Abstract
Odors are often associated with nuisance and health risks. In this study, the atmospheric levels of H2S and NH3 were determined in 5 sites near polluted urban rivers during rainy and dry periods in the city of Salvador, Brazil, as well as the relationships between these odorous compounds and meteorological and water quality parameters applying multivariate analysis, and the non-carcinogenic risks (as hazard quotient—HQ). The compounds H2S and NH3 were passively sampled and determined by molecular spectrophotometry. Average concentrations were lower in the rainy period (6.56 ± 0.83 μg m−3 for H2S; 8.67 ± 2.50 μg m−3 for NH3) than in the dry period (8.05 ± 1.44 μg m−3 for H2S; 10.62 ± 4.39 μg m−3 for NH3), probably due to lower water and air temperatures, which contribute to lower water–air transfer rates and higher precipitation and relative humidity values, thus favoring the removal of these compounds from the atmosphere. The PCA and HCA results confirmed these relationships, as well as showing strong positive correlations between NH3, N-NH4+, TN, Twater, BOD, and EC, and strong negative correlations between H2S, pH, and DO, indicating that these water quality parameters also influence the levels of H2S and NH3 in air, thereby confirming a common source for the gas emissions as being mainly from polluted rivers. The maximum H2S concentrations were above the odor threshold value established by the WHO in both periods. According to deterministic health risk assessment, the HQ values for NH3 were below the acceptable limit set by USEPA (HQ = 1). However, all HQ values found for H2S (4.28, 2.80, 1.46 at 95th percentile) for the 3 groups of the exposed population in the dry period using probabilistic risk assessment with Monte Carlo simulation were above this limit, therefore indicating human health risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In many countries, with the disorderly expansion of cities, rivers in urban areas are often disposal sites for domestic and industrial sewage, because the construction of sewage treatment systems has not kept pace with the increase in urbanization and industrialization, resulting in a significant increase in urban river pollution (Liang et al. 2018; Wan et al. 2014). This also affects the urban landscape, which is currently identified as a significant aspect of city development (Miao et al. 2020). In addition, it causes negative impacts on air quality on a local scale, mainly related to emissions of unpleasant odors and adverse effects on human health. Odors also contribute to negative economic effects through property depreciation and are considered the main causes of nuisances reported by exposed populations.
Odors are complex mixtures consisting mainly of reduced sulfur compounds (RSC) such as hydrogen sulfide, methyl sulfide, dimethyl sulfide, and dimethyl disulfide; of volatile organic compounds (VOCs), including aromatic, aliphatic, and chlorinated hydrocarbons, terpenes, short chain fatty acids, aldehydes, and ketones; and of nitrogen compounds such as ammonia and amines (Borrás et al. 2016; Sazakli and Leotsinidis 2021). Under anaerobic conditions, such as those found in urban rivers polluted predominantly by domestic sewage, hydrogen sulfide (H2S) resulting from dissimilatory sulfate reduction is the main RSC emitted into the atmosphere, causing unpleasant odors (Zhou et al. 2011). Ammonia (NH3), the major nitrogenous compound present under these conditions, is generated from the disintegration of nitrogen-rich compounds such as proteins and urea, also causing odors (Liang et al. 2018). In this paper, H2S and NH3 were chosen as typical odor-generating pollutants in areas near contaminated urban rivers due to the scarcity of studies to determine such compounds in these areas.
Hydrogen sulfide is a colorless, irritating, corrosive, flammable, asphyxiating gas, with a very unpleasant odor, perceptible at very low concentrations by humans, making smelly the areas near emission sources, in addition to causing corrosion in pipes, metallic, and concrete structures, due to the oxidation reaction of the H2S occurring in the presence of aerobic bacteria producing sulfuric acid (H2SO4) (Cruz and Campos 2008; Zuo et al. 2019). In the troposphere, H2S is oxidized mainly by hydroxyl radicals (OH•), being converted initially to sulfur dioxide (SO2), and then to H2SO4 and sulfate ion (SO42−), leading to degradation of air quality (Finlayson-Pitts and Pitts Jr 2000). The H2S acts on the central nervous system, eyes, and respiratory system, and acute exposure at high concentrations can cause paralysis of the olfactory nerve, pulmonary edema, convulsions, and even death (WHO 2000; Guidotti 2010). Studies have shown that long-term exposure to low concentrations also affects human health, causing symptoms such as eye irritation, nausea, headaches, coughing, and respiratory problems (Drimal et al. 2010; Aatamila et al. 2011).
Ammonia is also a colorless gas with an extremely strong odor, one of the main constituents of the nitrogen cycle and the principal alkaline compound with an important function in atmospheric chemistry, due to its participation in neutralization reactions of SO2, H2SO4, nitric acid (HNO3), and hydrochloric acid (HCl). The product of these reactions is a fine particulate material (PM2.5) of ammonium salts (NH4NO3, (NH4)2SO4, NH4Cl), present in submicron aerosols, cloud droplets, and precipitation, resulting in decreased visibility and risks to human health (Finlayson-Pitts and Pitts Jr 2000; Nair and Yu 2020). Furthermore, excessive deposition of nitrogen in ecosystems contributes to eutrophication aquatic environments and increased nutrients in soil (Behera et al. 2013a). Inhaled ammonia is almost completely retained in the upper respiratory tract and chronic exposure can cause respiratory irritation, coughing, chest tightness, wheezing, and impaired lung function (USEPA 2016a).
For sampling H2S, NH3, and other air pollutants, active or passive techniques are generally used in different environments. Unlike the active sampling technique, where air is sucked into the sampler with the aid of a pump, requiring a continuous supply of electrical energy or use of batteries, in addition to air flow meters, constant calibrations, as well as periodic maintenance and expensive equipment, the passive sampling is based on physical processes, such as diffusion and permeation, without the need for artificial pumping, where the driving force for the transport is the difference in chemical potential of the analyte between the sampled medium and the collection surface. Thus, the latter technique is useful for monitoring in different areas including sites where electricity is not available and therefore other techniques could not be used (Campos et al. 2010b; Zabiegała et al. 2010).
The application of the passive sampling, when compared to active sampling techniques (continuous and discontinuous), has also other significant advantages, such as low cost, small dimensions, portability, easy, and silent operation, usually which combines the sampling steps with analyte isolation and pre-concentration in a single step, and also no specialized training and maintenance are required for exposure of the samplers in the field, further reducing the operational costs. This technique is a potential tool for cost-effective monitoring of air pollutants with high spatial and temporal coverage, eliminating many disadvantages of active sampling and sample preparation techniques while producing results with high precision and accuracy (Huang et al. 2018; Marć et al. 2015; USEPA, 2014).
Principal component analysis (PCA) and hierarchical cluster analysis (HCA) are multivariate statistical techniques which are widely used to assess similarities and patterns among samples. These techniques have been applied in studies involving air quality and/or water quality to determine and evaluate possible relationships between different variables (sampling stations and samples; air pollutants and meteorological parameters; urbanization indicators, environmental variables, physicochemical and microbiological parameters of water quality; air pollutants and water quality parameters), as well as to identify pollution or emission sources (Calazans et al. 2018; Cruz et al. 2019, 2020a; Glińska-Lewczuk et al. 2016; Passos et al. 2021; Santana et al. 2019; Wan et al. 2014).
Health risk assessment (HRA) associated with the exposure to odorous compounds can be used as a tool to determine the influence of these compounds on human health (Niu et al. 2014). However, to our knowledge, no studies have so far been found in the literature assessing the risks associated with the health of exposed populations in areas near to polluted urban rivers.
In recent years, some studies have been carried out in order to determine the composition and concentration of odorous compounds emitted into the atmosphere mainly from wastewater treatment plants (WWTPs) and municipal solid waste (MSW) landfills (Chang et al. 2019; Godoi et al. 2018; Guo et al. 2017; Kim et al. 2013; Lasaridi et al. 2010; Niu et al. 2014), as well as to assess the associated health risks to the population of nearby areas and/or workers of WWTPs or MSW landfills exposed to odorous compounds (Cheng et al. 2019; Godoi et al. 2018; Niu et al. 2014; Ulutaş et al. 2021; Wang et al. 2020; Wu et al. 2018). However, studies conducted in areas near to polluted urban rivers to quantify odorous compounds and apply multivariate statistical techniques are scarce (Wan et al. 2014).
In Brazil, the urban population is over 160 million people, corresponding to about 84% of the total population, according to the latest census conducted by the Brazilian Institute of Geography and Statistics (IBGE 2010). Moreover, 45% of Brazilians do not have access to basic sanitation and 57% of the sewage generated per day (about 9.0 ton) does not receive adequate treatment (ANA 2017). Nevertheless, in Brazilian cities, few studies have been conducted to determine atmospheric levels of odorous compounds in urban areas near open sewers, wastewater treatment plants (WWTPs), or streams polluted by domestic sewage (Campos et al. 2010a; Godoi et al. 2018; Oliveira et al. 2019).
