a densely populated city center, semi-rural areas, and forested areas. Additionally, the ozone formation potentials of BTEXs were calculated, and toluene was found to be the most important compound in ground level ozone formation. The spatial distribution of BTEXs and nitrogen dioxide pollution maps showed that their concentrations were higher around the major roads and city center, while rural-forested areas were found to be rich in ozone. BTEXs and nitrogen dioxide were found to have higher atmospheric concentrations in winter. That was mostly related to the source strength and low mixing height during that season. The average toluene to benzene ratios demonstrated that there was a significant influence of traffic emissions in the region. Although there was no significant change in sulfur dioxide concentrations in the summer and winter seasons of 2017, the differences in the spatial distribution showed that seasonal sources such as domestic heating and intensive outdoor barbecue cooking were effective in the atmospheric presence of this pollutant. The lifetime cancer risk through inhalation of benzene was found to be comparable with the limit value (1 × 10–6) recommended by USEPA. On the other hand, hazard ratios for BTEXs were found at an acceptable level for different outdoor environments (villages, roadside, and city center) for both seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inorganic gaseous pollutants (such as ozone, nitrogen dioxide, and sulfur dioxide) and volatile organic compounds (VOCs) have been the focus of attention in many studies in the literature because of their serious effects on human health and the ecosystem (Bozkurt et al. 2018; De Marco et al. 2019; Demirel et al. 2014; Petracchini et al. 2016; Tecer et al. 2017). Especially, ozone (O3) is of primary interest because of its phytotoxicity at ambient concentrations and widespread existence in Europe, particularly in the Mediterranean area (Krupa and Manning 1988; UNECE 2010). It is known that O3 in the troposphere is a secondary pollutant that is produced from photochemical reactions between nitrogen oxides (NOx) and volatile organic carbons (VOCs) in the presence of sunlight. In order to reduce O3 concentrations, the emissions of its precursors, NOx and VOCs, must be brought under control which is rather difficult in the modern world. Primary sources of nitrogen dioxide (NO2) are motor vehicles and power plants emissions (Stranger et al. 2008). Volatile organic compounds (VOCs) are the O3 precursors and are the most common species present in urban air. They originate from various sources, such as industrial emissions, vehicular emissions, fossil fuel combustion, solvent usage, and biological processes (An et al. 2014; Song et al. 2007; Yan et al. 2017). Sulfur dioxide (SO2), another pollutant that affects air quality, has adverse effects on environmental and public health (Cotrozzi 2020; Hedley et al. 2002; Johns et al. 2011). Seasonal differences can be seen in the atmospheric amount of this pollutant, whose atmospheric existence is mostly based on fossil fuel burning (Petracchini et al. 2016).
BTEXs (benzene, toluene, ethylbenzene, and xylenes) that are a sub-group of VOCs have a high ozone formation potential in the atmosphere (Alghamdi et al. 2014). They are on the list of hazardous air pollutants (HAPs). HAPs are pollutants known for causing cancer or having other detrimental health effects (Presto et al. 2016). International Agency for Research on Cancer (IARC) classified benzene as a Group 1 carcinogen in 1987 (IARC 1987) and ethylbenzene as possibly carcinogenic to humans (Group 2B) in 2000 (IARC 2000). BTEXs also lead to a variety of adverse health effects such as birth defects, eczema, asthma, and bronchitis (Bolden et al. 2015). Moreover, insulin resistance and leukemia have been associated with benzene exposure (Choi et al. 2014). Toluene, ethylbenzene, and xylenes are found to be significantly related to cardiovascular diseases (Xu et al. 2009).
Passive sampling is a method frequently used in studies performed simultaneously at multiple points (Carmichael et al. 2003). With this technique, the concentrations of air pollutants sampled over a sampling period ranging from a few hours to a few weeks are determined as total or average values (Bozkurt et al. 2018). In this study, the authors focused on the determination of O3, NO2, SO2, and BTEX concentrations in the Bolu plateau. The main objectives of this study are as follows: (1) to determine the atmospheric concentrations of O3, NO2, SO2, and BTEXs in Bolu plateau and evaluate their seasonal variations; (2) to determine the relationship between the air pollutants; (3) to examine the spatial distributions and identify possible pollution sources; and (4) to estimate the health risks due to BTEXs.
Material and methods
Study area
Bolu is a small city located in the western Black Sea region of Turkey. The city has a total area of 8323.39 km2 and a population of around 300,000 (BDEU 2018). It has a bowl-like topography in which the city center is surrounded by mountains. The average altitude of the plateau is 1000 m, and the altitude of the city center is 725 m. The typical Black Sea climate, which is rainy in all seasons, is effective in the region. According to long-term meteorological data for 89 years, average annual precipitation and average annual temperature are 388.1 mm and 12.0 °C, respectively (Turkish State Meteorological Service 2018). The prevalent wind direction is west-southwest. Compared to summer (ca. 750 m), there is a low mixing height (ca. 150 m) in winter. Airflows coming from the prevalent wind direction sector carry contaminants from the industrialized cities of Turkey and Eastern European countries. Vehicular emissions and coal usage in domestic heating in winter can be considered as effective anthropogenic sources in the sampling area although some small-scale industrial activities such as cement, metal, woodwork, poultry, and glass are carried out in the city (BDEU 2018). Since 2009, natural gas has been allocated to residences in the city and Bolu Abant Izzet Baysal University and the industrial zone started to use natural gas in 2010. However, coal has been still used for residential heating both in the city center and the villages.
Sampling strategy
In this study, the spatial distribution of the concentrations of O3, NO2, SO2, and BTEXs in the Bolu atmosphere at different time intervals was determined using the passive sampling method. In the first sampling campaign, only inorganic pollutant (NO2, SO2, O3) samples were collected simultaneously at 47 sampling points between 5 and 13 March 2016. In the other sampling campaigns, the sampling was conducted in winter (28 January to 12 February 2017) and summer (7–23 July 2017); BTEX samples were also collected in addition to the inorganic pollutants. The second sampling campaign was carried out at 47 points in the winter, and the number of sampling points was increased to 63 in the third campaign (summer campaign). Locations of the sampling points for each campaign are pointed out on the map in Fig. 1.
