Abstract
Background
Posterior reversible encephalopathy syndrome (PRES) is associated with variable predisposing risk factors including preeclampsia and eclampsia since it proposed. However, studies of large case series focusing on pregnancy-related PRES are still limited. We performed a large retrospective study of patients with pregnancy-related PRES admitted to our institution.
Methods
This was a single-center, 2010–2015 retrospective cohort study of patients with pregnancy-related PRES who underwent neuroimaging via magnetic resonance imaging or computerized tomography from mainland China.
Results
26 of 28 women with eclampsia and 7 of 59 women with preeclampsia had confirmed PRES. A total of 36 patients were finally included as confirmed pregnancy-related PRES in this research. Acute hypertension was present in 31 patients (86%). Headache was the most common presenting symptom (81%) followed by seizures (73%), altered mental status (57%), nausea/vomiting (47%) and visual disturbance (33%). Atypical involved regions included frontal lobe (72%), temporal lobe (67%), basal ganglia (50%), cerebellum (47%), brain stem (14%) and thalamus (8%). Atypical neuroimaging features included restricted diffusion (33%), contrast enhancement (19%) and hemorrhage (19%). Comorbidities included thrombocytopenia (25%), pulmonary infection (25%), anemia (19%), fever (17%), acute renal failure (8%), HELLP syndrome (6%), DIC (6%). Most of patients recovered completely with timely diagnosis and treatment. Two patients who suffered DIC finally died.
Conclusions
Patients with pregnancy-related PRES may present with atypical neuroimaging findings. Moreover, our data supported the view that nearly all imaged patients with eclampsia had clinical and radiologic findings of PRES.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posterior reversible encephalopathy syndrome (PRES), first described as reversible posterior leukoencephalopathy syndrome (RPLS) in 1996 by Hinchey et al. [1], is an uncommon clinical-radiological entity characterized by a variety of symptoms, including headache, seizures, visual abnormalities and altered mental status, accompanied by radiologic findings of reversible vasogenic edema, typically located in bilateral parieto-occipital areas and subcortical white matter [2]. This syndrome has been recognized in a variety of predisposing conditions, including acute hypertension, preeclampsia or eclampsia, renal disease, sepsis, autoimmune disease, and exposure to immunosuppressants [2].
Preeclampsia is described as new onset hypertension after 20 weeks of gestation, accompanied by a large amount of protein in the urine (>300 mg/dl in 24 h) [3]. Eclampsia is the onset of seizures/convulsions in a woman with preeclampsia in the absence of any other cause. Both preeclampsia and eclampsia are associated with many serious cardiovascular and neurological complications, including PRES [4,5,6]. In 2003, a retrospective cohort study showed that nearly all (25 of 27) patients with eclampsia who underwent neuroimaging had clinical and radiologic findings of PRES [7], which is further confirmed by other studies [4, 8], demonstrating that PRES is a core component of the pathogenesis of eclampsia. However, some studies showed conflicting results that only half of imaged patients with eclampsia can be diagnosed as PRES [5, 9, 10]. Furthermore, the limited literature has focus on how often PRES occurs in association with preeclampsia. So this study aimed to present the first evidence on which percentage of PRES occurs among patients with eclampsia and/or preeclampsia from mainland China.
In addition, recent reports indicated that different predisposing risk factors may cause some discrepancies in MRI appearance of PRES patients. For example, preeclampsia-eclampsia associated PRES has been shown to have higher prevalence of intracranial hemorrhage (ICH) [11, 12] and more basal ganglia involvement [13,14,15] in contrast with those PRES with hypertension as the only cause. But other studies showed inconsistent results, so we also sought to investigate the clinical and radiologic findings of patients with pregnancy-related PRES admitted to our institution.
Patients and methods
This project was a single-center, retrospective cohort study inclusive of imaged patients with pregnancy-related PRES between January 2010 and December 2015, which was approved by the institutional review board of our hospital.
Patients selection
Inclusion criteria consisted of the following three criteria: (1) pregnancy or within 6 weeks’ postpartum; (2) diagnosis of preeclampsia, eclampsia or PRES; (3) initial neuroimaging via MRI or CT was performed within 96 h from symptoms onset;
Exclusion criteria included the following three criteria: (1) not being pregnant or longer than 6 weeks postpartum; (2) known seizure disorder; and (3) diagnosis of PRES with other predisposing risk factors.