Thus, the objectives of the present study were to determine the concentrations of H2S and NH3 in air ambient of five sites near polluted rivers in the city of Salvador, Bahia, Brazil, during two different seasons (rainy and dry) using passive sampling, and to evaluate the relationships between the concentrations of these compounds, physicochemical parameters of water quality and meteorological parameters using multivariate statistical techniques. Furthermore, estimating the non-carcinogenic risks for three population groups associated with exposure to studied odorous compounds by inhalation was also part of these objectives.
The results of this study can also contribute to encourage governments on the need to implement public policies in order to achieve the Sustainable Development Goals (SDGs) contained in the United Nations Agenda 2030 (UN 2019), linked specifically to goal 6 which aims to ensure availability and sustainable management of water and sanitation for all, since the lack of basic sanitation has been identified as one of the main causes of pollution of water resources, besides also influencing air quality.
Material and methods
Sampling
The city of Salvador, capital of the state of Bahia, in northeastern Brazil, has a tropical climate and presents a precipitation profile with two well-defined periods, the rainy one occurring from April to the first half of September, and the dry one from the second half of September to March. This city has about 3.0 million inhabitants and twelve watersheds that are currently impacted by anthropogenic activities, mainly by the discharge of domestic sewage. River waters are generally dark and have an unpleasant odor, indicating severe pollution of these aquatic environments.
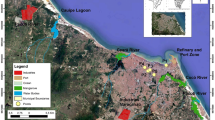
Five sampling sites R1–R5 (Fig. 1a) were selected near polluted urban rivers in the city of Salvador. The first and second sampling sites (R1 and R2) are located in areas of the Pedras and Pituaçu rivers watershed, being R1 (12° 56′ 45.1″ S, 38° 27′ 05.0″ W) and R2 (12° 58′ 09.5″ S, 38° 26′ 13.7″ W) in the Tancredo Neves and the Imbuí neighborhoods, respectively. This watershed has an area of 27,050 km2, which corresponds to 8.76% of the municipal territory, being considered the fourth largest watershed of Salvador, in terms of surface area. The third sampling site (R3—12° 59′ 50.6″ S, 38° 26′ 58.0″ W) is situated in the Camarajipe River watershed area, in the Costa Azul neighborhood. This watershed has a total area of 35,877 km2, corresponding to 11.62% of the entire territory of Salvador, being the third largest watershed in extension of this city (Santos et al. 2010). The fourth sampling site (R4—13° 00′ 23.0″ S, 38° 29′ 20.8″ W) is situated in the area of the Lucaia River watershed, in the Rio Vermelho neighborhood. Located in the south of the city of Salvador, this watershed has an area of 14,740 km2, and concentrates about 11% of the population in its surroundings. The fifth sampling site (R5—13° 00′ 13.8″ S, 38° 30′ 32.5″ W) is in the Ondina river basin area, within the campus of the Federal University of Bahia (UFBA) in the Ondina neighborhood. This river basin is situated in the extreme south of Salvador, with an area of 3.08 km2, and is the smallest basin in extent, corresponding to 1% of the territory of Salvador (Santos et al. 2010).
Sampling for determination of H2S and NH3 in ambient air from five sites near polluted urban rivers in the city of Salvador was conducted in the rainy and dry seasons of 2019, using diffusive passive samplers. At each sampling site and for each compound studied, three passive samplers were simultaneously exposed at a minimum height of 2 m (Fig. 1b). These samplers based on gas molecular diffusion through static air layer (Fig. 1c) were developed locally and validated in tropical environments for exposure periods of 1 and 2 weeks to the passive sampler for H2S, and only 1 week to the passive sampler for NH3, according to previous studies (Campos et al. 2010b; Lima 2011). Thus, in this study, as the samplings of H2S and NH3 were carried out simultaneously, the exposure period used was 1 week. The detailed description and preparation of the passive samplers have been presented in previous study (Campos et al. 2010b).
Analysis and quality control
After exposure of the passive samplers, each filter was transferred to a microtube and the analytes fixed on impregnated filters were extracted with 1.5 mL of deionized water in an unheated ultrasonic bath, followed by centrifugation for 5 min (13,500 rpm).
The H2S fixed on the filters impregnated with the solution of 5.5 × 10−2 mol L−1 zinc acetate/1.0 × 10−1 mol L−1 sodium hydroxide/1.0 × 10−2 mol L−1 trisodium citrate was determined as sulfide ion by molecular spectrophotometry (λ = 670 nm) using the methylene blue method (Shanthi and Balasubramanian 1996). The NH3 attached to the filters impregnated with 3.3 × 10−3 mol L−1 oxalic acid was analyzed as ammonium ion by molecular spectrophotometry (λ = 630 nm) using the indophenol blue method (Kothny et al. 1989).
Calibration curves for H2S and NH3 based on the external standardization method were constructed from dilution in water of standardized solution of sodium sulfide 37 μg L−1 and NH4Cl 1.0 × 10−4 mol L−1, respectively. They were composed of seven points, with a linear dynamic range between 0.2 and 2.0 μg mL−1 for H2S and 18 and 540 μg L−1 for NH3. The regression equation for each calibration curve was calculated using the linear regression method. Good linear correlations were found for both analytes, with R2 > 0.9965 for H2S and R2 > 0.9980 for NH3. The LOD and LOQ values were determined by statistical analysis of the data, and the following values were obtained: 0.027 and 0.090 μg mL−1 for H2S, and 0.011 and 0.040 μg mL−1 for NH3, respectively.
Statistical analysis
PCA and HCA are multivariate statistical techniques classified as exploratory and unsupervised. The PCA is based on reducing the dimensionality of the data with the least possible loss of information, aiming to redistribute the observed variation on the original axes to obtain a set of orthogonal axes, and denominated principal components (PCs). In this analysis, the grouping of samples sets the data structure by constructing the graphs of scores and loadings, whose axes are the PCs on which the data are projected. The HCA is a multivariate technique which allows samples to be grouped based on their similarities or differences, and the results of the groupings are shown through a two-dimensional graph called dendrogram (Cruz et al. 2020a; Granato et al. 2018).
In addition to the concentrations of H2S and NH3 determined in this study, secondary data of physicochemical parameters of water quality were obtained from technical reports of environmental quality of the rivers of the city of Salvador on the official website of the Institute for the Environment and Water Resources of Bahia (INEMA) (http://www.inema.ba.gov.br), as well as meteorological parameters provided by the official website of the Brazilian National Institute of Meteorology (INMET) (https://portal.inmet.gov.br) were also used in the statistical analysis.
Information concerning meteorological parameters were obtained from the automatic meteorological station of the city of Salvador, whose operation is under the responsibility of INMET. Thus, in this study, for periods of 1 week in the dry and rainy seasons, the sum of the values of precipitation (PPT) and average values calculated from hourly values of wind speed (WS), relative humidity (RH), air temperature (Tair), and solar irradiation (SI) were used. Physicochemical parameters of water quality were monitored by INEMA at different points in each watershed. Thus, mean concentrations of ammoniacal nitrogen (N-NH4+), total nitrogen (TN), dissolved oxygen (DO), biochemical oxygen demand (BOD), mean values of hydrogen potential (pH), water temperature (Twater), and electrical conductivity (EC) obtained from locations next to the five sampling sites chosen in this study were used.
Thus, the relationships between the average measured concentrations of H2S and NH3 compounds, of seven physicochemical parameters of water quality and five meteorological parameters, were determined and evaluated in the five sampled sites. The data were submitted to multivariate analysis, applying PCA and HCA, using Statistica 7.0 software. The sampling sites were identified as R1–R5 and the letters RN and D were used to define the rainy and dry seasons, respectively.
Health risk assessment (HRA)
Constant exposure to various toxic compounds found in different environmental matrices contributes to deteriorate the health of the population directly or indirectly. Health risk assessment is an estimation of potential adverse effects on human health caused by exposure to toxic compounds and has been used to estimate potential adverse carcinogenic and non-carcinogenic health effects (Cruz et al. 2020b; Ulutaş et al. 2021; USEPA 2016b).
According to the USEPA classification system for carcinogenicity, the H2S and NH3 compounds were classified in group D, with inadequate or inexistent evidence of human carcinogenicity (USEPA 2003a, 2016a), and therefore, only the non-carcinogenic risks could be estimated regarding these compounds.
The HRA method recommended by USEPA was used in this study to estimate the health risks to the adult population due to exposure to H2S and NH3 compounds by inhalation over 25 years, at the five sites near polluted rivers in the city of Salvador (Bahia, Brazil), considering the non-carcinogenic risks, expressed as the hazard quotient (HQ) (USEPA 2016b). Three different groups of exposed population were considered: P1 represents residents who live and work near sampling sites; P2 is for residents living near the sampling sites but studying or working elsewhere; and P3 is for persons who work or study near sampling sites but reside in another location with a certain distance, as suggested by Godoi et al. (2018).
