Abstract
In this work, a new and carbon-free reductant, i.e., secondary aluminum dross, is used to replace coal to recycle valuable metals from copper slag by smelting reduction. The influence of substitution ratio on slag properties and metal recovery was investigated. The results show that the metal recovery is mainly determined by the settlement of the generated metals after reduction because of the rapid increase of slag viscosity. Although the viscosity of the final slag was 0.3–0.4 Pa·s when using coal as the primary reductant at 1550°C, only 95.4% Fe was recovered because of the slag foaming effect. The viscosity was still kept at an acceptable level at the substitution ratio of 50% but the foaming phenomenon disappeared, leading to an almost complete recovery of iron (98.2%). However, the final slags became extremely viscous with a further increase of aluminum dross content, greatly impeding the separation of metal droplets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper slag is an important by-product of copper metallurgy. In general, 2.2–3.0 tons slag will be produced for each ton of refined copper.1 Every year, tens of millions of tons of copper slag is discharged into the environment in China.2 Unfortunately, these industrial wastes are mainly disposed of by landfilling in specific sites of copper smelters, causing management and environmental issues.3 Therefore, the recycling of copper slag into useful and clean products is of great importance to the sustainable development of the copper industry.
Copper slag is a fayalite-based system and contains abundant valuable resources, such as iron and copper (~40 wt% total iron and 0.5–3.0 wt% total Cu).4 The iron grade in copper slag is even higher than that of some natural ores. Besides, the mining process required for the primary iron ores can be saved if iron and steel are produced from secondary resources, showing advantages in cost and environmental aspects.5 Thus, the extraction of iron from copper slag has great economic value and is considered a promising way to promote the large-scale utilization of this industrial waste. In the past 2 decades, many technologies have been applied to the iron recovery process, mainly including physical,6 hydrometallurgical,7 and pyrometallurgical methods.8,9,10,11 Among them, the direct reduction-magnetic separation based on pyrometallurgical principles is the most commonly used one because of its high recovery efficiency.12 By this method, > 90% iron can be recovered from copper slag after magnetic separation.13,14 To further improve the recovery efficiency of valuable metals, the recycling of copper slag by using the smelting reduction method has been investigated by many researchers.15,16 The greatest advantage of this technique is its short process since the reduced metals can be directly separated from the slag during smelting.
Despite many advantages, a major concern about the above two recovery processes is the usage of carbonaceous materials. In practice, excessive solid carbon is usually required to supply heat because this process is an endothermic reaction, increasing both cost and carbon emission, which is disadvantageous to achieving China’s carbon peak and neutrality goals. To solve this issue, Heo et al. proposed a novel iron recovery process based on aluminothermic smelting reduction, which is found to be more efficient and environmentally friendly.17 However, its high cost is a big concern for this recovery process. Aluminum dross is an industrial waste produced from aluminum smelters. It can be divided into two types according to aluminum content, i.e., primary aluminum dross (Al content ≥ 50 wt%) and secondary aluminum dross (Al content: 5–35 wt%).18 The latter is the residue of the primary aluminum dross after aluminum extraction. The disposal of secondary aluminum dross is also a focus problem because it is difficult to further extract aluminum from this type of waste.18
The chemical composition of secondary aluminum dross indicates that it may be an excellent reductant for iron and copper oxides in copper slag. Its usage as a replacement for coal in the smelting reduction of copper slag not only greatly reduces the carbon emissions, but also brings about the advantages of aluminothermic reduction at an acceptable cost. However, great changes in composition and thermo-physical properties of the liquid slag would also be expected compared to the traditional carbothermic and aluminothermic reduction processes due to the dissolution of other oxides from aluminum dross. These changes would significantly influence the reduction and separation efficiency. To understand these issues, an attempt has been made to recover valuable metals from waste copper slag by using coal and aluminum dross as co-reductants in this work. The influences of substitution ratio and smelting temperature on the slag properties, metal recovery, and slag-refractory interaction were investigated by experimental and thermodynamic approaches to determine the optimal design of reductant proportion and smelting temperature for iron recovery.