The diagnosis of PRES was made by radiologists using the following three criteria: (1) clinical presentations of acute neurologic change, including headache, seizures, visual abnormalities, altered mental status, or focal neurologic deficits; (2) brain imaging showed cortical or subcortical vasogenic edema with posterior predominance (in patients with ICH, we assigned the diagnosis of PRES only if edema was present in at least one area in addition to that surrounding the hemorrhagic focus or foci.); and (3) clinical or radiologic proof of reversibility.
Imaging analysis
MRI was performed on 1.5- or 3-T scanners, standard sequences, including sagittal and axial T1-weighted images, T2-weighted images and fluid-attenuated inversion recovery (FLAIR) were obtained in all patients. Specific sequences included diffusion-weighted images (DWI), apparent diffusion coefficient (ADC) maps, gadolinium-enhanced T1-weighted images, MR angiography (MRA) and MR venography (MRV) were performed in most patients, respectively. Brain CT and MRI were reviewed jointly by two neuroradiologists, who would determine via consensus which cases were truly consistent with PRES.
Clinical data
According to inclusion criteria, a total of 90 imaged patients (including 28 women with eclampsia, 59 women with preeclampsia and 3 PRES patients without history of gestational hypertension) were initially included for suspected subjects in this study. 26 of 28 women with eclampsia (92%) and 7 of 59 women with preeclampsia (12%) had confirmed PRES. Adding to three previously healthy women (all of them had no preeclampsia or eclampsia during pregnancy), a total of 36 patients with confirmed pregnancy-related PRES were finally included in this study. These patients were assessed for demographic, clinical and laboratory data, comorbidities, neuroimaging and prognosis (Fig. 1). Demographic and clinical findings are expressed as mean ± SD, or number (percentage).
Results
Clinical findings
A total of 36 patients with confirmed pregnancy-related PRES were finally included in this research. Mean ± SD age at presentation was 26.5 ± 6.40 [minimum–maximum (min–max), 16–41 years]. The mean gestational age of all patients was 34.7 ± 4.06 weeks (min–max, 30–39 weeks). Interestingly, all of 14 postpartum eclamptic patients had PRES, whereas 9 (82%) of 11 antepartum eclamptic patients had PRES. All of patients with antepartum PRES (n = 15, 100%) had cesarean delivery, whereas 17 of 21 patients with postpartum PRES (81%) had cesarean delivery. The median onset time of postpartum PRES was on day 3 (min–max, 0–26 days).
Acute hypertension was present in 31 patients (86%), with nine patients (25%) had a systolic blood pressure of 180 mmHg or more. Whereas normal blood pressure (BP < 140/90 mmHg) was noted in five patients. In addition, mean peak systolic blood pressure was 160 mmHg (min–max, 100–220 mmHg), and mean peak diastolic blood pressure at presentation was 100 mmHg (min–max, 66–150 mmHg).
Headache was the most common presenting symptom (n = 29, 81%), followed by seizures (n = 26, 72%), altered mental status (n = 19, 53%), nausea/vomiting (n = 16, 44%) and visual disturbance (n = 12, 33%). Common complications included: thrombocytopenia (defined as platelet counts <150, n = 9, 25%), pulmonary infection (n = 9, 25%), anemia (n = 7, 19%), mild to moderate fever (defined as body temperature ≥37.3 °C, n = 6, 17%), acute renal failure (n = 3, 8%), HELLP syndrome (n = 2, 6%), and disseminated intravascular coagulation (DIC, n = 2, 6%).
As for the three PRES patients without history of eclampsia or preeclampsia, the presenting symptoms included headache in three patients, altered mental status in two patients, nausea/vomiting in two patients and visual disturbance in one patient. Interestingly, acute hypertension was also noted in two patients after disease onset (156/82 and 148/110 mmHg, respectively).
Of the 17 patients who underwent lumbar puncture examination, eight (47%) revealed increased intracranial pressure, the cerebrospinal fluid (CSF) examination was mostly normal, except for one case of increased white cell counts and three cases of elevated protein levels. D-dimer abnormality (defined as D-dimer >0.5 g/L) was noted in 22 patients (73%), with its average level to be 2.5 ± 1.7 g/L (min–max, 0.14–7.25 g/L).