The hazard quotient (HQ) was calculated according to the following equations:
Regarding Eq. 1, EC is the exposure concentration (μg m−3); C is the concentration of the contaminant in ambient air (μg m−3); ET is the daily exposure time (h day−1); EF is the exposure frequency (day year−1), using the values of ET = 24, 15, and 8 h day−1, and EF = 350, 350, and 225 day year−1, considering P1, P2, and P3, respectively; ED is the exposure duration (25 years); and AT is the average lifetime in hours (ED × 365 day year−1 × 24 h day−1). Equation 2 includes the variables EC (previously defined in Eq. 1), and RfC, the reference concentration (μg m−3), which is specific for each compound, a level below the one at which adverse health effects are unlikely to occur during a lifetime (USEPA 2013).
Based on the hazard quotient estimate, if HQ ≤ 1, exposure to a given chemical compound is unlikely to cause adverse effects on human health (acceptable level), while values of HQ > 1 correspond to exposure to a certain compound that is likely to cause adverse effects on human health (USEPA 2016b).
HRA can be carried out by deterministic and probabilistic methods (Rajasekhar et al. 2020). Deterministic health risk assessment considers that all variables are known constants, and it was used in this study to ammonia, which has a much higher RfC value (500 μg m−3) when compared to the RfC value for H2S (2.0 μg m−3). Probabilistic risk assessment (PRA) is a general term for risk assessments which use models to represent the probability of different risk levels in a population (i.e., variability) or to characterize uncertainty in risk estimates (USEPA 2001). Monte Carlo simulation is the technique widely used for PRA, in which random values are repeatedly generated for each uncertain variable to simulate a model. Each uncertain variable is assigned a possible value that is associated with a probability distribution (Abdo et al. 2017; Yaghmaien et al. 2019).
In the case of H2S, the Monte Carlo simulation (MCS) was applied to quantify the uncertainties and sensitivity of the parameters used in the HRA associated with HQ values, using a probabilistic approach, according to the parameters and distribution models shown in Table 1. The Crystal Ball software (version 11.1.2.4) was used to calculate the probability distributions of these risks, and a total of 10,000 repetitions were performed to simulate the Monte Carlo method and sensitivity analysis.
Results and discussion
Concentrations of the hydrogen sulfide and ammonia compounds in the ambient air in areas near polluted urban rivers and comparison with previous studies
The distributions of the concentrations of H2S and NH3 considering each of the five sampling sites near polluted urban rivers in the city of Salvador during rainy and dry periods are represented by the amplitude of the boxplot diagrams in Fig. 2. Table 2 presents a statistical summary of H2S and NH3 concentrations considering all sampling sites chosen in this study for the two sampling periods. According to this table, the average concentrations found showed a seasonal variation with lower values in the rainy period (6.56 ± 0.83 μg m−3 for H2S and 8.67 ± 2.50 μg m−3 for NH3) than in the dry period (8.05 ± 1.44 μg m−3 for H2S and 10.62 ± 4.39 μg m−3 for NH3), probably due to lower water and air temperature values, which contribute to less evaporation of these compounds from the water surface into the air. Moreover, higher values of precipitation and relative humidity favor the removal of these compounds from the atmosphere during the rainy season. Assessing water and air temperatures helps to better understand seasonal variations as these influence emissions of odorous compounds from liquid sources in open areas, such as polluted urban rivers and WWTPs. The increase mainly in water temperature contributes to greater volatilization and the consequent increase in emissions of odorous compounds into the atmosphere during the dry season. The results of previous studies confirm these observations (Invernizzi et al. 2019; Rong et al. 2009, 2012; Rumsey and Aneja 2014; Santos et al. 2012).
Boxplot diagrams for the distribution of H2S and NH3 concentrations (μg m−3) in the air of five different sites near polluted urban rivers in the city of Salvador, Brazil, during dry and rainy periods. The upper and lower bars show the maximum and minimum values. Lines inside the boxes show the median values and the “x” represents the average values
Current Brazilian environmental legislation (CONAMA 2018), which establishes national air quality standards in outdoor environments, despite establishing that compounds inconvenient to public welfare due to the unpleasant odors generated can be considered air pollutants, does not establish limits for the compounds H2S and NH3, thus requiring the use of international legislation or guidelines for the evaluation of the data obtained in this study. Table 2 also presents a comparison of the results obtained in this study with the recommended exposure limits by the World Health Organization (WHO), United States Environmental Protection Agency (USEPA), Ontario Ministry of the Environment (OME), and Texas Commission on Environmental Quality (TCEQ).
The World Health Organization (WHO) recommends as a guideline to avoid substantial complaints about odor annoyance among the exposed population that H2S concentrations should not exceed 7.0 μg m−3 in an average period of 30 min (WHO 2000). The results obtained in this study showed that maximum H2S concentrations in both periods are above this established limit, thereby confirming the frequent complaints of the population in the sampled areas. Additionally, the average H2S concentrations exceeded about 3.3 times in the rainy period and 4 times in the dry period, the level recommended by USEPA (2.0 μg m−3) for daily inhalation exposure to ensure lifetime risk reduction, indicating potential adverse effects on human health (USEPA 2003b). However, according to the WHO air quality guideline, the allowable limit of H2S in air (150 μg m−3) over an average 24-h period (WHO 2000) was not exceeded at the sampling sites (Table 2).
For ammonia, the limits set by the Texas Commission on Environmental Quality (TCEQ) in outdoor environments are 180 μg m−3 and 92 μg m−3 for short-term and long-term exposure, respectively (TCEQ 2016). The Ontario Ministry of the Environment (OME) sets a limit of 100 μg m−3 for a 24-h exposure period (OME 2012). At all sites studied, the average NH3 concentrations were below these limits for the sampling periods in both periods.
The odor threshold for H2S is quite low (0.7 μg m−3), in comparison with the odor threshold concentration for ammonia (26.6 μg m−3) (Ruth 1986). In this study, the maximum NH3 concentrations obtained were not above the odor threshold in both periods. However, the maximum H2S concentrations correspond to about 12 and 15 times the odor threshold in the rainy and dry periods, respectively. These results are in accordance with complaints from the population due to odor nuisance in these areas and with previous studies where H2S was indicated as the main compound responsible for odors in areas near to WWTPs and open sewage (Lasaridi et al. 2010), as well as in urban areas near stormwater catch basins (Kabir et al. 2010).
Hence, this evaluation corroborates the need to implement actions for minimizing the release of untreated sewage into urban rivers in the city of Salvador, as well as a revision of the Brazilian environmental legislation to introduce air quality standards for odorous compounds, to avoid complaints from population due to discomfort caused by odors.
Since studies in urban areas near polluted rivers are scarce, the results obtained in this work were compared with few studies conducted near wastewater treatment plants (WWTPs) and streams polluted by domestic sewage in Brazilian cities in recent years, and it was possible to observe that the concentrations were in the same order of magnitude (Campos et al. 2010a; Godoi et al. 2018; Oliveira et al. 2019).
Godoi et al. (2018) in a study carried out in the city of Curitiba, in southern Brazil, monitored H2S concentrations in air from indoor and outdoor areas of two WWTPs (A and B) using Radiello® diffusive passive samplers during winter and summer. The average H2S concentrations found ranged from 0.14 to 32.0 µg m−3, and the concentrations at most of the WWTP-A points and also at all of the WWTP-B sampling points were above the concentration established by the USEPA guideline (2.0 μg m−3), thus suggesting potential adverse effects on human health and odor nuisance. The highest H2S concentrations were found in the summer at points located closer to the WWTPs (Godoi et al. 2018).
Oliveira et al. (2019) reported that among thirteen sites sampled of urban recreation areas (URA) in the city of Fortaleza, in northeastern Brazil, only two sites, one next to a stream heavily polluted by domestic sewage and another near one of the largest WWTPs in this city, have the occurrence of H2S which was detected, and the average concentrations varied between 0.76 and 7.53 µg m−3. They also determined ammonia concentrations at the thirteen sites of the URA and reported average NH3 concentrations ranging from 2.78 to 11.07 μg m−3, with the highest mean value found at the site near the WWTP, which according to Liu et al. (2019) is one of the main sources of NH3 in urban environments, besides vehicle traffic and industries. The authors emphasized the importance of monitoring at sites impacted by emissions of these compounds, due to odor problems and the potential harmful to human health.
Campos et al. (2010a) applied an optimized analytical methodology using cryogenic capture and gas chromatography to determine H2S and other RSC at some sites with open sewers in the city of Salvador and found H2S concentrations varying from 0.44 to 2.1 µg m−3.
However, higher concentrations of H2S and NH3 (maximum 90 µg m−3 and 9.3 mg m−3, respectively) emitted from the indoor of WWTP in urban area have been reported by Kim et al. (2013) in South Korea and only of H2S (in the undetected range, 87.50 µg m−3) by Niu et al. (2014) in Tianjin, North China. Wan et al. (2014) also reported higher concentrations of H2S and NH3 (mean 0.71 and 1.42 mg m−3, respectively) emitted from polluted recreational rivers in the city of Nanjing, China.
