Abstract
In this work, an innovative and carbon-free smelting reduction process is proposed to recycle valuable metals from waste copper slag using secondary aluminum dross as a reductant. Experimental and thermodynamic investigations were carried out to understand the reaction and separation process and its correlation with smelting temperature, modified basicity (CaO/SiO2 mass ratio), and dross/slag ratio. The interaction between MgO crucible and liquid slag was also revealed, which was found to have a critical role in metal recovery. The reduction rates of iron and copper are close to 100 pct, but their separation from slag during smelting is highly sensitive to temperature and basicity. Higher modified basicity (0.9 to 1.1) is essential to enhance the recovery of valuable metals because it can reduce both MgO dissolution and slag viscosity. At optimal conditions, 98.6 pct Fe and 98.3 pct Cu in the slag can be recovered as a high-purity alloy. Meanwhile, ~ 99 pct of hazardous elements in the slag have been removed by a reduction-evaporation mechanism. From the results, it is clear that aluminum dross is an efficient, green, and cost-effective reductant for copper slag, by which these two industrial wastes can be transformed into clean and value-added products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper slag is a by-product produced in the copper smelting and converting processes.[1] Every year, more than 40 million tons of copper slag is discharged into the environment worldwide.[2] Dumping in specific landfill sites is still the main way to dispose of the copper slag for most copper smelters.[3] The disposal of such a huge amount of copper slag not only occupies a large area of land but also causes environmental problems because it contains hazardous elements, such as heavy metals and arsenic. Therefore, the recycling of copper slag as useful products is gaining increasing interest in the last two decades.[4,5,6]
Typically, copper slag is a FeO–SiO2-based system and contains around 40 wt pct total iron and 0.5 to 3.0 wt pct total Cu.[7] Thus, the recovery of valuable metals from copper slag is regarded as an economically effective way to address sustainability and environmental issues, especially considering the shortage of natural resources. Current technologies applied to the recovery process mainly include physical,[8] hydrometallurgical,[9] and pyrometallurgical methods.[10,11,12,13,14] The direct reduction by carbonaceous materials based on pyrometallurgical principles at 1200 °C to 1300 °C followed by magnetic separation is the most commonly used one because of its high recovery efficiency.[15,16] For example, Li et al.[17] reported an innovative technology for coal-based reduction of copper slag by the co-addition of Na2CO3 and CaO. The recovery rates of iron and copper after magnetic separation reached 94.3 and 86.5 pct, respectively, with CaO/SiO2 of 0.5 and Na2CO3 content of 8 wt pct. To decrease the use of fossil-based reductants, walnut shell char with a high content of fixed carbon has been explored as a green reductant in the recycling of copper slag, as reported by Zhou et al.[18] A high recovery of iron (95.56 pct) was also obtained by this method.
Based on the new techniques of the ironmaking process, a large number of researchers have investigated the feasibility of iron recovery from copper slag by smelting reduction at 1450 °C to 1600 °C.[12,19,20,21] The effects of carbon type, C/FeO ratio, reaction time, and composition modification on the reduction behavior of FeO in molten copper slag have been systematically studied. The biggest strength of this method is the direct separation of the reduced metals during reduction, significantly shortening the recovery process. Despite this advantage, a major concern for smelting reduction is the high energy consumption and CO2 emissions due to the excessive addition of solid carbon. Aluminothermic reduction is a more efficient and environmentally friendly alternative for metal recovery from slag.[22] However, the high price of aluminum is a huge obstacle to the application of this process.
Aluminum dross is an industrial solid waste generated in large quantities in aluminum smelters.[23] In terms of aluminum content, it is divided into two major types. The type with high metal content is called primary aluminum dross (Al content ≥ 50 wt pct) that is produced during aluminum smelting and processing. Secondary aluminum dross is the residue generated after aluminum extraction of primary aluminum dross (Al content: 5 to 35 wt pct). It is difficult to further recover aluminum from this type of dross owing to its complex composition.[24] The disposal of secondary aluminum dross has become a focus problem because aluminum nitride in it would release ammonia as a toxic by-product.[25] Although many technologies have been developed to recycle this waste as clean and safe products,[24] the utilization amount of aluminum dross is far less than the annual generation.
Relatively high Al content in the secondary aluminum dross suggests a promising reductant for iron and copper oxides in copper slag. Besides, the usage of aluminum dross will significantly reduce the cost of the aluminothermic reduction process, by which these two wastes can be co-recycled. Moreover, AlN in the aluminum dross can also act as a reductant for copper slag.[26] However, the introduction of other oxides from the dross into the liquid slag would influence the slag property and the recovery efficiency of iron and copper. Besides, the reaction mechanism between these two wastes and the separation behavior of the reduced metals are unclear. To understand these issues, an attempt has been made to recycle valuable metals from waste copper slag by using secondary aluminum dross as a novel reductant in this work. The influences of smelting temperature, modified basicity, and dross/slag ratio on the recovery of iron and copper were investigated in depth. The purpose of this work is to find out an efficient, green, and sustainable method of recycling waste copper slag.