Experimental Procedure
Raw Materials
The industrial copper slag used in this work was collected from a copper-smelting plant in Ningde, China. Secondary aluminum dross and pulverized coal were used as the reductants for iron oxides in the copper slag. The chemical compositions of these raw materials are presented in Table I. To increase the activity of FeO in slag at elevated temperatures, the chemical reagent of CaO was used as a modifier to release more FeO from fayalite.3,13,15,19 According to our previous study,20 an initial modified basicity of 0.9 is applied in this experiment, at which the activity of FeO reaches the maximum value. Based on the material balance calculation, 16 g coal or 44 g aluminum dross is required for the complete reduction of iron oxides in the copper slag. Considering the consumption of C and Al by the reduction of Cu2S and SiO2 in the smelting reduction, the actual amount of the reductants is around 1.1 times the theoretical values. To find out the optimal proportion of these two reductants, five raw material proportions were designed, as shown in Table II.
Reduction and Recovery Procedure
The reduction experiments were performed in an electric tube furnace at 1500°C and 1550°C, respectively. First, 100 g copper slag and 27 g CaO reagent were mixed, and then the mixtures were pressed to cylindrical samples by a hydraulic press to increase their melting speeds during heating. Each pressed block was charged into a MgO crucible (purity ≥ 99.5 wt%, 48 mm inner diameter and 90 mm depth), and then the crucible was placed in the hot zone of the furnace and heated up to the target temperature. A holding time of 30 min was employed to ensure the complete melting of the pressed blocks and the formation of homogeneous liquid slag.20,21 After that, a specified amount of coal and aluminum dross (according to Table II) was added into the molten slag quickly via a quartz tube. After reduction for 60 min, the crucible was taken out quickly from the furnace and quenched into water. The whole process was conducted under a purified Ar atmosphere (flow rate of 0.5 L/min). During the smelting reduction process, the recovery of the reduced metals will be achieved by a sedimentation process.
Characterization
The chemical compositions and the microstructures of the reduced slags were detected by x-ray fluorescence (XRF) and field-emission scanning electron microscopy (FE-SEM), respectively. The weights and compositions of the ingots separated from the slag were measured by a high-accuracy electronic balance and an inductively coupled plasma-atomic emission spectroscopy (ICP-AES), respectively. The iron recovery was defined as the ratio of iron in the ingot to the total iron in the initial slag (43.4 g Fe/100 g slag), as described in Eq. 1.15 Besides, the interaction of slag with MgO crucible and the change of slag viscosity during reduction were analyzed by thermochemical software FactSage (version 8.0, the modules of “Equilib” and “Viscosity” and the databases of FToxid, FactPS, and Melts were utilized).
where mingot is the weight of the separated ingot, g; ωFe refers to the mass fraction of iron in the ingot, wt%; and mTFe is the weight of total iron in the initial slag, g.
Results and Discussion
Recovery of Fe and Cu
The photographs of the reduced slags and the metal ingots obtained by smelting reduction at different conditions are presented in Fig. 1. The separation of the reduced metals from slag has been successfully achieved during smelting for all the cases, confirming the effectiveness of this extraction method. However, these metal ingots differ in their weights and chemical compositions, as shown in Table III. For example, the weight of the ingot obtained by reduction at 1550°C is significantly higher than 1500°C under the same material proportion design, suggesting a better separation performance. Besides, this value exhibits an obvious increase with the substitution of aluminum dross for pulverized coal at both 1500 and 1550°C, which reaches a peak at the substitution ratio of 50% and then shows a significant decline with further increase of the amount of aluminum dross. According to the chemical analysis (Table III), the ingots obtained by coal reduction are typical Fe-C alloys with small amounts of Cu and Si. The carbon content in the ingot is remarkably decreased with increasing the substitution ratio of aluminum dross, while the silicon content shows an opposite behavior. Eventually, the iron ingots reduced by aluminum dross are transformed into Fe-Si-Cu alloys. These Cu-bearing alloys can be used as the raw materials for the smelting of weathering steel, antibacterial stainless steel, and high-strength steel.