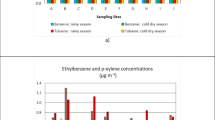
Figure 3 presents a more comprehensive comparison of the maximum concentrations (μg m−3) of H2S and NH3 in this study and other localities in the last decade in different areas: industrial or affected by industries (Campos et al. 2010b; Lima, 2011; Drimal et al. 2010; Rattanapan et al. 2014); urban (Reche et al. 2012; Kim et al. 2012; Behera et al. 2013b; Teng et al. 2017); near polluted recreational rivers (Wan et al. 2014); near open sewers (Campos et al. 2010a); next to stream heavily polluted by domestic sewage and WWTP (Oliveira et al. 2019); indoor and/or outdoor of WWTPs (Godoi et al. 2018; Kim et al. 2013; Niu et al. 2014; Ulutaş et al. 2021); near geothermal power plants (Cabassi et al. 2017; Cichowicz and Dobrznski 2021; Dumanoglu 2020); near large concentrated animal feeding operations (Li et al. 2017); agricultural and remote non-agricultural areas (Yao and Zhang 2013); indoor and outdoor of the municipal solid waste landfill (He et al. 2021).
Maximum concentrations (μg m−3) of H2S and NH3 in different areas of other locations in the period 2010–2021, compared with this study. a, Teng et al. 2017; b, Li et al. 2017; c, Yao and Zhang 2013; d, Behera et al. 2013b; e, Kim et al. 2012; f, Reche et al. 2012; g, He et al. 2021; h, Ulutaş et al. 2021; i, Cichowicz and Dobrzanski 2021; j, Dumanoglu 2020; k, Cabassi et al. 2017; l, Rattanapan et al. 2014; m, Drimal et al. 2010; n, Wan et al. 2014; o, Niu et al. 2014; p, Kim et al. 2013; q, Oliveira et al. 2019; r, Godoi et al. 2018; s, Lima, 2011; t, Campos et al. 2010b; u, Campos et al. 2010a; v, this study (dry period); w, this study (rainy period)
Multivariate analysis
A data matrix (30 × 14) was generated in which the sampling results in triplicate at the five sites during the rainy (RNR1-RNR5) and dry (DR1-DR5) seasons were arranged in lines, while information about the variables H2S and NH3 compounds, physicochemical water quality parameters (TN, N-NH4+, DO, BOD, pH, EC, and Twater), and meteorological parameters (WS, RH, Tair, PPT, and SI) were disposed in columns. The data were pre-processed by autoscaling, eliminating the influence of order of magnitude differences between the data.
The first two principal components (PC1 and PC2) were selected for data characterization because they presented eigenvalues greater than 1 and together explained 75.81% of total data variance; therefore, more than half of the variance described (Fig. 4a and Table 3). Through the PCA, the loading and score plots of the data were obtained from the correlations between the selected variables (Fig. 4a and b). The PC1 has accumulated 42.66% of the data variance and the variables that most contributed to data characterization were physicochemical parameters of water quality and the compounds H2S and NH3, as they were the variables with the highest absolute weights on this PC, according to Table 3.
In analyzing PC1, strong positive correlations are observed between NH3, N-NH4+, TN, BOD, Twater, and EC. The BOD parameter is used to quantify biodegradable organic matter in water bodies. N-NH4+ can originate through the hydrolysis of urea present in polluted aquatic environments. Thus, the parameters BOD, N-NH4+, and TN are strongly related to the presence of untreated domestic sewage discharges in aquatic environments (Glińska-Lewczuk et al. 2016; Mizukawa et al. 2019), which justifies the positive correlation of these parameters with NH3. Similar results were found by Wan et al. (2014), who applied PCA to evaluate the relationships between atmospheric levels of odorous compounds and some water quality parameters of 22 polluted recreational rivers in Nanjing, China. Correlations between H2S and S2−(aq) could not be established, since the latter parameter was not evaluated in the technical reports on environmental quality of rivers in the city of Salvador from the Institute of Environment and Water Resources of Bahia. The positive correlation between H2S and BOD indicates that this compound probably was emitted from polluted rivers. The H2S and NH3 compounds also are positively correlated, thereby confirming a common source for these gases, in the case of this study, emissions from urban rivers polluted mainly by domestic sewage.
It was also observed in PC1 that the H2S and NH3 compounds are positively correlated with Twater (Fig. 4a). The positive correlations between these odorous gases and Twater are justified on the basis that volatilization of these compounds from the water surface to the atmosphere occurs in accordance with Henry’s law and Fick’s first law of molecular diffusion (Invernizzi et al. 2019; Santos et al. 2012). Under these laws and the two-film theory, the total mass transfer coefficient (K) is the parameter that incorporates the effects of Henry’s law along with those mass transfer coefficients of the gaseous phase (kG) and liquid phase (kL). According to Hudson and Ayoko (2008), values of the Henry constant (H) indicate the phase that controls the total mass transfer. Thus, for compounds with H values > 1 × 10−1, the volatilization is faster and controlled by kL. For compounds with H values < 1 × 10−3, volatilization is slower and limited by kG. For compounds with intermediate H values ranging from 1 × 10−3 to 1 × 10−1, volatilization is moderate and influenced by kL and kG. The magnitude of mass transfer is a function of the properties of each compound studied. The emission rates of H2S and NH3 depend mainly on the liquid phase (Rong et al. 2009, 2012; Santos et al. 2012). Therefore, increasing water temperatures leads to higher water–air transfer rates, and consequent increases in H2S and NH3 concentrations in the atmosphere.
Regarding conductivity, positive correlations were found with H2S and NH3 compounds. Conductivity is a measure of the ability of water to conduct an electric current, and high conductivity values are related to high salinity. In water bodies polluted by domestic sewage, conductivity is related to organic matter of food and fecal origin and residues in general that incorporates salts into the water (Di Blasi et al. 2013), therefore explaining the positive correlations between H2S and NH3 compounds and conductivity.
Furthermore, strong negative correlations between H2S, DO, and pH were also observed in PC1 (Fig. 4a). In polluted urban rivers, the presence of organic matter is high, mainly due to the discharge of untreated domestic sewage. To degrade this organic matter, microorganisms consume dissolved oxygen (DO), which causes a decrease in its concentration, until reduction processes occur producing compounds with unpleasant odors, thus increasing the emissions of these compounds into the atmosphere. According to Chen et al. (2019) and Liang et al. (2018), blackening and odorization of urban rivers are generally caused by the large amount of organic pollutants from untreated sewage, which result in disequilibrium in the supply and consumption of DO in aquatic systems due to the high load of organic matter that rapidly consumes DO, leading to anaerobic conditions. The emissions of H2S from the aqueous phase to the gas phase are also influenced by pH. The sulfide solubilized in polluted water reacts with the hydrogen ion forming HS− and H2S (Eq. 3). Decreasing pH values favor the reaction towards H2S formation, increasing the water–air transfer rate and hence the concentrations of this compound in the atmosphere.
With respect to PC2, 33.15% of data variance was accumulated. In Table 3 and Fig. 4a, it can be noted that the variables that contributed most to the discrimination of the sample set in PC2 were the meteorological parameters (PPT, RH, Tair, WS, and SI). The following correlations were observed: (a) PPT, RH, and WS had positive correlation; (b) Tair and SI had positive correlation; and (c) PPT/RH/WS and Tair/SI had negative correlation. Similar correlations have also been found in a previous study conducted in the city of the Salvador (Cruz et al. 2020a).
The score plot of PC1 versus PC2 for the 5 sampling sites near polluted urban rivers in the city of Salvador during the rainy (RNRx) and dry (DRx) periods is shown in Fig. 4b. According to this figure and considering PC2, it was possible to note a tendency towards separation of the samples in two groups, according to the seasonal period. The samples collected in rainy period (RNR1-RNR5) were displaced to the region of negative scores, while samples collected in the dry period (DR1-DR5) were displaced to the region of positive scores on this PC.
Comparing the loading and score plots (Fig. 4a and b), it appears that samples from the rainy period presented higher values of precipitation, relative humidity, and wind speed, whereas samples from the dry period showed higher values of air temperature and solar irradiation. These five variables contributed the most to the discrimination of the samples in PC2, corresponding to average values for RH and PPT of 75.3% and 81.1% and 28.6 mm and 60.6 mm in the dry and rainy periods, respectively. Higher RH and PPT values indicate that higher amounts of water in the atmosphere during the rainy period contributed to the removal of the H2S and NH3 compounds through wet deposition, since they are water soluble. Increased WS contributed to the transport of these compounds to others sites this period as well. Although the air temperatures at the sampled sites were high in both periods (means = 24.2 °C and 26.5 °C in the rainy and dry periods, respectively), the maximum temperature values reached 31.7 °C in the dry period, thus contributing to a greater evaporation of H2S and NH3 compounds from the polluted rivers and the consequent increase in the concentrations during this period. Solar irradiation and air temperature showed a positive correlation according to PC2, since SI is one of the factors that directly influence air temperature.