Experimental Section
Materials
The raw materials used in this study mainly included copper slag and secondary aluminum dross (Figures 1(a) and (b)), which were collected from Zijin Copper Co., Ltd. (Fujian, China) and Zhengzhou Aluminum Co., Ltd. (Henan, China), respectively. The chemical compositions of these two industrial wastes are listed in Table I. The X-ray diffraction (XRD) pattern indicates that the major mineral phases in copper slag are fayalite (Fe2SiO4) and magnetite (Fe3O4), as shown in Figure 2. To obtain more free FeO in the slag at elevated temperatures, the CaO reagent (purity ≥ 98.5 wt pct, particle sizes: ≤ 37.4 μm) was used to modify the copper slag.[3,17,21] In general, the whole FeO in the slag can be released from fayalite when the modified basicity (CaO/SiO2 mass ratio, R) is around 1.0. To understand the influence of the modified basicity on metal recovery and its underlying mechanism, four CaO/SiO2 mass ratios were designed in the reduction experiment, as presented in Table II. The design of the mass ratio of aluminum dross/copper slag (AD/CS) in this study was based on the material balance calculation. For example, the stoichiometric AD/CS (0.44) in the reduction experiment was calculated based on the complete reduction of iron oxides in the copper slag. Besides, higher AD/CS ratios (i.e., 0.48, 0.52, 0.56, and 0.60) were also designed under the basicity of 1.1 considering the consumption of Al by reducing Cu2S and SiO2 as well as the loss of the aluminum dross during the charging process.
Procedure
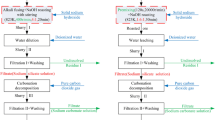
The smelting reduction experiments were conducted in an electric tube furnace at 1500 °C and 1600 °C, respectively, as displayed in Figure 3. First, 100 g copper slag was mixed with different quantities of CaO reagent according to Table II, and then the mixtures were pressed to cylindrical samples (45 mm in diameter and ~ 50 mm in height) under 150 MPa by a hydraulic press. Each pressed block was charged into a MgO crucible (purity ≥ 99.5 wt pct, 48 mm in inner diameter and 90 mm in depth), and then the crucible was placed in the hot zone of the furnace and heated up to the target temperature. A holding time of 30 minutes was employed to ensure the formation of homogeneous liquid slag. After that, a specified amount of aluminum dross was added to the molten slag quickly via a quartz tube. Before that, the aluminum dross was pressed into blocks and then crushed into small particles. After reaction for 60 minutes, the crucible was taken out quickly from the furnace and quenched into water. The whole process was performed under a purified Ar atmosphere (flow rate of 0.5 L/min) to avoid oxidation of the reduced metals. During smelting reduction, the reduced metals will be separated from the slag by a sedimentation process. After cooling, the bonding between the crucible and slag becomes weak, and the products can be taken out from the crucible by knocking the crucible wall down.
Characterization
The compositions of the secondary slags were determined by X-ray fluorescence (XRF). The weight of the iron ingot separated from the slag was measured by a high-accuracy electronic balance. The compositions of the alloys and the contents of hazardous elements in the reduced slags were analyzed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). The reduction rate of FeO was obtained by comparing its content in the slag before and after reduction. Meanwhile, the recovery rates of iron and copper were calculated from the measured results of the weight and chemical composition of the separated ingot, as described in Eq. [1].[21] Besides, a concept of “reduction degree” was proposed in this work to calculate the variation of slag composition and viscosity during the reduction process, which is a theoretical value and defined as the ratio of the amount of the reduced FeO compared to the total FeO in the initial slag. The mineral phases in the initial and reduced slags were identified by X-ray diffraction (XRD; X'pert PRO, PANalytical, Netherlands). The secondary slags were cut, ground, and polished for microstructure observation using field-emission scanning electron microscopy (FE-SEM; Model Ultra Plus, Carl Zeiss, Germany). Besides, the interaction between slag and crucible, and the changes of equilibrium phase assemblage as well as apparent viscosity of the slag during reduction were analyzed by thermochemical software FactSage (version 8.0, the modules of “Equilib” and “Viscosity” and the databases of FToxid, FactPS, and Melts were utilized).
where malloy is the weight of the separated alloy, g; ωFe/Cu refers to the mass fraction of iron or copper in the alloy, wt pct; and mTFe/TCu is the weight of total iron or total copper in the initial slag, 43.4 g Fe, and 0.275 g Cu per 100 g of slag.
