Abstract
The whitefly, Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae), is considered one of the most invasive and destructive pests for agriculture worldwide. Whitefly populations are maintained throughout the year by continuous exploitation of different plant species and, in this context, weeds can serve as alternative hosts, making permanent populations possible in the field with the migration of individuals to cultivated plant species. Invasive plant species can also serve as inoculum sources of whitefly-transmitted viruses, being more favorable to disease dissemination in agricultural fields. Thus, studies investigating B. tabaci performance on different hosts are highly relevant for a better understanding of the insect’s population dynamics. Further study may assist in directing management actions and eradication of the most suitable plants for the whitefly development. With these goals in mind, the present study assessed biological aspects of B. tabaci MEAM1 on 14 weed species commonly found in Brazilian agricultural fields, in addition to five cultivated plant species. It was verified that the species Ipomoea grandifolia, Solanum lycopersicum and Emilia sonchifolia required the shortest development periods (egg-adult) (23.90 to 24.67 days), indicating high susceptibility. High nymphal viability rates (98.33 to 80.83%) were observed in S. lycopersicum, Gossypium hirsutum, Raphanus raphanistrum, Glycine max, Amaranthus viridis, Euphorbia heterophylla, Commelina benghalensis, Galinsoga parviflora, Sida rhombifolia, E. sonchifolia, Merremia aegyptia and I. grandifolia, also indicating susceptibility. These plant species were revealed to be suitable hosts for whitefly development and, with the exception of the cultivated species, should be monitored and eradicated, expanding the management strategies for B. tabaci MEAM1 populations in agricultural scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the more than 1,500 known whitefly species (Martin and Mound 2007), Bemisia tabaci (Hemiptera: Aleyrodidae) stands out among the most destructive to crops worldwide (Nauen et al. 2014). This pest causes direct damage to plants through feeding and indirect damage, especially from the transmission of several viruses, being capable to generate losses of up to 100% of production (Navas-Castillo et al. 2011; Polston et al. 2014; Lourenção et al. 2015).
After decades of study on this hemipteran, it was found that B. tabaci corresponds to a complex of cryptic species with wide genetic diversity and that, although morphologically identical, they differ in some biological aspects, such as the ability to transmit viruses, expression of insecticide resistance, ability to induce physiological disorders, and host range (Dinsdale et al. 2010; De Barro et al. 2011; Tay et al. 2012). Bemisia tabaci became globally distributed during the 1980s, after multiple invasions of the cryptic Middle East Minor-Asia Minor 1 (MEAM1) (formerly referred to as biotype B), likely via ornamental plant trade between countries (Cheek 1994; De Barro et al. 2011). As a result, this whitefly started to cause severe damage to several crops of economic importance since it promoted a significant increase in the incidence of begomoviruses on a global scale (Brown 1994; Jones 2003; Gilbertson et al. 2015).
The cryptic species MEAM1 stands out for its high degree of polyphagia, being able to infest a wide range of plant species including agricultural crops, vegetables, ornamental plants, as well as weed species (Abd-Rabou and Simmons 2010). Given the extensive range of hosts, and the notorious ability to adapt to different environments, B. tabaci MEAM1 is considered one of the most invasive and predominant cryptic species worldwide, with a long history of displacement of native cryptic species (Chen et al. 2002; Wang et al. 2017). As it is a multivoltine insect, which does not go through diapause or inactive periods during its life cycle, B. tabaci populations are maintained throughout the year by continuous exploitation of a wide variety of hosts, with the insect’s dispersal being an important factor for the colonization in different environments (Naranjo et al. 2010). Thus, aspects of the interaction between B. tabaci and different hosts are of great relevance for understanding the population dynamics of this insect and its management in agricultural crops (Zalom et al. 1995). Although traditionally the cultivated plants have received greater focus from studies regarding the biological performance of B. tabaci, it is known that some weeds play an important role as alternative hosts of the insect, offering an opportunity to maintain populations during the year and enabling the migration of this pest to cultivated plants (Chu et al. 1995; Gachoka et al. 2005). In addition, several weed species can act as inoculum sources for a variety of viruses transmitted by whiteflies who favor the spread of diseases in crop areas (Silva et al. 2010; Barreto et al. 2013; Fariña et al. 2019).