Figure 5 shows a dendrogram generated by hierarchical cluster analysis (HCA). The result represents the groups joined in decreasing order of similarity (x-axis) and the distances between them (y-axis). The data were grouped according to Ward’s method, and the metric used to calculate the degree of similarity was the Euclidean distance. The formation of five groups was observed, whose similarities were consistent with the data obtained through PCA.
The dendrogram (Fig. 5) shows well-established linear correlations between NH3, N-NH4+, TN, and EC, in addition to H2S with Tair, SI, pH, and DO. The NH3 and N-NH4+ exhibited greater similarity, followed by the group formed by TN and EC. Furthermore, Tair and SI formed a more similar group to H2S than the group formed by pH and DO. These data corroborate with PCA and suggest that N-NH4+, TN, and EC are the most influential parameters for atmospheric NH3 levels in those sites. The correlations of H2S with pH and DO, as discussed earlier, indicate that polluted rivers are predominant sources of this pollutant.
Assessment of population health risks associated with exposure to hydrogen sulfide and ammonia
According to a deterministic health assessment for NH3, the estimated HQ values at all sites studied varied from 9.0 × 10−3 to 3.0 × 10−2 and are below the USEPA acceptable level, i.e., HQ < 1, indicating that non-carcinogenic risks to the population health due to exposure to only this compound are not observed considering an exposure period of 25 years. Individuals belonging to the three considered groups of the exposed population (P1, P2, and P3) may only suffer adverse health effects if the average NH3 concentrations exceed 521, 834, and 2433 μg m−3, respectively.
Table 4 presents the HQ values (average, median, minimum, and maximum) and their specific percentiles (10th–90th) obtained from Monte Carlo simulations for H2S emissions in the five sampled sites near polluted urban rivers in the city of Salvador, considering the three groups of exposed population P1, P2, and P3. The maximum HQ values were about 6, 4, and 2 times higher than the limit set by USEPA (HQ = 1) in the dry period for P1, P2, and P3, respectively, and about 4 and 3 times in the rainy period for P1 and P2. Maximum risk corresponds to the acute chronic exposure condition, which uses the maximum values of all input variables in the risk models used for exposure assessment. This maximum exposure condition is less likely to occur and therefore maximum risks can be overestimated (Rajasekhar et al. 2020). According to the risk assessment guideline (USEPA 2001) and other studies (Niu et al. 2014; Ghaderpoury et al. 2021; Rajasekhar et al. 2020; Yaghmaien et al. 2019), the 95th percentile value was adopted as the upper-bound estimate of the potential risk.
Figure 6 presents the probability distributions of the non-carcinogenic risks of hydrogen sulfide for three groups of the exposed population within a 5 to 95% confidence interval at the five studied sites near polluted urban rivers in the city of Salvador during dry and rainy periods. The results showed HQ values of 4.28, 2.80, and 1.46 for the dry period and 3.31, 2.15, and 7.36 × 10−1 for the rainy period at the 95th percentile for P1, P2, and P3, respectively, i.e., the non-carcinogenic risks were higher in the dry period than in the rainy one. Except for P3 in the rainy period, all other values were higher than the limit allowed by USEPA, meaning that the risks are high considering 25 years of exposure and, therefore, the three groups of the exposed population considered in this study may suffer adverse health effects, such as eye irritation, headache, nausea, and respiratory problems. Hence, urgent actions are required for the recovery of polluted rivers in the city of Salvador.
A sensitivity analysis was also performed in this study to determine the contributions of the input variables in estimating the non-carcinogenic risk values for H2S. The results of the sensitivity analyses of the non-carcinogenic risks (Fig. 7) revealed that H2S concentration was the most important contributing variable to the non-carcinogenic risk values, accounting for 64.9%, 81.7%, and 77.4% in the dry period and 47.5%, 67.8%, and 60.3% in the rainy period for P1, P2, and P3, respectively. As the contributions of H2S concentrations to sensitivity were positive values in all cases, there was a positive correlation between concentrations and non-carcinogenic risks. For ET, the sensitivity contributions were 28.8%, 10.2%, and 15.7% in the dry period and 42.8%, 17.3%, and 26.9% in the rainy period considering P1, P2, and P3, respectively. The results showed that ET also had positive correlations with the non-carcinogenic risks. The variables contributing least to HQ values were AT, ED, and EF, with positive correlations being verified between ED and EF and non-carcinogenic risks, but a negative correlation between AT and these risks.
There are few studies using the probabilistic approach to health risk assessment (HRA) for populations and/or workers due to exposure to H2S by inhalation. Niu et al. (2014) performed HRA for workers associated with exposure to H2S and other odorous compounds emitted from wastewater treatment plants (WWTPs) in Tianjin, northern China, using Monte Carlo probabilistic analysis and considering daily exposure time of 8 h. The 95th percentile, average, and median HQ values obtained for were 1.60, 1.14, and 1.13, respectively, thus higher than the limit established by USEPA (HQ = 1), representing a human health threat to workers at the WWTPs. The contributions of the input variables to the non-carcinogenic risk values were also evaluated through sensitivity analysis, and H2S concentration was the most influential variable. These results are in accordance with those obtained in the present study.
Other studies have used the deterministic method to perform the HRA associated with exposure to H2S. Godoi et al. (2018) showed that non-carcinogenic risks due to H2S emissions are significant (HQ > 1) for workers and local residents, especially those closest to anaerobic WWTPs in the city of Curitiba, Brazil. These authors warn that these results are important from a social and public health point of view, and that WWTPs operating with anaerobic technology without rigorous control, process optimization, and H2S emission reduction must be recognized as a significant source of air pollution, causing adverse health effects to local populations. Ulutaş et al. (2021) carried out a study on the assessment of H2S and BTEX concentrations in indoor and outdoor areas of one of the largest WWTPs in Istanbul, as well as HRA for workers and local residents due to exposure to these compounds. The non-carcinogenic risks found of all pollutants were less than 1 (HQ < 1), except benzene, showing that these compounds have no adverse health effect under three different exposure time scenarios (8, 12, and 24 h day−1).
Wu et al. (2018) conducted a study aiming to perform HRA for workers due volatile compound emissions from one of the largest municipal solid waste (MSW) landfills in Beijing, China. The results indicated that the potential risks for workers were not negligible in the waste areas, and that the non-carcinogenic risks were mainly due to the compounds H2S, trichloropropane, and naphthalene, since the corresponding HQ values (maximums of 2.3, 2.0, and 1.2, respectively) found in these areas exceeded the limit set by USEPA (HQ > 1) during all seasons of the year. Cheng et al. (2019) also carried out a study for the identification and HRA of odor emissions from waste landfilling and composting in southeast Shanghai, China. The results showed that with respect to contributions for occupational exposure, the carcinogenic risk was negligible in both facilities; however, H2S emissions from the landfill pose a threat to workers’ health, as non-carcinogenic risks (HQ = 1.2 × 101) are high for on-site workers.
Limitations and uncertainties of the passive sampling
Although passive sampling presents significant advantages for application in monitoring of gases and vapors, this technique has some disadvantages, because it does not provide instantaneous concentrations and cannot distinguish transient episodes of high and low concentrations in a particular period, the results are given in form of time-weighted average (TWA) concentrations, and therefore the results obtained are more useful for comparisons with long-term air quality standards (Marć et al. 2015; Zabiegała et al. 2010).
Uncertainties also accompany the results obtained with passive diffusive samplers operating according to Fick’s first law, because although the sampling rate (SR) which is theoretically calculated from the defined geometry (area and length) of the sampler and the diffusion coefficient must be constant for a given compound, theoretical SR values generally differ from experimental values due to the influence of some environmental variables such as temperature, air velocity, and relative humidity. Furthermore, the performance of a passive sampler can also be affected by the sampling time required to capture an adequate amount of the analyte to ensure its subsequent quantification through an analytical method with minimal interference from other compounds (Leiva G et al. 2013; USEPA, 2014).
Studies aiming to assess the uncertainties of measurements performed with passive samplers for air quality investigations based on the application of the Guide to the Expression of Uncertainty in Measurement are still scarce (Leiva G et al. 2013). Uncertainties of the passive measurements have generally been evaluated by comparing the results obtained through parallel measurements with a reference method (USEPA, 2014).
The performance of the measurements carried out with the passive samplers for H2S and NH3 used in this study has been evaluated in previous studies by comparison with data obtained simultaneously with continuous analyzers calibrated in tropical industrial areas, which are influenced by complex odors and fluctuations in concentrations of these compounds, and generally have high values of temperature and relative humidity. The results showed that there was good agreement between active and passive methods. In addition, uncertainty values were found in the range of 10–19%, therefore within the European Union recommended limit of ± 25% (EU, 2008), and repeatability values, based on the relative standard deviation for each set containing three passive samplers, below the maximum value of 20% (Campos et al. 2010b; Lima 2011). This confirms the possibility of applying these passive samplers also in areas near polluted urban rivers in the city of Salvador in the present study.