Results
Characterization of the Secondary Slag
Figure 4 displays the photographs of the secondary slags and the iron ingots after reduction at different temperatures and basicity values. The reduced metals have been successfully separated from the slag, confirming the availability of using aluminum dross to recover iron from molten copper slag. As can be seen in Table III, the content of iron oxide is below 1.5 wt pct in all the secondary slags after magnetic separation, indicating extremely high reduction rates. XRD analysis of the slag tailings reveals similar results, in which no iron-containing phases but alumina-rich phases are detected, as shown in Figure 5.
Some reduced iron particles with sizes of 30 to 300 μm remained in the slag after reduction at 1500 °C, as shown in Figure 6(a). This is because the fine iron droplets could not fall to the bottom easily in a limited time if the slag is very viscous,[21] which will be discussed in-depth in Section IV. With the smelting temperature rising to 1600 °C, the iron particles were also evident in the slag at the basicity of 0.5 and 0.7, but disappeared with further increasing basicity, as shown in Figure 6(b). In Table III, it is also observed that MgO content in the slag is higher compared to the calculated values after reduction at 1600 °C, suggesting an interaction between slag and crucible. This reaction was particularly strong under lower basicity values, leading to the generation of considerable amounts of spinel (Figure 6(b)). However, the slag showed a more homogeneous structure and a lower volume fraction of solid phases at higher basicity values, implying a better flowability during reduction and thus improving the separation of metal droplets.
Recovery of Valuable Metals
The direct recovery of iron is an important indicator to evaluate the effectiveness of the smelting reduction process of waste slags. Considering this aspect, the influences of smelting temperature and modified basicity on the metal recovery were investigated, as illustrated in Figure 7. It is worth noting that the weight of the iron ingot was even higher than that of the total iron in the initial slag in some cases, indicating that at least two types of oxides in the slag have been reduced during smelting. The chemical analysis reveals that these ingots are Fe–Si alloys with a small amount of Cu, as presented in Table IV. These carbon-free and high-purity alloys can be used as the raw materials for the production of Cu-bearing steels, such as weathering steel, antibacterial stainless steel, and high-strength steel.
Although the reduction rate of FeO was close to 100 pct for all the cases, the Fe recovery was only 89.5 pct at the temperature of 1500 °C and the basicity of 0.5 because some metal particles were dispersed in the slag (Figure 6(a)). The settlement of the iron droplets was greatly enhanced with the increase of basicity. The Fe recovery increased to 94.3 pct when the basicity was elevated to 0.9, and then it remained almost constant with a further increase in basicity. A similar change trend was observed at 1600 °C, but a higher temperature contributed to a further significant improvement in the slag-metal separation, especially under higher basicity values. At this temperature, the recovery rates of iron and copper were calculated to be 98.6 pct and 98.3 pct, respectively, under the basicity of 0.9. These data strongly suggest that aluminum dross is a highly effective reductant for the recovery of valuable metals from waste copper slag.
Figure 8 shows the changes in the weight of separated metals and Fe recovery after reduction at 1600 °C as a function of the mass ratio of AD/CS. It can be seen that the recovery of iron is highly sensitive to AD/CS. The Fe recovery was 93.2 pct when the Al/FexO was designed in a stoichiometric ratio (AD/CS of 0.44), and then it reached a peak of 98.3 pct when AD/CS increased to 0.48. However, the recovery rate showed a sharp decline with the further increase of aluminum dross. This is because the reductant used in this work contains considerable amounts of alumina and magnesia. Their dissolution into the slag would produce solid precipitations during reduction, such as corundum and spinel.[27] This phenomenon was particularly strong at higher additions of aluminum dross, as demonstrated in Figure 9. Although all the iron oxides were transformed into the metallic form at AD/CS of 0.60, their separation from slag was difficult due to the massive formation of MgAl2O4 spinel around (black phases).
Discussion
Thermodynamic Analysis and Reaction Mechanism
The following reactions are expected to take place in the reduction process according to the thermodynamic calculation[28]:
(*) means that the component is in the liquid slag, and [*] means that the component is in the molten iron
According to Eqs. [2] and [3], the addition of CaO greatly increases the content of free FeO in the slag and, thus, improves the reduction of iron oxides in the presence of Al. The Gibbs free energy change of Eq. [3] is very negative even if the activities of both FeO and Al decrease to 0.001 at 1500 °C to 1600 °C (− 115 to − 70 kJ/mol), suggesting that iron oxides in the copper slag can be fully reduced in terms of thermodynamics. This hypothesis can be confirmed by the XRF and EDX analysis of the slag tailings, in which almost no iron oxide was detected (Table III; Figure 9).