Preparation of the samplers and analyses of the samples
BTEXs
BTEXs were collected by using stainless steel tubes (5 mm inner diameter and 15 mm diffusion path length) filled with Tenax-TA sorbent with 35/60 mesh particle size (Perkin Elmer, USA). The sampling procedure and the analytical methods were published in detail previously (Dörter et al. 2020) and mentioned briefly in this article. Before sampling, Tenax-TA sorbent tubes were conditioned in thermal desorber under high purity helium for 60 min at 320 °C. The tubes were stored in screw-capped falcon tubes containing silica gel and activated carbon. At the beginning of the sampling, the cover of the tubes in the diffusion direction was replaced by diffusion headers which allow air but prevent the entrance of dust and insects. The tubes were mounted vertically with the open end downward in aluminum shelters placed at about 2.5 m from ground level at the sampling points for 2 weeks. At the end of the sampling period, the tubes were closed, sealed, and stored in falcon tubes. They were stored at − 18 °C until analysis.
BTEXs were analyzed using thermal desorption (TD) (Perkin Elmer, TurboMatrix 300, USA) and gas chromatography-mass spectrometry (GC–MS) system (Shimadzu, GCMS-QP2010SE, Japan). Five-point (10 ng, 20 ng, 50 ng, 100 ng, and 200 ng) calibration curves were prepared for quantitative analyses.
Inorganic gaseous pollutants: NO2, SO2, and O3
Passive samplers developed by Eskişehir Technical University Air Quality Research Group were used to collect inorganic compounds (Gaga et al. 2012; Özden 2005; Özden and Döğeroğlu 2012). NO2 and SO2 gases were collected in the same sampler with the same trapping medium, whereas O3 was collected in a separate sampler. The NO2-SO2 and the O3 passive samplers were made from Teflon and Delrin materials, respectively. Both have dimensions of 2.5 cm length and 2.0 cm inner diameter.
As the trapping medium for NO2-SO2 gases, 20% TEA (triethanolamine) (Merck, Germany) aqueous solution impregnated GF/A glass fiber filter papers (Whatman, USA) were used. However, for O3, a mixture of 1% sodium nitrite (Merck, Germany), 2% sodium carbonate (Merck, Germany), and 2% glycerin was used as impregnation solution. After drying impregnated filter papers, they were fixed at the bottom of the samplers by using a ring. They were closed with a cap and labeled. At the beginning of each sampling campaign, the cap was replaced with a stainless steel mesh barrier. All inorganic samplers were placed vertically with the open end downward in aluminum shelters. After the sampling period, all the samplers were capped, sealed, and carried to the laboratory.
Before analysis, the extraction of NO2-SO2 trapped filter papers was performed for 15 min with 10 mL ultra-pure water (Milli Q) and 20 µL %35 H2O2. For O3 samples, the filter papers were extracted with 10 mL ultra-pure water (Milli Q) for 15 min. Extracts were filtered through cellulose acetate filters having 0.22-μm pore sizes. Analyses of all inorganic samples were performed by using Dionex ICS 1100 Series Ion Chromatography. O3, NO2, and SO2 gases were determined as NO3−, NO2−, and SO42−, respectively. Six-point (0.25 ppm, 0.5 ppm, 1 ppm, 2.5 ppm, 5 ppm, and 10 ppm) calibration curves were used in the analyses.
Quality control/quality assurance (QC/QA) parameters
BTEXs
An intermediate calibration standard was analyzed after analyses of ten samples to check the method performance. Field blanks were prepared and analyzed together with the samples. They were transported to the field along with the samplers, brought back to the laboratory, stored during the sampling period, and then analyzed. The mean sample to blank ratios was determined as 21 ± 22 for benzene, 23 ± 21 for toluene, 97 ± 98 for ethylbenzene, 52 ± 52 for m,p-xylene, and 91 ± 107 for o-xylene. The repeatability of the method for all compounds was determined from the analyses results of three parallel samplers placed at six sampling points during the campaigns. Percent relative standard deviations (% RSD) were found as 6.7 ± 6.0 for benzene, 5.2 ± 3.4 for toluene, 7.4 ± 4.8 for ethylbenzene, 5.2 ± 4.4 for m,p-xylene, and 5.4 ± 4.4 for o-xylene. For the determination of method detection limit (MDL), the calibration standard with the lowest concentration was analyzed 10 times, and the standard deviation of 10 replicates was multiplied by three and converted to units of atmospheric concentrations. The MDL values were obtained as 0.03, 0.02, 0.0008, 0.002, and 0.005 µg m−3 for benzene, toluene, ethylbenzene, m,p-xylene, and o-xylene, respectively. The method limit of quantification value (MLOQ) was calculated by multiplying the standard deviation of the ten replicate analyses of the lowest calibration standard by ten. The MLOQ values of benzene, toluene, ethylbenzene, m,p-xylene, and o-xylene were determined as 0.12, 0.05, 0.003, 0.007, and 0.016 µg m−3, respectively.
Inorganic gaseous pollutants
The accuracy of ion analysis was evaluated using certified reference material (ERM-CA408). Nitrate and sulfate ions were determined with 0.79% and 2.18% errors, respectively. A total of twelve field blanks were collected during the campaigns, and the means of the sample to blank ratios were found as 6.91 ± 5.30 for O3, 7.20 ± 5.73 for NO2, and 1.86 ± 0.48 for SO2. The repeatability was tested by placing three parallel samplers at six sampling points. Hence, percent relative standard deviations (% RSD) of NO2, SO2, and O3 were found as 12.4 ± 8.1, 16.9 ± 9.9, and 9.2 ± 7.2, respectively. In the determination of the method detection limit (MDL), the standard deviation of the ten replicates of calibration standard with the lowest concentration was multiplied by three. The MDLs of nitrite, nitrate, and sulfate were found as 1.97, 2.47, and 1.75 µg m−3, respectively. The method limit of quantification (MLOQ) was calculated by taking ten times the standard deviation of ten replicates of calibration standard with the lowest concentration. The MLOQs were obtained as 6.58, 8.23, and 5.82 µg m−3 for nitrite, nitrate, and sulfate, respectively.
Mapping
Spatial distribution maps of the pollutants were drawn using GIS software (Mapinfo, Professional, version 7.5). The coordinates of the sampling points and the relevant pollutant concentrations were entered into the MapInfo program. Contour maps were prepared by applying Vertical Mapper V3 and “triangulation with smoothing” as an interpolation technique.
Calculations
Atmospheric concentrations
Atmospheric concentrations of the pollutants were calculated using Fick’s first law of diffusion (Eq. 1).
where Q is pollutant concentration at the open end of the sampler (ng), L is diffusion path (cm), A is sampler surface area (cm2), D is diffusion coefficient (cm2 s−1), C is the amount of absorbed pollutant (µg m−3), and t is sampling time (s). For the Tenax-TA samplers, diffusion path (L) is 1.5 cm, and surface area (A) is 0.1963 cm2. Diffusion coefficient for 298 K (D298) is specific for each compound. USEPA (2017) stated D298 values for benzene (0.0923 cm2 s−1), toluene (0.0828 cm2 s−1), ethylbenzene (0.0758 cm2 s−1), m,p-xylene (0.0758 cm2 s−1), and o-xylene (0.0758 cm2 s−1). Equation 2 was used to adjust the D298 to the mean temperature of the sampling period.