Given the significant importance of B. tabaci MEAM1 as a key pest in several crops, and the high potential of some weed species to act as alternative hosts of this insect, this study aimed to evaluate the biological aspects of this whitefly in 14 species of weeds present in crops in Brazil, and five species of cultivated plants. A greater knowledge of the differences in insect performance in invasive plant species commonly associated with agricultural cropping systems may guide management actions and eradication of the most suitable plants for the development of the insect.
Materials and methods
Selected weed species
To carry out the tests, 14 species of common weeds in crops of economic importance in Brazil were selected (Table S1). Five cultivated species were also evaluated, including tomato (Solanum lycopersicum L. – cv. Candieiro), soybean (Glycine max L. – cv. TMG 7062 IPRO), cotton (Gossypium hirsutum L. – cv. FMT 707), corn (Zea mays L. – cv. 30F53 VYHR), and bell pepper (Capsicum annuum L. – cv. Cascadura Ikeda).
Stock colony of Bemisia tabaci MEAM1
The insects used in the tests were provided from a previously established colony identified according to De Barro et al. (2003). The colony was kept in a greenhouse (2.5 × 2.5 × 2.0 m), with the sides and roof partially closed with glass and covered with an anti-aphid screen. Soybean and kale (Brassica oleracea var. acephala L.) plants were offered to maintain the insects and were kept in 2.5 L plastic pots. The deteriorated plants were replaced by healthy ones as needed.
Biological performance assessment
Pots with plants of the 19 plant species, with four to six expanded leaves, were protected by metallic cages covered with voile fabric to monitor the insect’s biological parameters. Four pots, each containing one plant of each species, were infested with 50 whitefly couples for 24 h to obtain oviposition. After checking the presence of eggs on the leaves under a stereoscopic microscope, areas with 30 eggs of B. tabaci MEAM1 were delimited (hydrographic pen) on one leaf of each plant. Each leaf corresponded to one repetition, in a total of four per treatment (n = 120), following a completely randomized design. Performance evaluations were performed daily, verifying the following biological parameters: duration of the incubation period, duration of nymphal instars (n1 to n4), nymphal viability, and duration of the period from egg to adult. The instar determination was made according to described by Naranjo and Ellsworth (2017). The test was carried out under greenhouse conditions (26.2 °C, with a maximum of 33.0 °C and a minimum of 19.3 °C; mean relative humidity of 54.14%, natural photophase).
Statistical analysis
Generalized linear mixed models employed in the statistical package PROC MIXED-SAS 9.2 (SAS Institute 2001) were used for analyzing the obtained data. The least squared means (LS-MEANS) statement of the GLIMMIX procedure in SAS, adjusted for Tukey, was used to compare treatment means at the 5% level of significance according to Fisher’s least significant difference (Fisher’s LSD).
Results
Biological performance assessment
Significant differences were found between the evaluated species in relation to all insect biological parameters (Table 1). The incubation period of B. tabaci MEAM1 on the different plants ranged from 7.40 to 9.16 days, with emphasis on bell pepper, C. benghalensis, I. grandifolia, soybean, E. sonchifolia and C. canadensis, which allowed the shorter incubation periods (7.40 to 7.80 days). The longest periods were observed in the species S. rhombifolia, G. parviflora and R. raphanistrum, with averages between 9.16 and 8.96 days.
The shortest duration periods (> 2.00 days) for the first instar of B. tabaci MEAM1 were observed in R. raphanistrum (1.60 days), G. parviflora (1.84) and tomato (1.98) (Table 1). These species differed from corn, bell pepper, C. benghalensis and C. canadensis, which provided the longest periods in this phase (3.39 to 5.02 days).
In the second nymphal instar, cotton stood out with the shortest time (1.67 days), followed by S. obtusifolia and M. aegyptia, which showed averages of 1.74 and 1.85 days, respectively. The longest periods observed were in bell pepper (5.13 days), corn (5.02 days) and C. canadensis (4.30 days), which also had the lowest averages in the first instar. The shortest periods of third instar duration were observed in C. benghalensis, S. latifolia, M. aegyptia and cotton, with averages between 2.12 and 2.72 days. Corn presented the highest average (5.02 days) of duration for the third instar of B. tabaci MEAM1, followed by G. parviflora (4.87) and S. obtusifolia (4.32).