As reported in the literature,[22,29,30,31] the interfacial reaction between FeO and Al particles in the liquid slag comprises the following steps: First, the reaction between the molten slag (FeO) and an Al particle is triggered by the high temperature. After that, an irregular Al2O3 layer is generated at the surface of the Al particles, and the reduced iron droplets spread out quickly and grow gradually. Meanwhile, the Al2O3 layer continuously dissolves into the liquid slag in the form of Al3+. FeO diffusion also occurs in the opposite direction because of the concentration gradient. As the reaction proceeds, FeO is continuously reduced by metal Al, and Al2O3 content is significantly increased in the slag, giving rise to the formation of Al2O3-rich areas or precipitations. The above series of reaction steps are extremely fast and is believed to complete within 5 min.[22]
The aggregated metal droplets then settle to the bottom of the crucible due to the density difference between liquid iron and slag. SiO2 starts to be reduced when the FeO activity in the slag decreases to a certain extent, at which the Gibbs free energy change of reaction [4] is more negative than reaction [3]. The reduction of SiO2 allows the formation of Fe–Si alloy, in which the Si content decreases with basicity because higher basicity means less free SiO2 in the slag due to the formation of CaSiO3. In general, an increase of 1 wt pct of silicon in the molten iron decreases its melting point by 6.2 °C. This change is beneficial to the coalescence and growth of the metal droplets, and thus, a good separation of the reduced metals can be achieved at only 1500 °C.
Influence of MgO Dissolution
MgO-based refractories are the dominant lining materials of both copper and ferrous metallurgy furnaces. Despite this fact, the dissolution of oxides from the MgO crucible into the slag is inevitable during smelting reduction because fayalite slag is highly corrosive.[32] In this case, the slag viscosity will increase sharply when MgO content in the slag is close to or even exceeds its solubility limit, thus, affecting the separation of the reduced metals. To understand this process, the saturation solubility of MgO under various basicity values at 1600 °C was calculated by FactSage, as demonstrated in Figure 10. It can be seen that MgO shows a relatively high solubility during the early stage of reduction (reduction degree of 0 to 40 pct). In this stage, the MgO solubility exceeds 20 wt pct at lower basicity values. Therefore, the MgO contents in the reduced slags were measured to be much higher than the theoretical values, as presented in Table III. However, the solubility decreased to around 15 wt pct with the basicity increasing to 0.9 and 1.1.
Previous studies on the MgO dissolution in typical metallurgical slags also exhibit the same trend.[33,34,35] Based on Eq. [5], the solubility of MgO is inversely proportional to the activity of (O2−) in the slag. Lower basicity, i.e., less CaO in the slag, means less free oxygen ions, thus, enhancing the dissolution of MgO. At higher reduction degrees, the increase of Al2O3 content in the slag significantly encourages the formation of spinel, according to Eqs. [6] and [7], and thus, a sharp decrease in the solubility is observed. This change implies a dissolution–precipitation phenomenon during the smelting reduction process. At lower basicity values, more dissolution of MgO at the early stage resulted in more precipitation of spinel in the later reduction, as confirmed by Figure 6(b). This behavior was detrimental to the settling of metal droplets, and thus, a lower recovery was obtained.
Influences of Modified Basicity and Reduction Temperature
In the smelting reduction of copper slag, the slag viscosity plays a critical role in recovery efficiency by controlling the settling velocity of the reduced metals. In general, basicity and temperature are the determining factors for slag viscosity.[36,37,38,39] Therefore, their correlations in this study are discussed in this section. First, the variation of the slag composition during smelting reduction has been revised considering the dissolution of MgO, based on which the changes in the mass fraction of each phase with reduction degree were calculated by FactSage, as shown in Figure 11. Then the evolution of the apparent viscosity of the slags during reduction was quantitatively estimated based on FactSage and the simplified Einstein–Roscoe equation (Eq. [8]), as shown in Figure 12. It is worth noting that the calculated results in Figure 11 are different from those detected by XRD and SEM, where the presence of aluminosilicate crystals (the long-shaped phases) is significant and more spinel is observed, as shown in Figures 5 and 6. This is because these phases show high melting points, and therefore, their precipitation is possible during the cooling process although a quenching process is applied.
where η is the apparent viscosity of the solid-containing slag, Pa s; η0 is the viscosity of the liquid phase in the mixture, Pa s; and ωsolid refers to the mass fraction of solids in the slag, wt pct.