Similar to the BTEXs, the ambient concentrations of inorganic gases were determined by using Fick’s first law of diffusion (Eq. 1). To obtain the pollutant concentration (C, µg m−3) of the O3, NO2, and SO2, the analysis results which in the form of their ions (NO3−, NO2− and SO42−) should be multiplied with factors 0.774 (48/62), 1 (46/46), and 0.667 (64/96), respectively. For the inorganic passive samplers, diffusion path (L) is 2.5 cm, and surface area (A) is 3.14 cm2. Diffusion coefficient (D) for each gas for 298 K was DO3: 0.155 cm2 s−1, DNO2: 0.154 cm2 s−1, DSO2: 0.127 cm2 s−1. The diffusion coefficients were adjusted to the mean sampling temperature by using Eq. 2.
Ozone formation potential (OFP)
Ozone formation potential (OFP) serves in determining the contribution of compounds to the atmospheric O3 concentrations. OFP of each compound is different because they have different reactivities and compete with their counterparts (Li et al. 2019). The maximum incremental reactivity (MIR) method was used to determine OFP for BTEXs (Carter 1994). The MIR method is given in Eq. 3.
where OFP(i) represents the ozone formation potential of compound i, conc(i) is the concentration of compound i, and MIRCoefficient(i) is the constant for compound i (Carter 1994).
Health risk assessment
To obtain a probabilistic approach for health risk assessment, Monte Carlo (MC) simulation method was applied to the benzene data using the Crystal Ball Risk analysis software (application 11.1). Inhalation cancer risk for benzene (R, unitless) was calculated by multiplying the pollutant intake (I, mg kg−1 day−1) with the slope factor of the dose–response curve (SF, mg−1 kg day) as given in Eq. 4 (Civan et al. 2015).
Slope factor was obtained from USEPA-IRIS (2015) as 1.5 × 10−2 mg−1 kg day. In the estimation of the lifetime pollutant intake (I) via the inhalation route, Eq. 5 was used.
where C is the concentration of the pollutant (μg m−3), CU is the conversion unit (10−3 mg µg−1), IR is the inhalation rate (m3 day−1), and BW is the body weight (kg).
Hazard ratios (HR) were also used in the risk assessment of BTEXs. It is the ratio of daily average concentration (C, µg m−3) to the reference concentration for inhalation exposure (RfC, µg m−3) (Eq. 6).
The RfC is the daily intake level of a substance that has no adverse health effects. The RfC values were obtained from USEPA-IRIS (2015) as 0.03 mg m−3 for benzene, 5 mg m−3 for toluene, 1 mg m−3 for ethylbenzene, and 0.1 mg m−3 for xylenes.
Results and discussion
Annual and seasonal evaluation
Three sampling campaigns were carried out on different dates (5–13 March 2016; 28 January–12 February 2017; and 7–23 July 2017) to compare pollutant concentrations between two seasons and 2 years. Statistical information of O3, NO2, SO2, benzene, toluene, ethylbenzene, and xylenes for all sampling campaigns is given in Table 1.
The mean O3 concentrations found in the present study were considerably higher than those obtained by other studies performed in Turkey (Bozkurt et al. 2018) and South Korea (Kim and Kim 2002). However, in another study (Burley and Bytnerowicz 2011) conducted at different altitudes in a mountainous area, significantly higher O3 levels (50.98 ppb O3 at 3783 m and 52.68 ppb O3 in 4342 m altitudes) were detected. In Ontario Sandbanks, Provincial Park located at the seaside has thousands of visitors annually (> 600,000) especially during the summer gave an average of 39.4 ppb ozone concentration for 2-year sampling (Blanchard and Aherne 2019). This was comparable to our data. The mean O3 concentration obtained in the 2017-winter campaign was found to be higher than that of other campaigns. Although it is known that O3 concentration is maximum in summer due to its formation under high solar radiation (Civan et al. 2015; Sicard et al. 2013; Zhao et al. 2016), 2017 winter concentration was higher than the 2017 summer concentration in this study. However, the average concentration found in the 2017-summer campaign was higher than the 2016-winter campaign.
In the present study, the mean NO2 concentrations were found to be lower than those of many outdoor studies (Algar et al. 2004; Bozkurt et al. 2018; Hien et al. 2014). The concentrations of NO2 decreased gradually in one year due to the decrease in the number of motor vehicles with old equipment and/or fuel type. The ongoing decrease in summer was related to the reduction in traffic circulation with the beginning of the holiday season (Caballero et al. 2012). The seasonal difference may also be attributed to meteorological parameters. Increasing temperature and solar radiation favor photochemical activity and thereby the destruction of NO2 (Derwent et al. 1995). In addition, strong winds and higher mixing heights achieved in summer lead to dilution and, thereby, reduction of the concentration of the pollutant in the sampling area (Dörter et al. 2020; Gupta et al. 2008).
Although the mean SO2 concentrations determined in the present study were found to be lower than the results reported in the literature (Adon et al. 2016; Bozkurt et al. 2018; He et al. 2014; Hien et al. 2014), the results were comparable with the studies conducted in urban regions of Uganda (Kirenga et al. 2015) and Ghana (Arku et al. 2008). SO2 concentrations exhibited significant enhancement (threefold) between the two winter seasons. The inter-annual difference indicates that the use of natural gas and coal for domestic heating is more dominant in the 2017-winter campaign may be due to lower mean temperature (− 0.19 °C) compared to the 2016 campaign (9.21 °C). The contribution of industrial and anthropogenic activities to the SO2 concentrations in the summer season was not significant. Source differentiations between the sampling periods are revealed with the distribution maps in the following section.
The BTEX concentrations were found to be lower than those determined by Bozkurt et al. (2018), Demirel et al. (2014), Miri et al. (2016), and Monod et al. (2001) and comparable with the results of the study performed in the suburban region of Poland (Marć et al. 2014). In the study conducted in Kütahya (Artun et al. 2017), which is characterized as a thermal power plant city in Turkey, the atmospheric concentrations of benzene were found to be lower both in winter (2.4 times) and summer (1.3 times), and atmospheric concentrations of other VOCs were obtained at comparable levels with the present study. BTEX concentrations in winter were remarkably higher than in summer because of increased anthropogenic sources and poor dispersion due to stagnant air conditions. Moreover, in winter, the absence of favorable conditions for photochemical reactions such as high temperatures and intense solar radiation limits the photochemical depletion of BTEXs, and this leads to higher concentrations in winter compared to summer.