For the fourth nymphal instar of B. tabaci MEAM1, a shorter duration was observed in I. grandifolia, with an average of 4.73 days. The highest time averages in this phase were observed in S. latifolia, M. aegyptia, (A) viridis, corn and R. raphanistrum (15.89 to 9.56 days). Regarding the total duration of the nymphal period of (B) tabaci MEAM1, I. grandifolia stood out again with the lowest average (16.21 days) among the evaluated species, followed by tomato, E. sonchifolia and (C) benghalensis (16, 29; 16.93 and 17.50 days, respectively). On the other hand, the longest nymphal periods for the whitefly were observed in S. latifolia, C. canadensis and bell pepper, with averages between 25.78 and 22.60 days.
The development period (egg-adult) of the insect ranged from 23.90 to 33.91 days among the evaluated species (Table 1). The lowest duration averages were observed in I. grandifolia (23.90 days), tomato (24.24 days) and E. sonchifolia (24.67 days), which differed from S. latifolia, C. canadensis, bell pepper, corn, A. viridis and S. obtsusifolia, species in which longer development periods were verified (33.91 to 29.03 days).
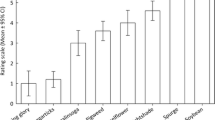
As for B. tabaci MEAM1 nymph viability, the highest percentage of emergence was found in nymphs confined to tomato (98.33%), followed by cotton, R. raphanistrum, soybean, (A) viridis, E. heterophylla, C. benghalensis, G. parviflora, S. rhombifolia, E. sonchifolia, M. aegyptia and I. grandifolia, which presented indices ranging from 95.83 to 80.83% (Fig. 1). The species that provided the lowest levels of nymphal viability were corn (6.67%), (B) pilosa (33.33%), S. obtusifolia (39.17%), and bell pepper (51.67%). Different effects of plant species were observed during the development of insect nymphs, with varying levels of impact on adult emergence (Fig. 2).
Bemisia tabaci MEAM1 nymphs observed under a stereoscopic microscope (40x) in a biological performance assessment with different plant species. A and B) fourth instar nymphs in normal development on Solanum lycopersicum and adult emerging on Ipomoea grandifolia, respectively; C) deformed fourth instar nymphs during development in Conyza canadensis; D) adult killed during adult emergence in Bidens pilosa; E) dry nymph during development in Richardia brasiliensis; F) nymph resected during development in Z. mays
Discussion
In the present study, variable performances of B. tabaci MEAM1 on different plant hosts were observed. All the species that were evaluated allowed the insect to complete the life cycle. However, some of them proved to be unsuitable hosts, requiring longer cycles (S. latifolia, C. canadensis and bell pepper) or reducing nymph viability (corn and B. pilosa) compared to the others.
Incubation periods ranging from 7.40 to 9.16 days were verified, while the nymphal period ranged from 16.21 to 25.78 days. In a study that evaluated the performance of B. tabaci MEAM1 on seven weed species, Sottoriva et al. (2014) found incubation periods between 8.20 and 9.10 days. In work carried out on the same whitefly species and kale genotypes, this period ranged from 6.08 to 7.03 days (Baldin et al. 2022). Bemisia tabaci eggs are laid on a pedicel, which is responsible for transporting water and solutes from the host plant to the eggs (Buckner et al. 2002; Walker et al. 2010). Thus, variations in the duration of the egg phase may be directly related to the temperature of the environment (Bonato et al. 2007) or even to specific characteristics of the epidermis’ surface in different plant tissues (Shah and Liu 2013).
Some of the weed species proved to be highly favorable to the development of B. tabaci MEAM1, providing short cycle durations (egg-adult) and high percentages of nymphal viability. For example, morning glory (I. grandifolia) stood out with the shortest egg-adult period of the whitefly (23.90 days), indicating high susceptibility, with an average similar to tomato, the insect’s preferred host. In this sense, Jiao et al. (2012) found that tomato was nutritionally superior to other hosts, justifying a shorter development period in the whitefly cycle. Besides tomato, cotton and soybean showed to be suitable hosts to B. tabaci MEAM1 among the cultivated species tested as expected, providing high nymphal viabilities and short development periods. The severe outbreaks of whiteflies on cotton fields observed in desert regions of North America, in 1990s, were attributed to the invasion of MEAM1 followed by the displacement of the native cryptic species, highlighting the great performance of the insect in this host (Ellsworth and Martinez-Carrillo 2001; Oliveira et al. 2001; Perring et al. 2001). Soybean is also known to harbor large whitefly populations in the field, with increasing outbreaks of this pest affecting Brazilian soybean production (Tamai et al. 2006; Arnemann 2018). Although the host-plant used for the rearing of whiteflies may potentially display influence in the performance on the subsequent host (Costa et al. 1991), the insects used in the present study were reared mostly in kale, besides soybean. Yet, other cultivated plant species (bell pepper, corn, cotton and tomato) were tested with no potential benefit of the condition of host adaption in advance, allowing robust comparisons between insect performance on weeds and cultivated plants.