In Figure 12, it is observed that the viscosity values of all the slags are kept below 0.15 Pa s at reduction degrees of 0 to 40 pct. However, the slag viscosity increases rapidly as the reaction further progresses. Our previous study has shown that Al2O3 with higher content in the slag acts as a network former and greatly increases its structural complexity by polymerizing [AlO4]- and [Si(Al)O4]-tetrahedra, thus, increasing the resistance of the liquid slag to flow.[40] This change suggests that the recovery efficiency is mainly determined by the settling velocity of the metal droplets in the final stage of reduction. Lower basicity not only caused higher Al2O3 content in the slag but also induced more precipitation of solids (Figures 6 and 11), thus, resulting in a more evident increase of viscosity. When the modified basicity is 0.5, the slag viscosity is higher than 2.3 Pa s at the end of reduction at both 1500 °C and 1600 °C, making it difficult for the generated droplets to separate from the slag by sedimentation. In this case, the terminal settling velocity of the droplets with diameters of 100 μm is only 0.75 to 0.96 × 10−5 m/s according to the Stokes equation (Eq. [9]), and the time required for their falling through the slag layer is more than 70 minutes, as shown in Figure 13. Consequently, certain amounts of metal particles with similar sizes remained in the cooling slag, as shown in Figure 6.
where vt refers to the terminal velocity of the metal droplet, m/s; g is the gravitational acceleration constant, 9.8 m/s2; d is the diameter of the droplet, m; ρFe and ρslag represent the density values of liquid iron (7040 kg/m3) and slag (3000 kg/m3), respectively; and ηslag is the viscosity of the slag, Pa s.
The slag viscosity decreases significantly with basicity. As reported in the literature, higher CaO content in the slag can break up the chain and sheet structures by releasing more free oxygen ions,[38,41] thus, improving the kinetics of slag–metal separation. Temperature is also a critical factor in slag viscosity. Although there are no solid phases generated at 1500 °C under basicity values of 0.9 and 1.1 (Figure 11), the slag viscosity is above 1.7 Pa s in the final stage, which requires around 50 minutes to separate the 100 μm-sized droplets from the slag. Fortunately, the viscosity values decrease to below 0.8 Pa s when increasing the smelting temperature to 1600 °C because it supplies sufficient energy for the aluminate structural units to migrate. This highly fluid slag creates favorable conditions for the growth and sedimentation of the reduced metals. In this condition, only 24 minutes is required for the separation of the same-sized droplets. Therefore, almost complete recovery of valuable metals has been achieved (Figures 6(b) and 7).
Influence of Dross/Slag Ratio
The reduction of SiO2 in copper slag is not ignorable when using aluminum dross as the reductant in the smelting reduction process (Table IV). Therefore, the usage of slightly over-stoichiometric amounts of aluminum dross is necessary to ensure the complete reduction of iron oxide. However, adverse effect appears with further increasing the amount of aluminum dross, as demonstrated in Figure 8. The excessive amount of aluminum dross means more intensive reactions between Al particles and molten slag, greatly increasing the transient temperature of the liquid slag and thus accelerating the dissolution of the MgO crucible. Besides, the increase of SiO2 content due to the excessive aluminum dross reduces the slag basicity and further improves the dissolution of MgO. In the later stage, the much higher content of alumina in the slag dramatically reduces the solubility of MgO and favors the formation of spinel, as shown in Table V and Figure 9. The extremely high solid/liquid ratio makes it difficult to separate the reduced metals from the slag. Therefore, a significant drop in the metal recovery was identified at higher additions of aluminum dross.
Elimination of the Hazardous Elements and Reuse of the Tailings
The waste copper slag contains various hazardous elements, such as Zn, Cu, Pb, and As. These elements have been largely removed in the smelting reduction process, especially at higher basicity values, as depicted in Table VI and Figure 14. The elimination of ZnO, PbO, and As2O3/As2S3 from the slag also took place by a reduction mechanism, as described in Eqs. [10] through [13], mainly owing to their much lower thermodynamic stability compared to Al2O3.[28] Then the Zn, Pb, and As species were evaporated from the melts due to their high volatility. Therefore, they were not detected in the reduced metals. The vapors can be easily collected by cooling the exhaust gases. After reduction at 1600 °C with basicity of 0.9, the volatilization rates of Zn, Pb, and As were as high as 99.8, 99.1, and 98.7 pct, respectively. Meanwhile, 98.3 pct of copper in the slag was reduced into the molten iron by forming Fe–Si–Cu crude alloy (Eqs. [14] and [15]). Besides, aluminum dross is also classified as hazardous waste because of the risk of generating NH3 from AlN. At high temperature and inert atmosphere conditions, AlN can be used as a reductant to recover iron from the copper slag, as shown in Eq. [16] and is eventually converted into harmless products. Undoubtedly, the elimination of these hazardous elements is favorable to the clean and safe utilization of the copper slag.