Pollutant correlations
Pearson correlation constants for O3, NO2, SO2, and BTEXs are given in Table 2. There were negative correlations of O3 with NO2 and BTEXs, especially in summer. The formation of O3 because of photochemical reactions between nitrogen oxides (NO + NO2 = NOx) and BTEXs in the presence of sunlight caused a reverse relationship between O3 and its precursors. NO2 and SO2, which have common sources such as domestic heating in the winter period, were found in an intermediate correlation in winter, but there was no correlation in summer. Strong correlations were obtained among toluene, ethylbenzene, and xylenes (TEX). These high correlations show that they are produced in the same way and/or emitted from the same sources. Relatively lower correlations were observed between TEX and benzene that might be because the benzene is mostly emitted from vehicles, while TEX compounds can also be emitted from various industrial activities (Artun et al. 2017).
Spatial analysis
The spatial variations of O3 concentrations for three sampling campaigns are given in Fig. 2. Ozone was found to have approximately the same spatial distribution throughout all campaigns. The higher concentrations were observed in rural and woodland regions and away from the main roads. Forests emit biogenic volatile organic compounds that have an essential role in the ozone formation mechanism (Wu et al. 2020). Ozone is a secondary pollutant resulting from photochemical reactions which can take several hours or days. High O3 concentrations are observed in the downwind of the source regions of precursors since O3 can be transported over long distances. Although its precursors (VOC and NOx) are commonly found in an urban atmosphere, the depletion of O3 by NOx causes lower O3 concentrations in urban sites (Gaga et al. 2012; Sicard et al. 2013). The concentrations of O3 were high around the city center in winter 2017 that might be due to very low mixing heights (as low as 150 m) observed in winter.
Contrary to O3, NO2 concentrations were higher at sampling sites located along the major roads (TEM Highway and D-100 Highway) in winter (Fig. 3). It is also noteworthy that NO2 concentrations gradually decreased with increasing distance from vehicular emissions. The rural sites had generally low NO2 concentrations. However, unlike the winter sampling, an increase in atmospheric NO2 concentrations was not detected on the highways in the summer. The concentrations of NO2 were lower in the summer sampling period which is a holiday time. The high concentrations were obtained along the roads of the Gölcük Lake and the Abant Lake Natural Parks. These natural parks are the very popular recreational and tourist area of Turkey. People generally come from the nearby cities (İstanbul, Ankara, Kocaeli) with tour service buses and private vehicles and visit the nature parks in the summertime.
The spatial variations of the SO2 concentrations obtained in all campaigns are given in Fig. 4. Since there was still usage of coal besides natural gas for domestic heating, the concentrations of SO2 were higher around populated settlement areas and industrial regions in winter. In summer, SO2 concentrations obtained from sampling points close to industrial sites and upland/picnic areas were higher than the city center. SO2 may be emitted in upland and recreational areas due to activities that use coal and wood, such as domestic heating and barbecue. Since the temperature in upland areas is very low (average 10–15 °C) in summer, people are using coal and wood for domestic heating.
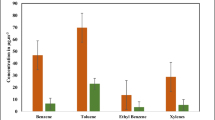
The spatial variations of BTEXs concentrations are given in Fig. 5. The highest BTEX concentrations were obtained in the city center as 4.53 µg m−3 for benzene, 5.25 µg m−3 for toluene, 0.88 µg m−3 for ethylbenzene, 1.30 µg m−3 for m,p-xylene, and 1.32 µg m−3 for o-xylene. There was a distinct difference between the seasons in the Kartalkaya ski center region, which is a winter tourism center. Traffic emissions significantly increased toluene, ethylbenzene and xylene concentrations during the winter period. On the other hand, the similarity in the distribution of TEX compounds suggests that there are common sources for these compounds. Their concentrations were found to be much higher in the city center where the traffic and population are high and, in the region, where small-scale factories are located. Benzene was found to be effective along the main roads and in the city center. The concentrations of BTEXs were low in the summer. Their concentrations decreased, while O3 was formed, as was mentioned previously.
Diagnostic ratios
Benzene and toluene are constituents of gasoline, and the toluene content in gasoline is 3–4 times higher than that of benzene. Since these compounds can be released into the atmosphere from motor vehicle exhaust, the ratio of toluene to benzene (T/B) is mostly used as an indicator of traffic emissions (Cerón-Bretón et al. 2015; Pekey and Yılmaz 2011). T/B ratios between 1.5 and 3 are assumed to be characteristic of traffic emission (Kelessis et al. 2006; Pekey and Yılmaz 2011), while higher T/B ratios can be explained with large additional sources of toluene (industrial applications, evaporation sources) (Artun et al. 2017; Bozkurt et al. 2018; Laowagul et al. 2008; Petracchini et al. 2016). The average T/B ratios for winter and summer seasons were found to be 1.56 ± 0.83 and 1.50 ± 0.69, respectively. The values show that traffic emissions could be an effective source in the Bolu plateau. The distributions of T/B ratios for both seasons are given in Fig. 6. Remarkably, the highest ratios (6.35 for winter and 4.10 for summer) were determined at the sampling points representing the surrounding villages. Non-traffic sources such as evaporation from paints and/or solvents are thought to be more effective at these points. In addition, the ratios below 3 were determined along the TEM and D100 highways, which can be seen quite clearly on the winter map.
Another diagnostic VOC ratio, xylene-to-ethylbenzene (X/E), is used to characterize the age of air mass. These compounds have constant emission rates, and the X/E ratio in the source samples is assumed to be 3.5 ± 0.5 (Monod et al. 2001). However, m,p-xylene is more reactive than ethylbenzene. As the air mass moves, the faster depletion of m,p-xylene causes the decrease of the X/E ratio. The average X/E ratios for winter and summer seasons were obtained as 1.00 ± 0.17 and 1.46 ± 0.37, respectively. The slightly higher X/E ratios in summer can be a result of increased mixing height and therefore relatively fresh emissions. The distributions of X/E ratios were similar in both seasons (Fig. 6). The higher values (not exceed 1.48 for winter and 2.33 for summer) around the city center indicate the presence of fresh emissions. On the other hand, the lower X/E ratios in rural sites showed that air mass was transported from distant sources to the region.