Conclusions
In this study, concentrations of H2S and NH3 compounds were measured simultaneously in areas near polluted urban rivers in the city of Salvador (State of Bahia, Brazil) for the first time using passive sampling. Although NH3 concentrations were below the limits established by TCEQ and OME at all sampled sites, the maximum H2S concentrations were above the limit set by the WHO guideline on the prevention of odor annoyance, corresponding to about 12 and 15 times the odor threshold in the rainy and dry periods, respectively. Furthermore, the average H2S concentrations exceeded the USEPA recommended level by about 3.3 times in the rainy period and 4 times in the dry period, thus indicating potential adverse health effects to the exposed population and confirming frequent community complaints due to odor nuisance in areas near polluted urban rivers.
Based on PCA and HCA analyses, important relationships were established between H2S and NH3 compounds and physicochemical parameters of water quality, which are indicators of pollution of aquatic environments, thereby pointing out that untreated domestic sewage discharges in urban rivers of the city of Salvador significantly interfere in the atmospheric levels of H2S and NH3 in areas near these rivers. The PCA also showed a tendency to separate the samples into two groups, depending on the seasonal period. These results demonstrated the importance of chemometric methods in identifying the emission source of H2S and NH3 compounds in the studied areas, as well as confirming the influence of seasonality on the concentrations of these odorous compounds.
Regarding non-carcinogenic health risks, whereas for NH3 the estimated HQ values were below the USEPA acceptable level (HQ = 1) at all studied sites, most HQ values for H2S were above the limit set by USEPA for both dry and rainy periods, thus confirming that the risks are high considering 25 years of exposure, and that three groups of population investigated in this study may suffer adverse health effects. Furthermore, the H2S concentration was the most influential variable on the HQ results according to the sensitivity analysis.
The results obtained in this study showed the need to adopt measures to decrease the release of untreated sewage into the urban rivers of the city of Salvador, as well as strategies to recover the rivers of this city, and a revision of the Brazilian legislation to introduce air quality standards for odorous compounds, in order to avoid the discomfort caused by odors and reduce the health impacts of the exposed population.
Data availability
Data will be made available on reasonable request.
References
Aatamila M, Verkesalo PK, Korhonen MJ, Suominen AL, Hirvonen MR, Viluksela MK, Nevalainen A (2011) Odour annoyance and physical symptoms among residents living near waste treatment centres. Environ Res 111:164–170. https://doi.org/10.1016/j.envres.2010.11.008
Abdo H, Flaus J-M, Masse F (2017) Uncertainty quantification in risk assessment - representation, propagation and treatment approaches: application to atmospheric dispersion modelling. J Loss Prev Process Ind 49:551–571. https://doi.org/10.1016/j.jlp.2017.05.015
ANA – Agência Nacional de Águas (2017) Atlas Esgotos. https://www.ana.gov.br/noticias/atlas-esgotos-revela-mais-de-110-mil-km-de-rios-com-comprometimento-da-qualidade-da-agua-por-carga organica/atlaseesgotosdespoluicaodebaciashidrograficas-resumoexecutivo_livro.pdf/view. Accessed 16 Jan 2021
Borrás E, Tortajada-Genaro LA, Muñoz A (2016) Determination of reduced sulfur compounds in air samples for the monitoring of malodor caused by landfills. Talanta 148:472–477. https://doi.org/10.1016/j.talanta.2015.11.021
Behera SN, Sharma M, Aneja VP, Balasubramanian R (2013a) Ammonia in the atmosphere: a review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ Sci Pollut Res 20:8092–8131. https://doi.org/10.1007/s11356-013-2051-9
Behera SN, Betha R, Balasubramanian R (2013b) Insights into chemical coupling among acidic gases, ammonia and secondary inorganic aerosols. Aerosol Air Qual Res 13:1282–1296. https://doi.org/10.4209/aaqr.2012.11.0328
Cabassi J, Tassi F, Venturi S, Calabrese S, Capecchiacci F, D’Alessandri W, Vaselli O (2017) A new approach for the measurement of gaseous elemental mercury (GEM) and H2S in air from anthropogenic and natural sources: examples from Mt. Amiata (Siena, Central Italy) and Solfatara Crater (Campi Flegrei, Southern Italy). J Geochem Explor 175:48–58. https://doi.org/10.1016/j.gexplo.2016.12.017
Calazans GM, Pinto CC, Costa EP, Perini AF, Oliveira SC (2018) The use of multivariate statistical methods for optimization of the surface water quality network monitoring in the Paraopeba river basin. Brazil Environ Monit Assess 190:491. https://doi.org/10.1007/s10661-018-6873-2
Campos VP, Oliveira AS, Cruz LPS, Borges J, Tavares TM (2010a) Optimization of parameters of sampling and determination of reduced sulfur compounds using cryogenic capture and gas chromatography in tropical urban atmosphere. Microchem J 96:283–289. https://doi.org/10.1016/j.microc.2010.04.003
Campos VP, Cruz LPS, Godoi RHM, Godoi AFL, Tavares TM (2010b) Development and validation of passive samplers for atmospheric monitoring of SO2, NO2, O3 and H2S in tropical areas. Microchem J 96:132–138. https://doi.org/10.1016/j.microc.2010.02.015
Chang H, Tan H, Zhao Y, Wang Y, Wang X, Li Y, Lu W, Wang H (2019) Statistical correlations on the emissions of volatile odorous compounds from the transfer stage of municipal solid waste. Waste Manag 87:701–708. https://doi.org/10.1016/j.wasman.2019.03.014
Cheng Z, Sun Z, Zhu S, Lou Z, Zhu N, Feng L (2019) The identification and health risk assessment of odor emissions from waste landfilling and composting. Sci Total Environ 649:1039–1044. https://doi.org/10.1016/j.scitotenv.2018.08.230
Chen Z, Zhu Z, Song J, Liao R, Wang Y, Luo X, Nie D, Lei Y, Shao Y, Yang W (2019) Linking biological toxicity and the spectral characteristics of contamination in seriously polluted urban rivers. Environ Sci Eur 31:84. https://doi.org/10.1186/s12302-019-0269-y
Cichowicz R, Dobrznski (2021) 3D spatial analysis of particulate matter (PM10, PM2.5 and PM1.0) and gaseous pollutants (H2S, SO2 and VOC) in urban areas surrounding a large heat and power plant. Energies 14:4070. https://doi.org/10.3390/en14144070
Cruz LPS, Campos VP (2008) Sampling and analytical methods for atmospheric reduced sulphur compounds. Quim Nova 31:1180–1189. https://doi.org/10.1590/S0100-40422008000500047
Cruz LPS, Mota ER, Campos VP, Santana FO, Luz SR, Santos DF (2019) Inorganic and organic acids in the atmosphere of the urban area of the City of Salvador, Brazil. J Braz Chem Soc 30:904–914. https://doi.org/10.21577/0103-5053.20180227
Cruz LPS, Santos DF, dos Santos IF, Gomes IVS, Santos AVS, Souza KSPP (2020a) Exploratory analysis of the atmospheric levels of BTEX, criteria air pollutants and meteorological parameters in a tropical urban area in Northeastern Brazil. Microchem J 152:104265. https://doi.org/10.1016/j.microc.2019.104265
Cruz LPS, Luz SR, Campos VP, Santana FO, Alves RS (2020b) Determination and risk assessment of formaldehyde and acetaldehyde in the ambient air of gas stations in Salvador, Bahia, Brazil. J Braz Chem Soc 31:1137–1148. https://doi.org/10.21577/0103-5053.20190278
CONAMA - Conselho Nacional do Meio Ambiente (2018) Resolução nº 491 de 19 de novembro de 2018. http://www2.mma.gov.br/port/conama/legiabre.cfm?codlegi=740. Accessed 15 Fev 2021
Di Blasi JIP, Torres JM, Nieto PJG, Fernández JRA, Muñiz CD, Taboada J (2013) Analysis and detection of outliers in water quality parameters from different automated monitoring stations in the Miño river basin (NW Spain). Ecol Eng 60:60–66. https://doi.org/10.1016/j.ecoleng.2013.07.054
Drimal M, Koppová K, Klöslová Z, Fabiánová E (2010) Environmental exposure to hydrogen sulfide in central Slovakia (Ružomberok area) in context of health risk assessment. Cent Eur J Public Health 18:224–229. https://doi.org/10.21101/cejph.a3610