The reuse of the tailings is quite necessary, or otherwise, it still occupies a huge area of land. In this study, the composition of the final slag is located in the CaO–SiO2–Al2O3–MgO system, which is similar to that of the blast furnace slag (BFS) but richer in alumina. BFS is one of the industrial solid wastes that have been successfully recycled for the production of structural materials, such as cement, concrete aggregates, ultrafine powders, and ceramics.[42,43] The absence of FeO, the appropriate basicity, and the ultralow impurities are the keys to the successful application of BFC waste in the building materials field. In this work, the high recovery of Fe and the minimization of hazardous elements in copper slag suggest that the tailings have great potential in the application of high-performance building materials, then allowing the full recycling of copper slag.
Practical, Environmental, and Economic Implications
Carbothermic reduction has been widely used to extract metals from waste slag. However, this process is an endothermic reaction. Therefore, excessive solid carbon is usually required to maintain the reaction temperature, increasing both cost and emission. Aluminothermic reduction is a more efficient and eco-friendly technique because this process is highly exothermic and produces no CO2, but it also has some limitations. On the one hand, metal aluminum is rather expensive (~3500 dollars/ton). Hence, it is uneconomical to use it to recycle Fe from copper slag. On the other hand, it is hard to control the reduction process because the exothermic reaction is extremely violent. In this condition, the temperature will increase to 1900 °C or even higher,[22] creating a big challenge for the refractory linings.
Although Al content in the aluminum dross is only 33.2 wt pct, its price is 1/10 of the metal aluminum (320 dollars/ton). Besides, the oxides in the aluminum dross can act as cooling agents during reduction, helping to adjust the temperature to a more appropriate range. The exothermic effect shows no significant influence on the slag viscosity and metal recovery in the experimental study because the duration of high temperature is very short due to the temperature control in the furnace.[22] However, this effect will play a critical role in the variation of slag temperature in an industrial-scale process. To understand the quantitative influence of the addition of aluminum dross on the temperature variation of copper slag during reduction (initial temperature of 1200 °C), a heat balance calculation has been performed, as illustrated in Eqs. [17] through [19]. The relevant parameters are presented in Table VII (the reduction of copper and silicon is not considered in this calculation).
where ΔHreduction and ΔHslagging are the enthalpy changes of the aluminothermic reaction (Eq. [3]) and the slagging reaction (Eq. [2]), respectively, kJ/mol; ni is the molar quantity of component i in the modifier or aluminum dross, mol; ΔHi refers to the enthalpy change for the dissolution of component i into copper slag from room temperature, kJ/mol; Qloss is the heat loss during smelting reduction, kJ (estimated as 15 pct of the total physical heat of the slag); ΔT is the change of the slag temperature after reduction, °C; mFe and mslag are the weights of the reduced iron and the liquid slag, respectively, kg; CP,Fe and CP,slag represent the specific heat capacity of liquid iron and slag, respectively, kJ/(kg °C).
Based on the above calculation, the variation of slag temperature as a function of reduction degree was obtained, as shown in Figure 15. The slag temperature increases significantly with the progress of aluminothermic reduction, and an increase of 390 °C to 533 °C is identified for the slag temperature at the end of reduction under various basicity values. The final temperature is sufficient for the separation of the reduced metals from the liquid slag, suggesting that no additional energy supply is needed. This is a great advantage compared to the carbothermic reduction process. Besides, the increase in slag temperature is significantly lower compared with that using metal aluminum as the reductant (700 °C to 900 °C),[22] thus, avoiding extremely high temperatures of the melts. Based on the above discussion, it is clear that the proposed recycling process is technically feasible and economically effective and also minimizes the environmental risks caused by metallurgical wastes.
Despite these advantages, there are also some issues to be addressed before putting this process into practice. First, the slag/metal mass ratio in the final reduction is more than 2.4. The large volume of slag increases the settling distance of the reduced metals and, thus, lowers the separation efficiency. This effect is more significant in a large-scale operation. Therefore, the control of slag viscosity in the later reduction is extremely important to ensure the growth and sedimentation of the metal droplets. To further decrease the adverse effect of high slag/metal ratios, reactors with large cross-sectional areas may be used to reduce the slag thickness and increase the interfacial area between slag and metal. This design has already been applied to the copper and lead smelting furnaces, in which the slag/metal ratio is also very high. Second, the use of aluminum dross as reductants requires additional safety measures and costs in practice because it is classified as hazardous waste. For example, moisture protection should be strictly followed in the transportation and storage of aluminum dross to prevent the hydration of AlN. Third, the separated ingot contains no carbon but higher Si content compared to the conventional one. Therefore, significant amounts of carbon should be injected during steelmaking. Besides, a large amount of lime is also required due to the oxidation of Si. These issues are challenges to its scale application in the steelmaking process. The produced alloy may be more suitable to be used as auxiliary material in the steelmaking process, such as deoxidizing agent and raw material for silicon or copper-bearing steel. Moreover, an in-depth study on the composition control of the reduced metals is needed in our future work.