Ozone formation potential (OFP)
The OFP values of BTEXs were calculated for winter and summer. The relationship between atmospheric O3 concentrations, the total OFPs of BTEXs, and the meteorological factors are given in Fig. 7. The total OFP value of BTEXs was found to be higher in winter. Also, the atmospheric concentration of O3 was higher in winter when the total OFP was higher. However, as mentioned in the previous sections, it is not expected that the O3 concentration will be low in the summer when the key factors in ozone formation such as temperature and solar radiation are at high levels. Although the total OFP value of BTEX compounds was found to be lower in summer, it was stated that especially biogenic compounds played a more effective role in ozone formation in the summer (Dörter 2021).
In both seasons, toluene made the highest contribution (39% for winter and 41% for summer) to the total OFP, while benzene was the compound that made the lowest contribution (5% for winter and 4% for summer). Winter and summer OFP values of toluene were 6.46 ± 3.42 μg m−3 and 1.22 ± 0.89 μg m−3, respectively. It was followed by o-xylene (5.02 ± 3.23 μg m−3 for winter and 0.56 ± 0.56 μg m−3 for summer), m,p-xylene (3.21 ± 2.07 μg m−3 for winter and 0.86 ± 0.93 μg m−3 for summer), ethylbenzene (1.13 ± 0.63 μg m−3 for winter and 0.19 ± 0.17 μg m−3 for summer), and benzene (0.75 ± 0.44 μg m−3 for winter and 0.12 ± 0.06 μg m−3 for summer).
Health risk assessment
The lifetime cancer risk (LCR) due to inhalation exposure to benzene was determined with the Monte Carlo simulation method. The mean and median cancer risks were found to be 4.41 × 10−6 and 2.47 × 10−6, respectively. As seen in Fig. 8, the difference between the mean and median values may be a result of the lognormal distribution of benzene concentrations. The mean LCR of benzene (4.41 × 10−6) is comparable with the limit value determined by USEPA (1 × 10–6). Moreover, it was found in the range of the limit recommended by the WHO (1 × 10−6 to 1 × 10−5). There are also studies that classify compounds having LCRs of more than 1 × 10−4 as “definite risk,” between 1 × 10−5 and 1 × 10−4 as “probable risk,” between 1 × 10−6 and 1 × 10−5 as “possible risk,” and less than 1 × 10−6 as “negligible risk” (Bretón et al. 2017; Kumar et al. 2018; Sexton et al. 2007; Zhang et al. 2018). Therefore, the population living in the Bolu plateau could be at possible risk of suffering cancer due to benzene exposure through inhalation.
Compounds assumed to have a hazard ratio equal to or less than 1 do not pose a non-carcinogenic risk to the community (USEPA 2011). The non-cancer hazard ratios (HR) of BTEXs for different outdoor environments (villages, roadside, and city center) for both seasons were documented in Table 3. For all regions, HR values of all compounds were lower than the threshold value defined by USEPA.
Conclusions
In this study, simultaneous sampling of air pollutants including O3, NO2, SO2, and BTEXs at many points was carried out by passive sampling method. There was a substantial spatial variation for all pollutants. Most of the pollutants were found in high concentrations in urban and industrial areas, while O3 was detected in high concentrations in rural/forest areas. This is due to the easy transport of O3, the richness of biogenic VOCs in the forested areas, and the lower NO concentrations in these areas. NO2 concentrations were mostly dependent on vehicular traffic. As moving away from the roads, their concentrations decreased gradually. The high SO2 concentrations obtained in populated settlement areas and industrial regions reflect emissions from domestic heating and industrial activities in winter. However, SO2 concentrations were higher around sampling sites close to industrial sites and upland/picnic areas in summer. As a result of the continuity of the SO2 sources throughout the year, which was also seen in the spatial analysis, there was no significant difference in the atmospheric concentrations of this pollutant between summer and winter. The highest BTEXs concentrations were obtained in the city center, followed by the industrial region. Winter concentrations of NO2 and BTEXs were found to be higher than that of summer. Their seasonal variability is mainly influenced by parameters such as atmospheric fate, meteorological conditions, and emission strength. Diagnostic ratios were used to estimate source profiles and characterize air mass. The average T/B ratios for both seasons were found in the range representing the traffic emission. According to the distribution maps, the effect of this source was much greater around TEM and D100 highways. Moreover, the average of X/E ratios showed that there was an aged air mass contribution in the settlement areas. Among the VOCs examined in both seasons, toluene was determined as the compound with the highest ozone formation potential and its contribution to the total OFP was determined as 39% and 41% for winter and summer, respectively. Therefore, atmospheric toluene concentrations should be considered to develop an effective strategy based on O3 reduction in the region. The mean LCR through inhalation of benzene was found to be comparable with the limit value recommended by USEPA, whereas it was in the acceptable range of WHO. With the help of another commonly used categorization, it was determined as a possible risk group. Also, HR values indicated that non-carcinogenic risks related to BTEXs were negligible for villages, roadside, and city center over the Bolu plateau.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.