Based on the data in Table III, the direct recovery rates of iron after reduction at different conditions were calculated, as exhibited in Fig. 2. The Fe recovery reaches 93.7% for the copper slag after reduction by coal at 1500°C. This value increases to 96.0% with the substitution ratio increasing to 50%, but then it shows a significant decrease with further increase of aluminum dross addition. The substitution of aluminum dross for coal shows the same effect on metal recovery at 1550°C as 1500°C, but the increase of temperature plays an active role in the metal separation during smelting. At 1550°C, the recovery rate of iron is as high as 98.2% at the substitution ratio of 50%, suggesting an extremely high separation efficiency of the reduced metals. Meanwhile, > 98% Cu in the copper slag is also recovered into the ingot. The above results clearly show that the smelting reduction of copper slag using co-reductants of coal and aluminum dross is an efficient way to recover valuable metals.
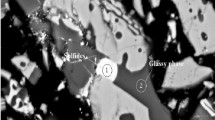
Actually, the reduction rates of iron oxides are nearly 100% for all the cases since almost no FeO or Fe2O3 is detected in the reduced slags, as confirmed by Fig. 3 and Table IV (the theoretical calculation for the composition of the reduced slags is made only based on the complete reduction of iron oxides in the copper slag). The relatively lower recovery rates suggest that some reduced iron droplets failed to settle to the metal pool and remained in the slag, as illustrated in Fig. 3a and e. Figure 3 also shows that the slag became less homogeneous but more compact with the substitution of aluminum dross for coal. Notably, the MgO contents in the reduced slags are much higher than the calculated values when using coal as the primary reductant, as shown in Table IV, suggesting a strong interaction between slag and MgO crucible. However, this interaction has been greatly suppressed by increasing the additional amount of aluminum dross. Besides, the Al dross used in this work contains various oxides, such as Al2O3, SiO2, and MgO, and their dissolution into the slag will greatly change the slag composition. Therefore, the theoretical composition of the reduced slag does not change linearly with the substitution ratio of Al dross/coal.
Thermodynamic Analysis
The main reactions occurred during the smelting reduction process are as follows:22
△Gθ = −109074−4.0 T J/mol
△Gθ= 141660−139.5 T J/mol
△Gθ= −919057 + 165.8 T J/mol
△Gθ= 117140−2.0 T J/mol
△Gθ= 13750−133.6 T J/mol
△Gθ= −1189650 + 131.4 T J/mol
△Gθ= 692940−357.5 T J/mol
△Gθ= −534170 + 60.7 T J/mol
(*) means that the component is in the liquid slag
[*] means that the component is in the molten iron
From Eqs. 2–7, it can be concluded that the reduction of FeO and Cu2S in the slag is quite feasible in terms of thermodynamics at the investigated temperatures (1773 K and 1823 K) whether using coal or aluminum dross as the reductant. Therefore, there were almost no iron or copper oxides/sulfides identified in the slags after smelting reduction (Table IV and Fig. 3). The feasibility of SiO2 reduction greatly increases when using Al as a reductant because of the much more negative Gibbs free energy change of reaction (9) compared to reaction (8). Consequently, no Al element is detected in the ingot although the actual amount of the reductants is slightly higher than the theoretical values, while the Si content in the ingot dramatically increases with the addition of aluminum dross, as shown in Table III.