The majority of patients got swift diagnosis and was treated timely by aggressive control of blood pressure and seizures. However, three patients were difficult to differentiate from cerebral venous sinus thrombosis (CVST); therefore anticoagulation therapy was administered until more clinical and radiologic evidence emerged. Most of those patients achieved complete neurological recovery except residual deficits in 4 (11%). However, two patients (6%) suffering DIC finally died. One death was due to severe coagulopathy from placental abruption and DIC, the other death was due to postpartum hemorrhage, acute renal failure requiring dialysis and DIC.
Radiologic findings
The diagnosis of PRES was made using one or more imaging modalities, including brain CT (n = 30), MRI (n = 36), MRI with contrast (n = 31), DWI imaging (n = 30), MRA (n = 18), MRV (n = 25). The parieto-occipital regions were the most commonly involved (n = 33, 92%) (Fig. 2), followed by frontal lobe (n = 26, 72%), temporal lobe (n = 24, 67%), basal ganglia (n = 18, 50%), and cerebellum (n = 17, 47%), brain stem (n = 5, 14%), thalamus (n = 3, 8%). For three patients who lacked parieto-occipital involvement, they all had bilateral basal ganglia edema, with one of them had additional brainstem involvement. Moreover, they all had a high BP (178/104, 155/84, 190/150 mmHg, respectively) after disease onset, and their manifestations dramatically improved after aggressive antihypertensive therapy, with edema nearly resolved on follow-up neuroimaging.
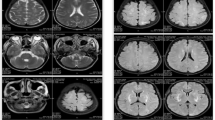
PRES in a 30-year-old woman with postpartum eclampsia who had gestational hypertension. a Axial unenhanced CT (day 1) demonstrate extensive low-density lesions in bilateral cerebral hemisphere, with a small hematoma in the right occipital regions. b, c Brain MRI (day 3) showed multiple cortical and subcortical hyperintense abnormalities in bilateral frontal and parieto-occipital lobes on fluid-attenuated inversion recovery (FLAIR) imaging, along with a well-defined hematoma in the right occipital lobe. d–f Repeated head CT and MRI (day 17) showed nearly complete resolution of brain edema, better seen in FLAIR. The hematoma is also greatly absorbed and dissipated
Of all patients, 34 (94%) had subcortical involvement and eight (22%) had cortical involvement. 10 of 30 patients underwent DWI imaging (33%) revealed restricted diffusion, which was usually punctate and surrounded by much larger areas of edema (Fig. 2). 6 of 31 patients (19%) underwent contrast enhancement imaging showed abnormal enhancement (Fig. 3), which included mild, punctate enhancement (n = 4); linear enhancement (n = 1) and leptomeningeal enhancement (n = 1).
PRES in a 28-year-old woman with postpartum eclampsia who was previously healthy. a FLAIR image showed symmetric hyperintense abnormalities in bilateral basal ganglion. B, DWI image showed the T2 abnormalities have two distinct diffusion patterns, the highly hyperintense signal (two arrows) in caudate nucleus indicates restricted water diffusion and ischemic/cytotoxic edema, while the nearly normal peripheral region indicates vasogenic edema that is caused by transient blood–brain barrier breakdown. c The ADC map revealed a significant reduction in ADC of a bilateral caudate nucleus (two arrows), compared with high signal in the peripheral area. d The contrast enhanced T1-weighted MRI showed symmetric punctate enhancement in bilateral caudate nucleus (two arrows)
ICH was present in seven (19%) of all patients: five patients had parenchymal hemorrhage, one case had subarachnoid hemorrhage and one had both types of hemorrhage. The hemorrhage could be noted at initial imaging in six of seven patients, with delayed hematoma identified on follow-up imaging in one patient. Six cases of ICH occurred near the parenchymal edema and associated no mass effect (Fig. 2), but one patient had large hematoma along with remarkable mass effect in the right basal ganglion (Fig. 4), who also had combined vesogenic edema and cytotoxic edema according to DWI/ADC images, together with multifocal vasoconstriction revealed on the cerebral vascular imaging.