References
Adon M, Yoboué V, Galy-Lacaux C, Liousse C, Diop B, Gardrat E, Doumbia E, Ndiaye S, Jarnot C (2016) Measurements of NO2, SO2, NH3, HNO3 and O3 in west African urban environments. Atmos Environ 135:31–40. https://doi.org/10.1016/j.atmosenv.2016.03.050
Algar OG, Pichini S, Basagana X, Puig C, Vall O, Torrent M, Harris J, Sunyer J, Cullinan P (2004) Concentrations and determinants of NO2 in homes of Ashford, UK and Barcelona and Menorca, Spain. Indoor Air 14:298–304. https://doi.org/10.1111/j.1600-0668.2004.00256.x
Alghamdi MA, Khoder M, Abdelmaksoud AS, Harrison RM, Hussein T, Lihavainen H, Al-Jeelani H, Goknil MH, Shabbaj II, Almehmadi FM, Hyvärinen AP, Hämeri K (2014) Seasonal and diurnal variations of BTEX and their potential for ozone formation in the urban background atmosphere of the coastal city Jeddah, Saudi Arabia. Air Qual Atmos Health 7:467–480. https://doi.org/10.1007/s11869-014-0263-x
An J, Zhu B, Wang H, Li Y, Lin X, Yang H (2014) Characteristics and source apportionment of VOCs measured in an industrial area of Nanjing, Yangtze River Delta, China. Atmos Environ 97:206–214. https://doi.org/10.1016/j.atmosenv.2014.08.021
Arku RE, Vallarino J, Dionisio KL, Willis R, Choi H, Wilson JG, Hemphill C, Agyei-Mensah S, Spengler JD, Ezzati M (2008) Characterizing air pollution in two low-income neighborhoods in Accra, Ghana. Sci Total Environ 402:217–231. https://doi.org/10.1016/j.scitotenv.2008.04.042
Artun GK, Polat N, Yay OD, Üzmez ÖÖ, Ari A, Tuygun GT, Elbir T, Altuğ H, Dumanoğlu Y, Döğeroğlu T, Dawood A, Odabaşı M, Gaga EO (2017) An integrative approach for determination of air pollution and its health effects in a coal fired power plant area by passive sampling. Atmos Environ 150:331–345. https://doi.org/10.1016/j.atmosenv.2016.11.025
Blanchard D, Julian Aherne J (2019) Spatiotemporal variation in summer ground-level ozone in the Sandbanks Provincial Park, Ontario. Atmosp Pollut Res 10:931–940. https://doi.org/10.1016/j.apr.2019.01.001
Bolden AL, Kwiatkowski CF, Colborn T (2015) New look at BTEX: are ambient levels a problem? Environ Sci Technol 49:5261–5276. https://doi.org/10.1021/es505316f
Bolu Directorate of Environment and Urbanization (BDEU) (2018) Bolu Provincial Environmental Status Report for 2018, Bolu
Bozkurt Z, Üzmez ÖÖ, Döğeroğlu T, Artun G, Gaga EO (2018) Atmospheric concentrations of SO2, NO2, ozone and VOCs in Düzce, Turkey using passive air samplers: sources, spatial and seasonal variations and health risk estimation. Atmos Pollut Res 9:1146–1156. https://doi.org/10.1016/j.apr.2018.05.001
Bretón JGC, Bretón RMC, Ucan FV, Baeza CB, Fuentes MDLLE, Lara ER, Marrón MR, Pacheco JAM, Guzmán AR, Chi MPU (2017) Characterization and sources of Aromatic Hydrocarbons (BTEX) in the atmosphere of two urban sites located in Yucatan Peninsula in Mexico. Atmosphere 8:107. https://doi.org/10.3390/atmos8060107
Burley JD, Bytnerowicz A (2011) Surface ozone in the White Mountains of California. Atmos Environ 45:4591–4602. https://doi.org/10.1016/j.atmosenv.2011.05.062
Caballero S, Esclapez R, Galindo N, Mantilla E, Crespo J (2012) Use of a passive sampling network for the determination of urban NO2 spatiotemporal variations. Atmos Environ 63:148–155. https://doi.org/10.1016/j.atmosenv.2012.08.071
Carmichael GR, Ferm M, Thongboonchoo N, Woo JH, Chan LY, Murano K, Viet PH, Mossberg C, Bala R, Boonjawat J, Upatum P, Mohan M, Adhikary SP, Shrestha AB, Pienaar JJ, Brunke EB, Chen T, Jie T, Guoan D, Peng LC, Dhiharto S, Harjanto H, Jose AM, Kimani W, Kirouane A, Lacaux JP, Richard S, Barturen O, Cerda JC, Athayde A, Tavares T, Cotrina JS, Bilici E (2003) Measurements of sulfur dioxide, ozone and ammonia concentrations in Asia, Africa, and South America using passive samplers. Atmos Environ 37:1293–1308. https://doi.org/10.1016/S1352-2310(02)01009-9
Carter WPL (1994) Development of ozone reactivity scales for volatile organic compounds. J Air Waste Manage Assoc 44:881–899. https://doi.org/10.1080/1073161X.1994.10467290
Cerón-Bretón JG, Cerón-Bretón RM, Kahl JDW, Ramírez-Lara E, Guarnaccia C, Aguilar-Ucán CA, Montalvo-Romero C, Anguebes-Franseschi F, López-Chuken U (2015) Diurnal and seasonal variation of BTEX in the air of Monterrey, Mexico: preliminary study of sources and photochemical ozone pollution. Air Qual Atmos Health 8:469–482. https://doi.org/10.1007/s11869-014-0296-1
Choi YH, Kim JH, Lee BE, Hong YC (2014) Urinary benzene metabolite and insulin resistance in elderly adults. Sci Total Environ 482:260–268. https://doi.org/10.1016/j.scitotenv.2014.02.121
Civan M, Elbir T, Seyfioğlu R, Kuntasal Ö, Bayram A, Doğan G, Yurdakul S, Andiç Ö, Müezzioğlu A, Sofuoğlu S, Pekey H, Pekey B, Bozlaker A, Odabaşı M, Tuncel G (2015) Spatial and temporal variations in atmospheric VOCs, NO2, SO2, and O3 concentrations at a heavily industrialized region in Western Turkey, and assessment of the carcinogenic risk levels of benzene. Atmos Environ 103:102–113. https://doi.org/10.1016/j.atmosenv.2014.12.031
Cotrozzi L (2020) Leaf demography and growth analysis to assess the impact of air pollution on plants: a case study on alfalfa exposed to a gradient of sulphur dioxide concentrations. Atmos Pollut Res 11:186–192. https://doi.org/10.1016/j.apr.2019.10.006
De Marco A, Proietti C, Anav A, Ciancarella L, D’Elia I, Fares S, Francesca Fornasier M, Fusaro L, Gualtieri M, Manes F, Marchetto A, Mircea M, Paoletti E, Piersanti A, Rogora M, Salvati L, Salvatori E, Screpanti A, Vialetto G, Vitale M, Leonardi C (2019) Impacts of air pollution on human and ecosystem health, and implications for the National Emission Ceilings Directive: Insights from Italy. Environ Int 125:320–333. https://doi.org/10.1016/j.envint.2019.01.064
Demirel G, Özden Ö, Döğeroğlu T, Gaga EO (2014) Personal exposure of primary school children to BTEX, NO2 and ozone in Eskişehir, Turkey: relationship with indoor/ outdoor concentrations and risk assessment. Sci Total Environ 473:537–548. https://doi.org/10.1016/j.scitotenv.2013.12.034
Derwent RG, Middleton DR, Field RA, Goldstone ME, Lester JN, Perry R (1995) Analysis and interpretation of air quality data from an urban roadside location in central London over the period from July 1991 to July 1992. Atmos Environ 29:923–946. https://doi.org/10.1016/1352-2310(94)00219-B
Dörter M, Odabasi M, Yenisoy-Karakaş S (2020) Source apportionment of biogenic and anthropogenic VOCs in Bolu plateau. Sci Total Environ 731:139201. https://doi.org/10.1016/j.scitotenv.2020.139201
Dörter M (2021) Investigation of spatial and seasonal variations of anthropogenic and biogenic volatile organic carbons (VOCs) determined by thermal desorption GC-MS technique and contribution of biogenic VOCs to ozone concentrations at high altitude semi-rural site. Ph.D. Thesis. Bolu Abant Izzet Baysal University, Bolu, Turkey