Conclusions
In this work, an efficient, green, and cost-effective method has been developed to recycle Fe and Cu from waste copper slag by using secondary aluminum dross as a reductant. The reduction and separation mechanisms during the recycling process were discussed, and the correlation between process variables and metals recovery was systematically investigated. The following are the main conclusions:
-
(1)
Iron oxides in the copper slag can be deeply reduced by aluminum dross, but some metal particles remained in the slag under lower basicity values due to limited settling velocity. The separation of the reduced metals was greatly enhanced by increasing temperature and basicity because of the significant reduction of slag viscosity. 98.6 pct iron and 98.3 pct copper in the slag have been recovered after reduction at 1600 °C with the basicity of 0.9.
-
(2)
Dissolution of MgO from the crucible into the slag was inevitable during reduction at 1600 °C, especially under lower basicity values (0.5 to 0.7). This interaction led to the precipitation of spinel in the later stage of reduction. The high solid/liquid ratio increased the slag viscosity and, thus, impeded the growth and sedimentation of the metal droplets. In contrast, this influence was negligible under the basicity of 0.9 to 1.1.
-
(3)
The addition of slightly over-stoichiometric amounts of aluminum dross is beneficial to the iron recovery because the reduction of SiO2 in copper slag is not ignorable, but the dross/slag ratio should be controlled to avoid the massive precipitation of spinel in the slag.
-
(4)
In the smelting reduction process, the removal of hazardous elements has also been achieved by a reduction-evaporation mechanism. The elimination rates of Zn, Pb, and As can be as high as 99.8, 99.1, and 98.7 pct, respectively. This change enabled the transformation of copper slag into clean resources and greatly reduced the environmental risks.
References
A. Potysz, E.D. van Hullebusch, J. Kierczak, M. Grybos, P.N.L. Lens, and G. Guibaud: Crit. Rev. Environ. Sci. Technol., 2015, vol. 45, pp. 2424–88.
D.Q. Wang, Q. Wang, and Z.X. Huang: Resour. Conserv. Recycl., 2020, vol. 162, p. 105037.
Z.Q. Guo, D.Q. Zhu, J. Pan, and F. Zhang: J. Clean. Prod., 2018, vol. 187, pp. 910–22.
İ Alp, H. Deveci, and H. Süngün: J. Hazard. Mater., 2008, vol. 159, pp. 390–95.
K. Murari, R. Siddique, and K.K. Jain: J. Mater. Cycles Waste Manag., 2015, vol. 17, pp. 13–26.
H.Y. Tian, Z.Q. Guo, J. Pan, D.Q. Zhu, C.C. Yang, Y.X. Xue, S.W. Li, and D.Z. Wang: Resour. Conserv. Recycl., 2021, vol. 168, p. 105366.
T.J. Chun, C. Ning, H.M. Long, J.X. Li, and J.L. Yang: JOM, 2016, vol. 68, pp. 2332–40.
S. Roy, A. Datta, and S. Rehani: Int. J. Miner. Process., 2015, vol. 143, pp. 43–49.
G.C. Shi, Y.L. Liao, B.W. Su, Y. Zhang, W. Wang, and J.J. Xi: Sep. Purif. Technol., 2020, vol. 241, p. 116699.
Z.L. Zuo, S.Y. Luo, S.H. Liu, J.K. Zhang, Q.B. Yu, and X.J. Bi: Chem. Eng. J., 2021, vol. 405, p. 126671.
B.S. Kim, S.K. Jo, D. Shin, J.C. Lee, and S.B. Jeong: Int. J. Miner. Process., 2013, vol. 124, pp. 124–27.
J. Zhang, Y.H. Qi, D.L. Yan, and H.C. Xu: J. Iron Steel Res. Int., 2015, vol. 22, pp. 396–401.
X.Y. Meng, Y. Li, H.Y. Wang, Y.D. Yang, and A. Mclean: J. Hazard. Mater., 2020, vol. 399, p. 122845.
P. Sarfo, A. Das, G. Wyss, and C. Young: Waste Manage., 2017, vol. 70, pp. 272–81.
L.Y. Yi, Z.C. Huang, T. Jiang, P. Zhao, R.H. Zhong, and Z.K. Liang: Minerals, 2017, vol. 7, p. 167.
D.Q. Zhu, J.W. Xu, Z.Q. Guo, J. Pan, S.W. Li, L.T. Pan, and C.C. Yang: J. Clean. Prod., 2020, vol. 250, p. 119462.