Atypical radiologic characters in a 25-year-old women presenting with lethargy after emergency cesarean delivery. a Axial head CT demonstrated a large hematoma in right basal ganglion and a low-density lesion in left basal ganglion. b, c Axial FLAIR MRI imaging showed the abnormalities in the left basal ganglion have two distinct diffusion patterns in DWI imaging, central area with hyperintense signal and normal peripheral area, representing cytotoxic edema and vesogenic edema, respectively. d Axial Flair imaging also showed vesogenic edema in bilateral temporal lobe and right frontal lobe. e MR angiography demonstrated multifocal stenoses in the proximal anterior, middle and posterior cerebral artery as well as internal carotid artery. f Follow-up CT on day 8 showed that hematoma in the right ganglion was partially absorbed, and the right ganglion ischemic lesion also resolved greatly
Interestingly, MRV imaging showed flow gaps in cerebral sinus of three patients, which were suspected as sinus thrombosis at one time but later turned out to be normal variation on following digital subtraction angiography (DSA).
Follow-up neuroimaging was performed in 30 of 36 cases; the overall median time to follow-up imaging was 9 days (min–max, 2–41 days). Follow-up imaging showed evidence of radiologic improvement in 29 cases (97%), with complete or near-complete resolution of brain edema in 20 patients (67%). One patient had no significant change probably due to a too early time (2 days) for follow-up imaging.
Discussion
The present series of patients is the largest single institutional series of imaged patients with pregnancy-related PRES from mainland China that has been published to date. Consistent with previous reports [4, 7, 8], our data also suggest that PRES occurred almost uniformly among patients with eclampsia. However, other studies showed that only 41–63% of imaged eclamptic women had clinical and radiologic findings of PRES [5, 9, 10]. These conflicting results, though may be partly caused by the selection bias, inconsistent diagnostic work-up and racial difference, remain to be further elucidated. In addition, our study found that PRES occurred among 12% of imaged patients with preeclampsia, emphasizing the importance of having a high index of suspicion among preeclampsia with neurologic signs. Considering that neuroimaging diagnosis would not change the key component in treatment—aggressive blood pressure control [5]— we do not recommend performing MRI and/or CT as standard practice in patients with preeclampsia for suspected PRES.
Consistent with previous studies [4, 15], our data showed that the majority (86%) of pregnancy-related PRES patients were complicated with abrupt hypertension, supporting the theory that hyperperfusion caused by abrupt hypertension that exceeds the retaining capacity of the brain capillary beds undoubtedly contributes to the development of PRES. However, due to the fact that PRES can develop when blood pressures were well within the usual range of autoregulation [2], endothelial cell dysfunction theory is now more widely accepted as the basic reason of pregnancy-related PRES. The synthesis and secretion of a variety of endothelial cell and neutrophil chemokines, and cytokines provoke a vicious cycle that results in disruption of vascular integrity and vasogenic edema, sometimes progressing to cytotoxic edema. As reveled in our data and other studies [7, 16], the prevalence of cytotoxic edema noted on DWI imaging can be up to 22–33% in pregnancy-related PRES. In addition, we reported a higher frequency (19%) of contrast enhancement, which also reflects the high degree of blood–brain barrier dysfunction in these patients, though our data conflicts with a lower frequency reported in a previous study [15].
Notably, our data revealed that pregnancy-related PRES often occurred in multiple areas of the brain, not necessarily the parietal-occipital region, occurring more commonly in basal ganglion (50%) than other predisposing factors induced PRES. Our results is consistent with the findings of many studies [13, 15], which suggests a tendency toward involvement of basal ganglia (33–60%) in preeclampsia-eclampsia-associated PRES, in contrast with a lower prevalence (18–21%) in PRES induced by other predisposing diseases. In addition, three patients noted with basal ganglia and/or brainstem involvement but sparing the parieto-occipital region, were considered to have a rare type of PRES—central variant, which is usually correlated with extreme hypertension, as reported in previous studies [17, 18].