Gaga EO, Döğeroğlu T, Özden Ö, Ari A, Yay OD, Altuğ H, Akyol N, Örnektekin S, Van Doorn W (2012) Evaluation of air quality by passive and active sampling in an urban city in Turkey: current status and spatial analysis of air pollution exposure. Environ Sci Pollut Res 19:3579–3596. https://doi.org/10.1007/s11356-012-0924-y
Gupta AK, Karar K, Ayoob S, John K (2008) Spatio-temporal characteristics of gaseous and particulate pollutants in an urban region of Kolkata, India. Atmos Res 87:103–115. https://doi.org/10.1016/j.atmosres.2007.07.008
He J, Xu H, Balasubramanian R, Chan CY, Wang C (2014) Comparison of NO2 and SO2 measurements using different passive samplers in tropical environment. Aerosol Air Qual Res 14:355–363. https://doi.org/10.4209/aaqr.2013.02.0055
Hedley AJ, Wong CM, Thach TQ, Ma S, Lam TH, Anderson HR (2002) Cardiorespiratory and all-cause mortality after restrictions on sulphur content of fuel in Hong Kong: an intervention study. The Lancet 360:1646–1652. https://doi.org/10.1016/S0140-6736(02)11612-6
Hien PD, Hangartner M, Fabian S, Tan PM (2014) Concentrations of NO2, SO2, and benzene across Hanoi measured by passive diffusion samplers. Atmos Environ 88:66–73. https://doi.org/10.1016/j.atmosenv.2014.01.036
IARC (1987) IARC monographs on the evaluation of carcinogenic risks to humans. In: Overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1 to 42. Suppl 7. World Health Organization, Lyons, France
IARC (2000) Some industrial chemicals. In: Ethylbenzene. IARC monographs on the evaluation of carcinogenic risks to humans volume 77. World Health Organization, International Agency for Research on Cancer, Lyon, France
Johns DO, Pinto JP, Kim JY, Owens EO (2011) Respiratory effects of short term peak exposures to sulfur dioxide. Encycl Environ Health 5:526–533. https://doi.org/10.1016/B978-0-444-63951-6.00726-9
Kelessis AG, Petrakakis MJ, Zoumakis NM (2006) Determination of benzene, toluene, ethylbenzene, and xylenes in urban air of Thessaloniki, Greece. Environ Toxicol 21:440–443. https://doi.org/10.1002/tox.20197
Kim KH, Kim MY (2002) The distributions of BTEX compounds in the ambient atmosphere of the Nan-Ji-Do abandoned landfill site in Seoul. Atmos Environ 36:2433–2446. https://doi.org/10.1016/S1352-2310(02)00191-7
Kirenga BJ, Meng Q, Van Gemert F, Aanyu-Tukamuhebwa H, Chavannes N, Katamba A, Obal G, Van der Molen T, Schwander S, Mohsenin V (2015) The state of ambient air quality in two Ugandan cities: a pilot cross-sectional spatial assessment. Int J Environ Res Public Health 12:8075–8091. https://doi.org/10.3390/ijerph120708075
Krupa SV, Manning WJ (1988) Atmospheric ozone: formation and effects on vegetation. Environ Pollut 50:101–137. https://doi.org/10.1016/0269-7491(88)90187-X
Kumar A, Singh D, Kumar K, Singh BB, Jain VK (2018) Distribution of VOCs in urban and rural atmospheres of subtropical India: temporal variation, source attribution, ratios, OFP and risk assessment. Sci Total Environ 613:492–501. https://doi.org/10.1016/j.scitotenv.2017.09.096
Laowagul W, Garivait H, Limpaseni W, Yoshizumi K (2008) Ambient air concentrations of benzene, toluene, ethylbenzene and xylene in Bangkok, Thailand during April-August in 2007. Asian J Atmos 2:14–25. https://doi.org/10.5572/ajae.2008.2.1.014
Li B, Ho SSH, Gong S, Ni J, Li H, Han L, Yang Y, Qi Y, Zhao D (2019) Characterization of VOCs and their related atmospheric processes in a central Chinese city during severe ozone pollution periods. Atmos Chem Phys 19:617–638. https://doi.org/10.5194/acp-19-617-2019
Marć M, Zabiegała B, Namieśnik J (2014) Application of passive sampling technique in monitoring research on quality of atmospheric air in the area of Tczew, Poland. Int J Environ Anal Chem 94:151–167. https://doi.org/10.1080/03067319.2013.791979
Miri M, Shendi MRA, Ghaffari HR, Aval HE, Ahmadi E, Taban E, Gholizadeh A, Aval MY, Mohammadi A, Azari A (2016) Investigation of outdoor BTEX: concentration, variations, sources, spatial distribution, and risk assessment. Chemosphere 163:601–609. https://doi.org/10.1016/j.chemosphere.2016.07.088
Monod A, Sive BC, Avino P, Chen T, Blake DR, Rowland FS (2001) Monoaromatic compounds in ambient air of various cities: a focus on correlations between the xylenes and ethylbenzene. Atmos Environ 35:135–149. https://doi.org/10.1016/S1352-2310(00)00274-0
Özden Ö (2005) Monitoring of air quality by use of passive samplers. MSc Thesis. Anadolu University, Eskişehir, Turkey
Özden Ö, Döğeroğlu T (2012) Performance evaluation of a tailor-made passive sampler for monitoring of troposheric ozone. Environ Sci Pollut Res Int 19:3200–3209. https://doi.org/10.1007/s11356-012-0825-0
Pekey B, Yılmaz H (2011) The use of passive sampling to monitor spatial trends of volatile organic compounds (VOCs) at an industrial city of Turkey. Microchem J 97:213–219. https://doi.org/10.1016/j.microc.2010.09.006
Petracchini F, Paciucci L, Vichi F, D’Angelo B, Aihaiti A, Liotta F, Paolini V, Cecinato A (2016) Gaseous pollutants in the city of Urumqi, Xinjiang: spatial and temporal trends, sources and implications. Atmos Pollut Res 7:925–934. https://doi.org/10.1016/j.apr.2016.05.009
Presto AA, Dallmann TR, Gu P, Rao U (2016) BTEX exposures in an area impacted by industrial and mobile sources: source attribution and impact of averaging time. J Air Waste Manage Assoc 66:387–401. https://doi.org/10.1080/10962247.2016.1139517
Sexton K, Linder SH, Marko D, Bethel H, Lupo PJ (2007) Comparative assessment of air pollution–related health risks in Houston. Environ Health Perspect 115:1388–1393. https://doi.org/10.1289/ehp.10043
Sicard P, De Marco A, Troussier F, Renou C, Vas N, Paoletti E (2013) Decrease in surface ozone concentrations at Mediterranean remote sites and increase in the cities. Atmos Environ 79:705–715. https://doi.org/10.1016/j.atmosenv.2013.07.042
Song Y, Shao M, Liu Y, Lu S, Kuster W, Goldan P, Xie S (2007) Source apportionment of ambient volatile organic compounds in Beijing. Environ Sci Technol 41:4348–4353. https://doi.org/10.1021/es0625982
Stranger M, Krata A, Kontozova-Deutsch V, Bencs L, Deutsch F, Worobiec A, Naveau I, Roekens E, Van Grieken R (2008) Monitoring of NO2 in the ambient air with passive samplers before and after a road reconstruction event. Microchem J 90:93–98. https://doi.org/10.1016/j.microc.2008.04.001
Tecer LH, Tagil S, Ulukaya O, Ficici M (2017) Spatial distribution of BTEX and inorganic pollutants during summer season in Yalova, Turkey. Ecol Chem Eng S 24:565–581. https://doi.org/10.1515/eces-2017-0037
Turkish State Meteorological Service (2018) Genel İstatistik Verileri. https://www.mgm.gov.tr/veridegerlendirme/il-ve-ilceler-istatistik.aspx?k=A. Accessed 16 June 2020.