Dumanoglu Y (2020) Monitoring of hydrogen sulfide concentration in the atmosphere near two geothermal power plants of Turkey. Atmos Pollut Res 11:2317–2326. https://doi.org/10.1016/j.apr.2020.08.015
EU - European Union (2008) Directive 2008/50/EC Official Journal of the European Communities, 2008L152.https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:152:0001:0044:en:PDF. Accessed 10 Feb 2021
Finlayson-Pitts BJ, Pitts JN Jr (2000) Chemistry of the upper and lower atmosphere: theory, experiments and applications. San Diego, USA
Ghaderpoury A, Hadei M, Hopke PK, Rastkari N, Kermani M, Shahsavani A (2021) Assessment of BTEX exposure and carcinogenic risks for mail carriers in Tehran, Iran. Air Qual Atmos Health. https://doi.org/10.1007/s11869-021-01027-5
Glińska-Lewczuk K, Gołaś I, Koc J, Gotkowska-Płachta A, Harnisz M, Rochwerger A (2016) The impact of urban areas on the water quality gradient along a lowland river. Environ Monit Assess 188:624. https://doi.org/10.1007/s10661-016-5638-z
Godoi AFL, Grasel AM, Polezer G, Brown A, Potgieter-Vermaak S, Scremim DC, Yamamoto CI, Godoi RHM (2018) Human exposure to hydrogen sulphide concentrations near wastewater treatment plants. Sci Total Environ 610–611:583–590. https://doi.org/10.1016/j.scitotenv.2017.07.209
Granato D, Santos JS, Escher GB, Ferreira BL, Maggio RM (2018) Use of principal component analysis (PCA) and hierarchical clusteranalysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: a critical perspective. Trends Food Sci Technol 72:83–90. https://doi.org/10.1016/j.tifs.2017.12.006
Guidotti TL (2010) Hydrogen sulfide: advances in understanding human toxicity. Int J Toxicol 29:569–581. https://doi.org/10.1177/1091581810384882
Guo H, Duan Z, Zhao Y, Liu Y, Mustafa MF, Lu W, Wang H (2017) Characteristics of volatile compound emission and odor pollution from municipal solid waste treating/disposal facilities of a city in Eastern China. Environ Sci Pollut Res 24:18383–18391. https://doi.org/10.1007/s11356-017-9376-8
He H, Wu T, Xu H, Lu Y, Qiu Z, Wang X, Zhang P (2021) Investigation on the emission and diffusion of hydrogen sulfide during landfill operations: a case study in Shenzhen. Sustainability 13:2886–2897. https://doi.org/10.3390/su13052886
Huang C, Shan W, Xiao H (2018) Recent advances in passive air sampling of volatile organic compounds. Aerosol Air Qual Res 18:602–622. https://doi.org/10.4209/aaqr.2017.12.0556
Hudson N, Ayoko GA (2008) Odour sampling 1: physical chemistry considerations. Bioresour Technol 99:3982–3992. https://doi.org/10.1016/j.biortech.2007.04.034
IBGE - Instituto Brasileiro de Geografia e Estatística (2010) Censo demográfico 2010. http://www.ibge.gov.br. Accessed 16 Jan 2021
Invernizzi M, Bellini A, Miola R, Capelli L, Busini V, Sironi S (2019) Assessment of the chemical-physical variables affecting the evaporation of organic compounds from aqueous solutions in a sampling wind tunnel. Chemosphere 220:353–361. https://doi.org/10.1016/j.chemosphere.2018.12.124
Kabir E, Kim K-H, Ahn J-W, Hong O, Chang Y-S (2010) Offensive odorants released from stormwater catch basins (SCB) in an urban area. Chemosphere 81:327–338. https://doi.org/10.1016/j.chemosphere.2010.07.028
Karageorgos P, Latos M, Kotsifaki C, Lazaridis M, Kalogerakis N (2010) Treatment of unpleasant odors in municipal wastewater treatment plants. Water Sci Technol 61:2635–2644. https://doi.org/10.2166/wst.2010.211
Kim JS, Bais AL, Kang S, Lee J, Park K (2012) A semi-continuous measurement of gaseous ammonia and particulate ammonium concentrations in PM2.5 in the ambient atmosphere. J Atmos Chem 68:251–263. https://doi.org/10.1007/s10874-012-9220-y
Kim KH, Jo SH, Song HC, Pandey SK, Song HN, Oh JM, Sunwoo Y, Choi KC (2013) Diagnostic analysis of offensive odorants in a large municipal waste treatment plant in an urban area. IJEST 10:261–274. https://doi.org/10.1007/s13762-012-0134-7
Kothny EL, Cook WA, Cuddeback JE, Dimitriades B, Ferrand EF, McDaniel PW, Nifon GD, Saltzman BE, Weiss FT (1989) Determination of ammonia in the atmosphere (indophenol method). In: Lodge J (ed) Methods of Air Sampling and Analysis, 3rd edn. Lewis Publishers, Michigan, pp 379–381
Kourtidis K, Kelesis A, Petrakakis M (2008) Hydrogen sulfide (H2S) in urban ambient air. Atmos Environ 42:7476–7482. https://doi.org/10.1016/j.atmosenv.2008.05.066
Lasaridi K, Katsabanis G, Kyriacou A, Maggos T, Manios T, Fountoulakis M, Kalogerakis N, Karageorgos P, Stentiford EI (2010) Assessing odour nuisance from wastewater treatment and composting facilities in Greece. Waste Manag Res 28:977–984. https://doi.org/10.1177/0734242X10372660
Leiva GMA, Gonzales B, Vargas D, Toro R, Morales SRGE (2013) Estimating the uncertainty in the atmospheric ammonia concentration in an urban area by Ogawa passive samplers. Microchem J 110:340–349. https://doi.org/10.1016/j.microc.2013.05.004
Li Y, Thompson TM, Van Damme M, Chen X, Benedict KB, Shao Y, Day D, Boris A, Sullivan AP, Ham J, Whitburn S, Clarisse L, Coheur P-F, Collett JL Jr (2017) Temporal and spatial variability of ammonia in urban and agricultural regions of northern Colorado, United States. Atmos Chem Phys 17:6197–6213. https://doi.org/10.5194/acp-17-6197-2017
Liang Z, Siegert M, Fang W, Sun Y, Jiang F, Lu H, Chen GH, Wang S (2018) Blackening and odorization of urban rivers: a bio-geochemical process. FEMS Microbiol Ecol 94:1–12. https://doi.org/10.1093/femsec/fix180
Lima MAO (2011) Development of passive sampler for ammonia in the atmosphere. Dissertation, Federal University of Bahia
Liu Y, Ngo HH, Guo W, Peng L, Wang D, Ni B (2019) The roles of free ammonia (FA) in biological wastewater treatment processes: a review. Environ Int 123:10–19. https://doi.org/10.1016/j.envint.2018.11.039
Marć M, Tobiszewski M, Zabiegała B, de la Guardia M, Namieśnik J (2015) Current air quality analytics and monitoring: a review. Anal Chim Acta 853:116–126. https://doi.org/10.1016/j.aca.2014.10.018
Miao S, Liu C, Qian B, Miao Q (2020) Remote sensing-based water quality assessment for urban rivers: a study in linyi development area. Environ Sci Pollut Res 27:34586–34595. https://doi.org/10.1007/s11356-018-4038-z
Mizukawa A, Filippe TC, Peixoto LOM, Scipioni B, Leonardi IR, de Azevedo JCR (2019) Caffeine as a chemical tracer for contamination of urban rivers. RBRH 24:e29. https://doi.org/10.1590/2318-0331.241920180184
Nair AA, Yu F (2020) Quantification of atmospheric ammonia concentrations: a review of its measurement and modeling. Atmosphere 11:1092. https://doi.org/10.3390/atmos11101092
Niu ZG, Xu SY, Gong QC (2014) Health risk assessment of odors emitted from urban wastewater pump stations in Tianjin, China. Environ Sci Pollut Res 21:10349–10360. https://doi.org/10.1007/s11356-014-2984-7
Oliveira MLM, Lopes MHPS, Policarpo NA, Alves CMAC, Araújo RS, Cavalcanti FSA (2019) Assessment of air pollutants in urban recreation areas of Fortaleza city. RBGU 11:e20180187. https://doi.org/10.1590/2175-3369.011.e20180187
OME - Ontario Ministry of the Environment (2012). Ambient air quality criteria. http://www.airqualityontario.com/downloads/AmbientAirQualityCriteria.pdf. Accessed 20 Fev 2021
Passos JBMC, de Sousa TDB, Campos JA, Lima RPC, Fernandes-Filho EI, da Silva DD (2021) Multivariate statistics for spatial and seasonal quality assessment of water in the Doce River basin. Southeastern Brazil Environ Monit Assess 193:125. https://doi.org/10.1007/s10661-021-08918-1
Rajasekhar B, Nambi IM, Govindarajan SK (2020) Human health risk assessment for exposure to BTEXN in an urban aquifer using deterministic and probabilistic methods: a case study of Chennai city. India Environ Pollut 265:114814. https://doi.org/10.1016/j.envpol.2020.114814
Rattanapan C, Suksaroj TT, Chumpikul J, Choosong T (2014) Health risk assessment of hydrogen sulfide exposure among workers in a Thai Rubber Latex Industry. EnvironmentAsia 7:25–31. https://doi.org/10.14456/ea.2014.5