In addition, we reported a 19% prevalence of ICH in pregnancy-related PRES, close to recent reports that ICH occurred with a frequency of 12.5–25% in patients with preeclampsia-eclampsia-associated PRES [12, 15], higher than the frequency (5.9–10.4%) in those with hypertension as the only cause of PRES [11, 12], but lower than the frequency (28–34%) in patients with sepsis/infection/shock related PRES [11, 12]. These discrepancies may relate to various factors, partly resulted from different imaging strategy, for example, new technology such as susceptibility-weighted imaging allowed for easier detection of blood products. But ICH does occur in preeclampsia-eclampsia related PRES with a relatively high frequency. We believe that endothelial/blood brain barrier breakdown partially contributed to the hemorrhage given the fact that hemorrhage often occurred near the parenchymal edema, though the cause of hemorrhage in PRES is not fully established [11].
Interestingly, MRA showed that a patient with combined ICH and vesogenic/cytotoxic edema also had multifocal vasoconstriction, which was also compatible with diagnosis of postpartum angiopathy (PPA). PPA is a rare cause of stroke in the puerperium, usually heralded by severe headaches within 1–2 weeks after delivery, demonstrating segmental vasoconstriction that often resolves spontaneously. Brain parenchymal abnormalities, including intracranial hemorrhage, vasogenic edema and infarction can occur in more than one third of PPA patients and can cause high proportion of unfavorable outcomes [19]. Hence, clinicians should be aware of the overlap of PRES and PPA [19, 20] and take cerebrovascular angiography such as MRA as conventional weapons.
Our data showed that two women finally died of DIC, which is more inclined to develop among severe preeclampsia patients than overall during pregnancy (incidence rate: 12.6–14 vs 0.03–0.35%) [21, 22]. Given that DIC remains one of the leading causes for maternal mortality worldwide [23], prompt diagnosis and rigorous treatment are needed to reduce the morbidity and mortality associated with DIC.
PRES is a clinico-radiological entity with a varying combination of symptoms and signs, including headache, seizures, visual abnormalities, nausea, vomiting, impaired consciousness, and focal neurologic deficits. However, several diseases mimicking PRES, including acute stroke, CVST, encephalitis, are also inclined to present during pregnancy and puerperium. Sometimes it is fairly difficult to establish an accurate diagnosis in an early stage on clinical grounds alone, which could result in delayed treatments, causing higher morbidity or mortality in these young individuals. For example, rigorous blood pressure control is desirable in PRES, whereas mild to moderate hypertension is permitted in acute stroke. In addition, the anticoagulation therapy aimed at possible CVST, which is associated with high rate of mortality and morbidity, can probably raise PRES-related hemorrhage frequency [11]. MRI, including DWI and MR angiography/venography, should be performed as soon as possible to exclude vascular lesions such as CVST and stroke. Of great importance, flow gaps observed on MRV should be judged with caution, and DSA should be done as early as possible to rule out the possibility of sinus thrombosis. Furthermore, analysis of the CSF is necessary to rule out intracranial infection and inflammation. In conclusion, timely imaging and broad differential diagnosis may lead to more appropriate decisions, thus preventing the possible development of long-term neurologic deficits.
Importantly, appropriate reduction in blood pressure may prevent progression from vasogenic to cytotoxic edema and the resultant permanent neurologic deficits. Because abrupt decreases in blood pressure may adversely affect uteroplacental perfusion and fetal status, treatment of hypertension should mandate close maternal blood pressure and fetal monitoring. The importance of magnesium sulfate for the prevention and treatment of eclamptic seizures is also emphasized.
The study shares the limitations of all retrospective studies, including the selection bias that neuroimaging was only performed in part of patients with preeclampsia and/or eclampsia because there was no consistent protocol for physicians to decide who would undergo neuroimaging. Another shortcoming is that the diagnostic work-up, therapeutic management and follow-up imaging were not standardized for all patients, which may have biased our results. Further research with consistent protocols is needed to identify predicting factors of PRES development, and to achieve earlier recognition and better blood pressure management of this highly variable disease.
In conclusion, in this study, we characterized in greater detail the clinical and radiologic characteristics of pregnancy-related PRES in a larger case series from mainland China. We showed that nearly all imaged women with eclampsia had neurologic abnormalities and accompanying radiologic findings of PRES. We also revealed that atypical distributions and imaging manifestations of pregnancy-related PRES have a higher incidence than has been commonly perceived. Early recognition of PRES together with aggressive comprehensive and meticulous medical management may facilitate complete maternal recovery.