United Nations Economic Commission for Europe Convention on long-range transboundary air pollution International Co-operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests (ICP FORESTS) manual on methods and criteria for harmonized sampling, assessment, monitoring and analysis of the effects of air pollution on forests (2010) Part XV: Monitoring of Air Quality, Hamburg, Germany
USEPA-IRIS (2015) https://cfpub.epa.gov/ncea/iris2/atoz.cfm. Accessed 16 June 2020.
USEPA (2011) Exposure factors handbook 2011 edition (Final). U.S. Environmental Protection Agency, Washington, DC EPA/600/R-09/052F. Accessed 16 June 2020
USEPA (2017) https://www3.epa.gov/ceampubl/learn2model/parttwo/onsite/estdiffusion-ext.html Accessed 16 June 2020
Wu K, Yang X, Chen D, Gu S, Lu Y, Jiang Q, Wang K, Ou Y, Qian Y, Shaoa P, Lu S (2020) Estimation of biogenic VOC emissions and their corresponding impact on ozone and secondary organic aerosol formation in China. Atmos Res 231:104656. https://doi.org/10.1016/j.atmosres.2019.104656
Xu X, Freeman NC, Dailey AB, Ilacqua VA, Kearney GD, Talbott EO (2009) Association between exposure to alkylbenzenes and cardiovascular disease among national health and nutrition examination survey (NHANES) participants. Int J Occup Environ Health 15:385–391. https://doi.org/10.1179/oeh.2009.15.4.385
Yan Y, Peng L, Li R, Li Y, Li L, Bai H (2017) Concentration, ozone formation potential and source analysis of volatile organic compounds (VOCs) in a thermal power station centralized area: a study in Shuozhou, China. Environ Pollut 223:295–304. https://doi.org/10.1016/j.envpol.2017.01.026
Zhang Z, Yan X, Gao F, Thai P, Wang H, Chen D, Zhou L, Gong D, Li Q, Morawska L, Wang B (2018) Emission and health risk assessment of volatile organic compounds in various processes of a petroleum refinery in the Pearl River Delta, China. Environ Pollut 238:452–461. https://doi.org/10.1016/j.envpol.2018.03.054
Zhao S, Yu Y, Yin D, He J, Liu N, Qu J, Xiao J (2016) Annual and diurnal variations of gaseous and particulate pollutants in 31 provincial capital cities based on in situ air quality monitoring data from China National Environmental Monitoring Center. Environ Int 86:92–106. https://doi.org/10.1016/j.envint.2015.11.003
Acknowledgements
This study was a part of the theses “Spatial Monitoring of Gaseous Pollutants in the City Center of Bolu by Passive Sampling Method” and “Investigation of Spatial and Seasonal Variations of Anthropogenic and Biogenic Volatile Organic Carbons (VOCs) Determined by Thermal Desorption GC-MS Technique and Contribution of Biogenic VOCs to Ozone Concentrations at High Altitude Semi-Rural Site.” The authors thank TUBITAK and BAIBU-BAP for financial support. The authors also thank Hatice Karadeniz, Tuğçe Demir, Ercan Berberler, Melike Büşra Bayramoğlu Karşı, Sait Karşı, Akif Arı, Pelin Ertürk Arı, Tuğçe Koçak, and Zafer Dörter for their help in the sampling studies. The authors also acknowledge Mihriban Civan for her help in the health risk assessment part.
Funding
It was supported by the Scientific and Technical Research Council of Turkey (TUBITAK) under the grant of 114Y632 and the Scientific Research Projects Coordination Unit of Bolu Abant Izzet Baysal University (BAIBU-BAP) under the grants of 2014.03.03.789 and 2015.03.03.891.
Author information
Authors and Affiliations
Contributions
MD participated in all sampling processes, analyzed and interpreted data, and was a major contributor in writing the manuscript. EMT participated in a large part of the sampling, analyzed and interpreted data, and approved the last version of the manuscript. TD developed, validated inorganic passive samplers and made resources available for the extraction, and approved the last version of the manuscript. ÖÖÜ developed and validated inorganic passive samplers and supervised the sample extraction process for inorganic pollutants, reviewed, edited, and approved the last version of the manuscript. EOG developed and validated inorganic passive samplers and made resources available for the extraction, and reviewed, edited, and approved the last version of the manuscript. DK participated in all sampling processes, conducted ion chromatography experiments, and reviewed, edited, and approved the last version of the manuscript. SYK participated in a large part of the sampling, interpreted data, supervised the research activity, and wrote, reviewed, edited, and approved the last version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gerhard Lammel
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dörter, M., Mağat-Türk, E., Döğeroğlu, T. et al. An assessment of spatial distribution and atmospheric concentrations of ozone, nitrogen dioxide, sulfur dioxide, benzene, toluene, ethylbenzene, and xylenes: ozone formation potential and health risk estimation in Bolu city of Turkey. Environ Sci Pollut Res 29, 53569–53583 (2022). https://doi.org/10.1007/s11356-022-19608-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19608-x