The development period averages obtained in this study ranged from 23.90 to 33.91 days and were most similar to those whitefly studies by other authors who studied the species’ interaction with soybean, cowpea, tomato, zucchini, cabbage, poinsettia and cassava (17–27 days) plants (Villas-Bôas et al. 2002; Lima and Lara 2004; Cruz et al. 2014). Variable fitness patterns of phytophagous insects might be influenced by factors such as host nutritional value and presence of defense compounds in the plant tissues (Bernays and Chapman 1994). In a study comparing the performance of B. tabaci MEAM1 and Mediterranean (MED) in three different hosts, it was verified lower survivorship and longer nymph development periods for both whiteflies fed in Euphorbia pulcherrima Wild., which was considered the most inferior host, presenting lower nitrogen and higher carbohydrate and phenolic compounds in comparison with tomato and cotton (Jiao et al. 2012).
The ingestion of deleterious compounds produced by non-host plants or resistant genotypes (antixenosis or antibiosis) can cause behavioral and/or physiological changes to the arthropod that tries to colonize it (Smith 2005; Baldin et al. 2019), resulting in different levels of mortality, as already documented for this whitefly species in other hosts (Baldin et al. 2005, 2022; Baldin and Beneduzzi 2010; Silva et al. 2012; Cruz et al. 2014; Cruz and Baldin 2017; Pantoja et al. 2018; Novaes et al. 2020; Santos et al. 2021). However, B. tabaci MEAM1 exhibits a remarkable host adaptability, which has been corroborated by recent findings regarding the ability of this insect to circumvent plant defenses, such as phenolic glucosides, by acquiring host-plant genes via horizontal transfer, enabling it to neutralize plant defense compounds (Xia et al. 2021). This expressive adaptation capacity was also observed in studies assessing B. tabaci Mediterranean (MED) in tobacco, a non-preferred host for this cryptic species. Therefore, Xia et al. (2017) found that whiteflies improved their performance in tobacco after 10 generations being reared in this host, with up-regulation of genes providing larger body volume and muscle, which allowed the insect to overcome plant morphological defenses. In a study that evaluated a wide variety of plant species as possible hosts of B. tabaci MEAM1, Simmons et al. (2008) identified the largest group of new hosts as belonging to the genus Ipomoea, with 32 species. Plants belonging to this genus have been identified as potential hosts for the maintenance of native cryptic species of B. tabaci in Brazil and Argentina (Alemandri et al. 2012; Barbosa et al. 2014). In addition, several species of geminiviruses associated with plants of the genus Ipomoea have been globally disseminated (Varma et al. 2011). Among the species belonging to this genus, I. grandifolia stands out for its greater interference on cultivated plants, due to the aggressiveness and long growing cycle (Barroso et al. 2019). In the present study, I. grandifolia induced the shortest development period, and provided 80% nymphal viability for the whitefly, which reinforces the importance of monitoring the insect in agricultural scenarios.
The species E. sonchifolia and C. benghalensis were also among the plants that provided the shortest development periods for the whitefly, with lower averages than those of soybean and cotton, allowing nymphal viability to reach higher than 83.00%. Sottoriva et al. (2014) also found high viability of the immature phase of B. tabaci MEAM1 on E. sonchifolia (89.00%); however, the duration of the nymphal period observed in this species was 19 days, differing from the 16.93 days verified in the present study. These same authors found the shortest nymphal (18.30) and egg-to-adult (26.70) periods in E. heterophylla among the evaluated species, obtaining duration averages similar to those obtained in the present study (18.07 and 26.37, respectively). In a study conducted in the Brazilian semiarid region, Bezerra et al. (2004) found that the species E. heterophylla was the most infested by B. tabaci MEAM1 among the evaluated weeds, proving to be highly favorable to the maintenance of this pest in the field.