Influence Mechanism of Substitution Ratio
After reduction, the reduced metal droplets settle to the bottom of the crucible because of the density difference between liquid iron and slag. In this case, the recovery efficiency of valuable metals is mainly determined by the settling velocity of the metal droplets, which is controlled by the slag viscosity.15,23,24 Based on the actual compositions of the slags in Table I and Table IV, their changes in viscosity during smelting reduction were quantitatively calculated by FactSage, as shown in Fig. 4. The viscosity of the initial slag is only ~0.04 Pa·s at both 1500 and 1550°C, providing an excellent environment for the reduction and separation process. However, the viscosity values dramatically increase in the later period of reduction. This is because FeO is a highly effective network-modifier for CaO-SiO2 system slag.25 Its disappearance will intensify the degree of polymerization of the slag structure and thus lower its fluidity. This change is extremely significant for the cases using aluminum dross as the primary reductant. For example, the slag viscosity values increase to 1.37 and 1.82 Pa·s at the end of reduction at 1500°C when the substitution ratios are 75% and 100%, respectively.
As reported in the literature,21,25 Al2O3 at higher content in the slag would behave as a network former and greatly decrease the fluidity of the liquid slag by the polymerization of [AlO4]- and [Si(Al)O4]-tetrahedra. This evolution is responsible for the 30-40 times increase in slag viscosity after the reduction of FeO by aluminum dross. In this condition, it is difficult for the generated metals to separate from the slag by sedimentation. According to the Stokes equation [Eq. 10], the terminal settling velocity of the droplets with diameters of 100 μm is only 1.2~1.6 × 10−5 m/s and the time required for their falling through the slag is more than 40 min, as shown in Fig. 5 (the height of the slag obtained after reduction is regarded as the maximum sedimentation distance in the calculation of separation time). In this condition, therefore, some metal particles produced in the later stage of reduction with similar sizes would remain in the slag, as shown in Fig. 3e. The slag viscosity decreases obviously with the smelting temperature because higher temperature supplies more energy to break up the complex structures of silicates or aluminates. Thus, the iron recovery is improved at 1550°C compared to 1500°C.
where vt refers to the terminal velocity of the metal droplet, m/s; g is the gravitational acceleration constant, 9.8 m/s2; d is the diameter of the droplet, m; ρFe and ρslag represent the density values of liquid iron (7040 kg/m3) and slag (3000 kg/m3), respectively; and ηslag is the viscosity of the slag, Pa·s.
The final slags exhibit the lowest viscosity values when using coal as the main reductant, as shown in Fig. 4, which are only 0.42 and 0.30 Pa·s at 1550°C when the substitution ratios are 0 and 25%, respectively. The highly homogeneous structures of these slags are good evidence of this result (Fig. 3a and b). In this case, only around 10 min is required to separate the 100-μm-sized droplets from the slag. However, this advantage did not bring about the best recovery efficiency of iron, as demonstrated in Fig. 2. This inconsistency is mainly attributed to the slag foaming effect. It is well known that the foaming of the liquid slag would occur during carbothermic reduction because of the evolution of CO bubbles.26 Therefore, many large-sized pores can be observed in the final slags, as shown in Fig. 3a and b. The mass transfer in the liquid slag during the reduction can be enhanced by the agitation effect of bubbles. However, the reduced metal droplets are easily trapped in the foamy slag, hindering their coalescence and growth.15
The viscosity of the final slag at 1550°C increases to 0.58 Pa·s when raising the substitution ratio to 50%. This value is also kept in an acceptable level because in this condition, only 16 min is required for the complete settlement of the same-sized droplets. Besides, the aluminothermic reduction is much faster than the carbothermic reduction. According to Heo et al.,15,17 the reduction of FeO is proved to occur within 5 min when using aluminum as the reductant, whereas > 30 min is needed for coal reduction. This difference means more time left for the settling separation of the metals reduced by aluminum dross. Moreover, the slag foaming effect disappears at this proportion design of reductants, as shown in Fig. 3c, which is advantageous for the growth and settlement of the reduced metals. Consequently, almost full recovery of iron from the waste copper slag has been achieved in this condition.