References
Hinchey J, Chaves C, Appignani B et al (1996) A reversible posterior leukoencephalopathy syndrome. N Engl J Med 334:494–500
Fugate JE, Rabinstein AA (2015) Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 14:914–925
Mol BW, Roberts CT, Thangaratinam S et al (2016) Pre-eclampsia. Lancet 387:999–1011
Brewer J, Owens MY, Wallace K et al (2013) Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol 208:461–468
Fisher N, Saraf S, Egbert N et al (2015) Clinical correlates of posterior reversible encephalopathy syndrome in pregnancy. J Clin Hypertens (Greenwich) 18:522–527
Cozzolino M, Bianchi C, Mariani G et al (2015) Therapy and differential diagnosis of posterior reversible encephalopathy syndrome (PRES) during pregnancy and postpartum. Arch Gynecol Obstet 292:1217–1223
Zeeman GG, Fleckenstein JL, Twickler DM et al (2004) Cerebral infarction in eclampsia. Am J Obstet Gynecol 190:714–720
Wagner SJ, Acquah LA, Lindell EP et al (2011) Posterior reversible encephalopathy syndrome and eclampsia: pressing the case for more aggressive blood pressure control. Mayo Clin Proc 86:851–856
Kurdoglu Z, Cetin O, Sayin R et al (2015) Clinical and perinatal outcomes in eclamptic women with posterior reversible encephalopathy syndrome. Arch Gynecol Obstet 292:1013–1018
Hossain N, Khan N, Panhwar N et al (2015) Clinical spectrum of Posterior Reversible Encephalopathy Syndrome (PRES) in patients with eclampsia. Pak J Med Sci 31:1121–1123
Hefzy HM, Bartynski WS, Boardman JF et al (2009) Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. AJNR Am J Neuroradiol 30:1371–1379
Sharma A, Whitesell RT, Moran KJ (2010) Imaging pattern of intracranial hemorrhage in the setting of posterior reversible encephalopathy syndrome. Neuroradiology 52:855–863
Mueller-Mang C, Mang T, Pirker A et al (2009) Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology 51:373–383
Demirtas O, Gelal F, Vidinli BD et al (2005) Cranial MR imaging with clinical correlation in preeclampsia and eclampsia. Diagn Interv Radiol 11:189–194
Liman TG, Bohner G, Heuschmann PU et al (2012) Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases. Eur J Neurol 19:935–943
Loureiro R, Leite CC, Kahhale S et al (2003) Diffusion imaging may predict reversible brain lesions in eclampsia and severe preeclampsia: initial experience. Am J Obstet Gynecol 189:1350–1355
McKinney AM, Short J, Truwit CL et al (2007) Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 189:904–912
Casey SO, Truwit CL (2000) Pontine reversible edema: a newly recognized imaging variant of hypertensive encephalopathy? AJNR Am J Neuroradiol 21:243–245
Fugate JE, Ameriso SF, Ortiz G et al (2012) Variable presentations of postpartum angiopathy. Stroke 43:670–676
Fletcher JJ, Kramer AH, Bleck TP et al (2009) Overlapping features of eclampsia and postpartum angiopathy. Neurocrit Care 11:199–209
Erez O, Novack L, Beer-Weisel R et al (2014) DIC score in pregnant women: a population based modification of the International Society on Thrombosis and Hemostasis score. PLoS One 9:e93240
Rattray DD, O’Connell CM, Baskett TF (2012) Acute disseminated intravascular coagulation in obstetrics: a tertiary centre population review (1980 to 2009). J Obstet Gynaecol Can 34:341–347
Erez O, Mastrolia SA, Thachil J (2015) Disseminated intravascular coagulation in pregnancy: insights in pathophysiology, diagnosis and management. Am J Obstet Gynecol 213:452–463
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical standards and patient consent
We declare that all human studies have been approved by the institutional review board of Xiangya Hospital, Central South University and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Wen, Y., Yang, B., Huang, Q. et al. Posterior reversible encephalopathy syndrome in pregnancy: a retrospective series of 36 patients from mainland China. Ir J Med Sci 186, 699–705 (2017). https://doi.org/10.1007/s11845-017-1567-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-017-1567-2