Reche C, Viana M, Pandolfi M, Alastuey A, Moreno T, Amato F, Ripoll A, Querol X (2012) Urban NH3 levels and sources in a Mediterranean environment. Atmos Environ 57:153–164. https://doi.org/10.1016/j.atmosenv.2012.04.021
Rong L, Nielsen PV, Zhang G (2009) Effects of airflow and liquid temperature on ammonia mass transfer above an emission surface: experimental study on emission rate. Biores Technol 100:4654–4661. https://doi.org/10.1016/j.biortech.2009.05.003
Rong L, Nielsen PV, Zhang G (2012) Experimental and numerical study on effects of airflow and aqueous ammonium solution temperature on ammonia mass transfer coefficient. Air & Waste Manage Assoc 60:419–428. https://doi.org/10.3155/1047-3289.60.4.419
Rumsey IC, Aneja VP (2014) Measurement and modeling of hydrogen sulfide lagoon emissions from a swine concentrated animal feeding operation. Environ Sci Technol 48:1609–1617. https://doi.org/10.1021/es403716w
Ruth JH (1986) Odor thresholds and irritation levels of several chemical substances: a review. Am Ind Hyg Assoc J 47:A-142-A-151. https://doi.org/10.1080/15298668691389595
Santana FO, Campos VP, Santos IF, Cruz LPS, Brito AVS (2019) Seasonal quimiometric study of formaldehyde and acetaldehyde atmospheric levels and health risk assessment, in urban areas of Salvador-Bahia, Brazil. Microchem J 147:524–531. https://doi.org/10.1016/j.microc.2019.03.069
Santos E, de Pinho JAG, Moraes, LRS, Fischer T (2010) O Caminho das águas em Salvador. CIAGS/UFBA. http://www.conder.ba.gov.br/sites/default/files/2018-08/O%20caminho%20das%20%C3%A1guas%20em%20Salvador%20-%20Bacias%20Hidrogr%C3%A1ficas%2C%20bairros%20e%20fontes.pdf. Accessed 20 Jan 2021
Santos JM, Kreim V, Guillot JM, Reis NC, Melo de Sá L, Horan NJ (2012) An experimental determination of the H2S overall mass transfer coefficient from quiescent surfaces at wastewater treatment plants. Atmos Environ 60:18–24. https://doi.org/10.1016/j.atmosenv.2012.06.014
Sazakli E, Leotsinidis M (2021) Odor nuisance and health risk assessment of VOC emissions from a rendering plant. Air Qual Atmos Health 14:301–312. https://doi.org/10.1007/s11869-020-00935-2
Shanthi K, Balasubramanian N (1996) Method for sampling analysis of hydrogen sulfide. Analyst 121:647–650. https://doi.org/10.1039/AN9962100647
TCEQ - Texas Commission on Environmental Quality (2016). https://www.tceq.texas.gov/toxicology/esl/list_main.html. Accessed 20 Fev 2021
Teng X, Hu Q, Zhang L, Qi J, Shi J, Xie H, Gao H, Yao X (2017) Identification of major sources of atmospheric NH3 in an urban environment in Northern China during wintertime. Environ Sci Technol 51:6839–6848. https://doi.org/10.1021/acs.est.7b00328
Ulutaş K, Kaskun S, Demir S, Dinçer F, Pekey H (2021) Assessment of H2S and BTEX concentrations in ambient air using passive sampling method and the health risks. Environ Monit Assess 193:399. https://doi.org/10.1007/s10661-021-09164-1
UN - United Nations (2019). The Sustainable Development Goals Report 2019. United Nations, New York. https://unstats.un.org/sdgs/report/2019/The-Sustainable-Development-Goals-Report-2019.pdf. Accessed 15 Fev 2021
USEPA – United States Environmental Protection Agency (2001) Risk assessment guidance for superfund: volume III – part A, process for conducting probabilistic risk assessment. Report EPA 540- R-02–002. https://www.epa.gov/sites/production/files/2015-09/documents/rags3adt_complete.pdf. Accessed 25 Fev 2021
USEPA - United States Environmental Protection Agency (2003a) Integrated Risk Information System (IRIS). Chemical Assessment Summary. Hydrogen sulfide. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0061_summary.pdf#nameddest=rfd. Accessed 25 Fev 2021
USEPA - United States Environmental Protection Agency (2003b). Toxicological review of hydrogen sulphide. EPA/635/R-03/005. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0061tr.pdf. Accessed 25 Fev 2021
USEPA - United States Environmental Protection Agency (2013) Terminology services, Vocabulary catalog, Vocabulary catalog list detail—Integrated Risk Information System (IRIS) Glossary, U.S. http://ofmpub.epa.gov/sor_internet/registry/termreg/searchandretrieve/glossariesandkeywordlists/search.do?details=&glossaryName=IRIS%20Glossary. Accessed 25 Fev 2021
USEPA - United States Environmental Protection Agency (2014) Passive samplers for investigations of air quality: method description, implementation, and comparison to alternative sampling methods. EPA/600/R-14/434. http://nepis.epa.gov/Adobe/PDF/P100MK4Z.pdf.
USEPA - United States Environmental Protection Agency (2016a) Integrated Risk Information System (IRIS). Toxicological review of ammonia noncancer inhalation. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0422_summary.pdf#nameddest=rfc. Accessed 25 Fev 2021
USEPA - United States Environmental Protection Agency (2016b) Guidelines for Human Exposure Assessment. https://www.epa.gov/sites/production/files/2016-02/documents/guidelines_for_human_exposure_assessment_peer_review_draftv2.pdf. Accessed 25 Fev 2021
Wan Y, Ruan X, Wang X, Ma Q, Lu X (2014) Odour emission characteristics of 22 recreational rivers in Nanjing. Environ Monit Assess 186:6061–6081. https://doi.org/10.1007/s10661-014-3840-4
Wang H, Wang H, Zhao C, Lv Z, Huang X, Liu X (2020) Linking health impact and post-environmental impact assessments: a case of municipal sewage treatment plant volatile organic compounds. Air Qual Atmos Health 13:421–433. https://doi.org/10.1007/s11869-020-00805-x
WHO - World Health Organization (2000) Air Quality Guideline for Europe. Ed 2. Regional Publications, European Series, No. 91, Copenhagen. https://www.euro.who.int/__data/assets/pdf_file/0005/74732/E71922.pdf. Accessed 25 Fev 2021
Wu C, Liu J, Liu S, Li W, Yan L, Shu M, Zhao P, Zhou P, Cao W (2018) Assessment of the health risks and odor concentration of volatile compounds from a municipal solid waste landfill in China. Chemosphere 202:1–8. https://doi.org/10.1016/j.chemosphere.2018.03.068
Yaghmaien K, Hadei M, Hopke P, Gharibzadeh S, Kermani M, Yarahmadi M, Emam B, Shahsavani A (2019) Comparative health risk assessment of BTEX exposures from landfills, composting units, and leachate treatment plants. Air Qual Atmos Health 12(4):443–451. https://doi.org/10.1007/s11869-019-00669-w
Yao XH, Zhang L (2013) Analysis of passive-sampler monitored atmospheric ammonia at 74 sites across southern Ontario, Canada. Biogeosciences 10:7913–7925. https://doi.org/10.5194/bg-10-7913-2013
Zabiegała B, Kot-Wasik A, Urbanowicz M, Namieśnik J (2010) Passive sampling as a tool for obtaining reliable analytical information in environmental quality monitoring. Anal Bioanal Chem 396:273–296. https://doi.org/10.1007/s00216-009-3244-4
Zhou J, He Q, Hemme CL, Mukhopadhyay A, Hillesland K, Zhou A, He Z, Van Nostrand JD, Hazen TC, Stahl DA, Wall JD, Arkin AP (2011) How sulphate-reducing microorganisms cope with stress: lessons from systems biology. Nat Rev Microbiol 9:452–466. https://doi.org/10.1038/nrmicro2575
Zuo Z, Chang J, Lu Z, Wang M, Lin Y, Zheng M, Zhu DZ, Yu T, Huang X, Liu Y (2019) Hydrogen sulfide generation and emission in urban sanitary sewer in China: what factor plays the critical role. Environ Sci: Water Res Technol 5:839–848. https://doi.org/10.1039/C8EW00617B
Acknowledgements
The authors gratefully acknowledge the Foundation for Research Support of the State of Bahia (FAPESB) for fellowships.
Funding
This study was partially supported by FAPESB with two research grants (registration numbers 4241/2018 and 4247/2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cruz, L.P.S., Alves, R.S., da Rocha, F.O.C. et al. Atmospheric levels, multivariate statistical study, and health risk assessment of odorous compounds (H2S and NH3) in areas near polluted urban rivers in the city of Salvador, in Northeastern Brazil. Air Qual Atmos Health 15, 159–176 (2022). https://doi.org/10.1007/s11869-021-01095-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-021-01095-7