Interaction of Slag with MgO Crucible
In the smelting reduction process, the interaction of slag with lining materials is also an important issue because the liquid slag usually is highly corrosive.27 Although MgO-based refractory materials have high corrosion resistance to many metallurgical slags, the dissolution of MgO from the crucible into the slag is very evident in this work. To determine the effect of the substitution of aluminum dross for coal on the slag-refractory interaction during reduction, the MgO solubility versus reduction degree curves under various substitution ratios at 1550 °C were calculated by FactSage, as demonstrated in Fig. 6. In this calculation, the reduction degree is defined as the ratio of the amount of the reduced FeO compared to the total FeO in the initial slag. It can be observed that MgO exhibits very high solubility (≥ 20 wt%) in the later stage of reduction when using pulverized coal as the primary reductant. The sharp decrease of free oxygen ions owing to FeO reduction is the main reason for this phenomenon.28 As a result, the contents of MgO in these slags are much higher than the theoretical values after reduction (Table IV). This change may show no significant effect on the slag viscosity and metal recovery since the MgO content in the final slag is significantly lower than the solubility values, as shown in Table IV and Fig. 6, but it increases the safety risks during production.
The MgO solubility in the slag at the later reduction stage dramatically decreases with the introduction of aluminum dross. For example, the solubility values in the final slags are 12.3% and 7.2%, respectively, when 50% and 100% coal is replaced by aluminum dross. This is because the increase of Al2O3 content in the slag would encourage the precipitation of spinel, which is unfavorable for the dissolution of MgO.29 Therefore, MgO contents in the reduced slags are very close to the theoretical values when the substitution ratio is > 50%, as shown in Table IV. Undoubtedly, this change is beneficial to protecting the refractory linings and ensuring the slag quality.30
Conclusion
In this work, a novel smelting reduction method has been developed to recover Fe from copper slag by using pulverized coal and secondary aluminum dross as co-reductants. The influence of the substitution ratio of aluminum dross for coal on slag properties, metal recovery, and slag-refractory interaction was investigated. The main conclusions are as follows:
-
1)
The slag viscosity increases rapidly at the end of reduction because of the sharp decrease in FeO content. This change is particularly significant when using aluminum dross as the primary reductant, greatly impeding the settling separation of reduced metals. Although the final slags exhibit the lowest viscosity values by coal reduction, it does not bring about the best recovery efficiency of iron due to the formation of foamy slag.
-
2)
The direct recovery of iron increases to 98.2% for copper slag when 50% coal is replaced by aluminum dross. The key reasons for this improvement can be revealed as follows: (i) the much faster reaction rates of aluminothermic reduction compared to carbothermic reduction; (ii) the relatively lower slag viscosity at the end of reduction (0.58 Pa·s); (iii) the disappearance of slag foaming effect.
-
3)
The interaction of slag with crucible materials is extremely strong when using coal as the main reductant, causing the massive dissolution of MgO into slag, which is disadvantageous to production safety and slag quality control. This phenomenon has been greatly suppressed by the introduction of aluminum dross because of the decrease of MgO solubility.
References
A. Harvey, Resour. Conserv. Recycl. 43, 353 (2005).
D.Q. Wang, Q. Wang, and Z.X. Huang, Resour. Conserv. Recycl. 162, 105037 (2020).
Z.Q. Guo, D.Q. Zhu, J. Pan, and F. Zhang, J. Clean. Prod. 187, 910 (2018).
T.J. Chun, C. Ning, H.M. Long, J.X. Li, and J.L. Yang, JOM 68, 2332 (2016).
J. Shi, Y. Qiu, B. Yu, X. Xie, J. Dong, C. Hou, J. Li, and C. Liu, JOM. https://doi.org/10.1007/s11837-021-05040-y (2022).
S. Roy, A. Datta, and S. Rehani, Int. J. Miner. Process. 143, 43 (2015).
G.C. Shi, Y.L. Liao, B.W. Su, Y. Zhang, W. Wang, and J.J. Xi, Sep. Purif. Technol. 241, 116699 (2020).