S.W. Li, J. Pan, D.Q. Zhu, Z.Q. Guo, J.W. Xu, and J.L. Chou: Powder Technol., 2019, vol. 347, pp. 159–69.
S.W. Zhou, Y.G. Wei, B. Li, and H. Wang: J. Clean. Prod., 2019, vol. 217, pp. 423–31.
D. Busolic, F. Parada, R. Parra, M. Sanchez, J. Palacios, and M. Hino: Min. Process. Extract. Metall. Rev., 2011, vol. 120, pp. 32–36.
P. Sarfo, G. Wyss, G.J. Ma, A. Das, and C. Young: Miner. Eng., 2017, vol. 107, pp. 8–19.
J.H. Heo, B.S. Kim, and J.H. Park: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 1352–63.
J.H. Heo, Y.S. Chung, and J.H. Park: J. Clean. Prod., 2016, vol. 137, pp. 777–87.
T.R. Mankhand: J. Sustain. Planet, 2012, vol. 3, pp. 86–94.
H.L. Shen, B. Liu, C. Ekberg, and S.G. Zhang: Sci. Total Environ., 2021, vol. 760, p. 143968.
A. Abdulkadir, A. Ajayi, and M.I. Hassan: Energy Proced., 2015, vol. 75, pp. 2099–2105.
R. Krüger, A. Roosen, and W. Schaper: J. Eur. Ceram. Soc., 1999, vol. 19, pp. 1067–70.
E. Haccuria, T. Crivits, P.C. Hayes, and E. Jak: J. Am. Ceram. Soc., 2016, vol. 99, pp. 691–704.
Y.J. Liang and Y.C. Che: Handbook of Thermodynamic Data of Inorganic Compounds, Northeastern University Press, Shenyang. 1993, pp. 449–479 (in Chinese).
J.H. Heo and J.H. Park: Calphad, 2017, vol. 58, pp. 229–38.
J.H. Heo and J.H. Park: Calphad, 2017, vol. 58, pp. 219–28.
G.Z. Zhang, N. Wang, M. Chen, and Y.Q. Cheng: ISIJ Int., 2020, vol. 60, pp. 602–09.
A. Malfliet, S. Lotfian, L. Scheunis, V. Petkov, L. Pandelaers, P.T. Jones, and B. Blanpain: J. Eur. Ceram. Soc., 2014, vol. 34, pp. 849–76.
S.M. Jung, D.J. Min, and C.H. Rhee: ISIJ Int., 2007, vol. 47, pp. 1718–22.
C.M. Yoon, Y. Park, and D.J. Min: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 2322–31.
C. Gu, M. Wang, X.F. Cai, and P. Gan: Chin. J. Eng., 2018, vol. 40, pp. 73–76. (In Chinese).
Z.L. Zuo, Q.B. Yu, J.X. Liu, Q. Qin, H.Q. Xie, F. Yang, and W.J. Duan: ISIJ Int., 2017, vol. 57, pp. 220–27.
H.P. Zhang, B. Li, Y.G. Wei, H. Wang, Y.D. Yang, and A. Mclean: Metall. Mater. Trans. B, 2020, vol. 51B, pp. 2663–72.
H.S. Park, Y.S. Han, and J.H. Park: ACS Sustain. Chem. Eng., 2019, vol. 7, pp. 14119–25.
G.R. Qu, Y.G. Wei, B. Li, H. Wang, Y.D. Yang, and A. McLean: J. Alloys Compd., 2020, vol. 824, p. 153910.
G.Z. Zhang, N. Wang, M. Chen, and H. Li: Steel Res. Int., 2018, vol. 89, p. 1800272.
J.H. Heo, T.S. Kim, V. Sahajwalla, and J.H. Park: Metall. Mater. Trans. B, 2020, vol. 51B, pp. 1201–10.
L.V. Fisher and A.R. Barron: Resour. Conserv. Recycl., 2019, vol. 146, pp. 244–55.
K. Onoue, M. Tokitsu, M. Ohtsu, and T.A. Bier: Constr. Build. Mater., 2014, vol. 70, pp. 231–42.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [Grant Numbers 51804075, 51974080, 52074077 and 52074081] and the Fundamental Research Funds for the Central Universities [Grant Number N2225020].
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted December 14, 2021; accepted May 13, 2022.
Rights and permissions
About this article
Cite this article
Xu, L., Liu, Y., Chen, M. et al. Efficient Recycling of Valuable Metals from Waste Copper Slag by Using Secondary Aluminum Dross as a Novel Reductant. Metall Mater Trans B 53, 2824–2837 (2022). https://doi.org/10.1007/s11663-022-02567-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02567-6