Z.L. Zuo, S.Y. Luo, S.H. Liu, J.K. Zhang, Q.B. Yu, and X.J. Bi, Chem. Eng. J. 405, 126671 (2021).
J. Zhang, Y.H. Qi, D.L. Yan, and H.C. Xu, J. Iron Steel Res. Int. 22, 396 (2015).
H. Wang, L. Shen, H. Bao, W. Zhang, X. Zhang, L. Luo, and S. Song, JOM 73, 703 (2021).
P. Sarfo, A. Das, G. Wyss, and C. Young, Waste Manage. 70, 272 (2017).
B.S. Kim, S.K. Jo, D. Shin, J.C. Lee, and S.B. Jeong, Int. J. Miner. Process. 124, 124 (2013).
S.W. Li, J. Pan, D.Q. Zhu, Z.Q. Guo, J.W. Xu, and J.L. Chou, Powder Technol. 347, 159 (2019).
S.W. Zhou, Y.G. Wei, B. Li, and H. Wang, J. Clean. Prod. 217, 423 (2019).
J.H. Heo, B.S. Kim, and J.H. Park, Metall. Mater. Trans. B 44B, 1352 (2013).
D. Busolic, F. Parada, R. Parra, M. Sanchez, J. Palacios, and M. Hino, Min. Process. Extract. Metall. Rev. 120, 32 (2011).
J.H. Heo, Y.S. Chung, and J.H. Park, J. Clean. Prod. 137, 777 (2016).
H.L. Shen, B. Liu, C. Ekberg, and S.G. Zhang, Sci. Total Environ. 760, 143968 (2021).
Z.Q. Guo, D.Q. Zhu, J. Pan, W.J. Yao, W.Q. Xu, and J. Chen, JOM 69, 1688 (2017).
G.Z. Zhang, N. Wang, M. Chen, and Y.Q. Cheng, ISIJ Int. 60, 602 (2020).
G.Z. Zhang, N. Wang, M. Chen, and H. Li, Steel Res. Int. 89, 1800272 (2018).
Y.J. Liang, and Y.C. Che, Handbook of Thermodynamic Data of Inorganic Compounds (Northeastern University Press, Shenyang, 1993), pp 449–479 (in Chinese).
H.S. Park, Y.S. Han, J.H. Park, and A.C.S. Sustain, Chem. Eng. 7, 14119 (2019).
J.H. Heo, T.S. Kim, V. Sahajwalla, and J.H. Park, Metall. Mater. Trans. B 51B, 1201 (2020).
Z.M. Yan, X.W. Lv, D. Liang, J. Zhang, and C.G. Bai, Metall. Mater. Trans. B 48B, 1092 (2017).
R. Corbari, H. Matsuura, S. Halder, M. Walker, and R.J. Fruehan, Metall. Mater. Trans. B 40B, 940 (2009).
A. Malfliet, S. Lotfian, L. Scheunis, V. Petkov, L. Pandelaers, P.T. Jones, and B. Blanpain, J. Eur. Ceram. Soc. 34, 849 (2014).
S.M. Jung, D.J. Min, and C.H. Rhee, ISIJ Int. 47, 1718 (2007).
C.M. Yoon, Y. Park, and D.J. Min, Metall. Mater. Trans. B 49B, 2322 (2018).
J. Shi, Y. Qiu, X. Wan, B. Yu, M. Chen, F. Zhao, J. Li, C. Liu, and P. Taskinen, JOM. https://doi.org/10.1007/s11837-021-05049-3 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51804075 and 51974080) and the Fundamental Research Funds for the Central Universities (Grant nos. N2025030 and N2225028).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, L., Zhang, D., Liu, Y. et al. Iron Recovery from Waste Copper Slag by Using Coal and Secondary Aluminum Dross as Co-Reductants. JOM 74, 2029–2036 (2022). https://doi.org/10.1007/s11837-022-05218-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05218-y