Corn, bell pepper, C. canadensis and S. latifolia caused the longest cycle lengths (29.73 to 33.91 days) and were among the least suitable hosts for B. tabaci MEAM1 evaluated in the present study. Results from corn, especially, showed the lowest nymphal viability (6.67%). Despite the fact that some studies have indicated corn as a potential host for B. tabaci MEAM1 (Quintela et al. 2016), grass species are generally unsuitable hosts for B. tabaci, barely allowing the insect to complete its cycle (Simmons et al. 2008). The unsuitability of grass species for B. tabaci was also observed in this work.
Brazil has no intense climatic amplitudes between regions and weed populations vary in different locations due to different edaphic factors. Plants and insects have adapted around Brazilian agricultural systems and their landscapes. This could justify differences in the occurrence of insect-host plant species between the northern, midwestern and southern regions of the country, as well as the transmission of associated pathogens. Additionally, in regions where significant temperature drops are common over the winter, the presence of alternative hosts such as ornamental plants and weeds is of great importance for the survival of B. tabaci MEAM1 populations throughout the year. In China, for example, a strong influence of alternative hosts was observed in protected crops for B. tabaci to survive over the winter, since the maintenance of the insect and plants under field conditions is unlikely. In many cases, the whitefly migrated to field crops during the summer, returning to be more problematic in greenhouses in the winter (Lin et al. 2007).
In general, the results obtained in this study reveal that, although there are variations in the performance of B. tabaci MEAM1 depending on the hosts evaluated, all the plant species that were studied allowed the insect to reach the adult stage. This indicated that these plants have variable potential as alternative hosts for the whitefly, especially in situations where there are no preferred plants. Species such as I. grandifolia, E. sonchifolia and C. benghalensis were highly susceptible and favorable to the insect, providing short development periods and high nymphal viability. In the case of E. heterophylla, it should be noted that this invasive species has already been identified as a reservoir of Tomato severe rugose virus (ToSRV) in the state of Goiás, Brazil (Barreto et al. 2013). This increases its importance, especially in areas destined for tomato cultivation, since the begomovirus is predominant in tomato-growing regions of south-central Brazil (Federal District, Minas Gerais, São Paulo and Goiás) (Inoue-Nagata et al. 2016). Eradication programs and periods without the presence of hosts can play an important role in the integrated management of whitefly and insect-transmitted viruses, reducing inoculum sources within and adjacent to the crop (Gilbertson et al. 2011). In this sense, the monitoring and control of these species can contribute to management strategies aimed at controlling populations of B. tabaci MEAM1 and its associated diseases under field conditions.
References
Abd-Rabou S, Simmons AM (2010) Survey of reproductive host plants of Bemisia tabaci (Hemiptera: Aleyrodidae) in Egypt, including new host records. Entomol News 121:456–465. https://doi.org/10.3157/021.121.0507
Alemandri V, De Barro P, Bejerman N, Argüello-Caro EB, Dumón AD, Mattio MF, Rodrigues SM, Truol G (2012) Species within the Bemisia tabaci (Hemiptera: Aleyrodidae) complex in soybean and bean crops in Argentina. J Econ Entomol 105:48–53. https://doi.org/10.1603/EC11161
Arnemann JA (2018) Ocorrência de mosca-branca em soja e hortícolas no Sul do Brasil aumenta na safra 2017/18. Mais Soja. https://maissoja.com.br/ocorrencia-de-mosca-branca-em-soja-e-horticolas-no-sul-do-brasil-aumenta-na-safra-2017-18/. Accessed 28 February 2023
Baldin EL, Beneduzzi RA (2010) Characterization of antibiosis and antixenosis to the whitefly silverleaf Bemisia tabaci B biotype (Hemiptera: Aleyrodidae) in several squash varieties. J Pest Sci 83:223–229. https://doi.org/10.1007/s10340-010-0289-2
Baldin EL, Vendramim JD, Lourenção AL (2005) Resistência de genótipos de tomateiro à mosca-branca Bemisia tabaci (Gennadius) biótipo B (Hemiptera: Aleyrodidae). Neotrop Entomol 34:435–441. https://doi.org/10.1590/S1519-566X2005000300012
Baldin EL, Vendramim JD, Lourenção AL (2019) Resistência de plantas a insetos: fundamentos e aplicações. Fealq, Piracicaba
Baldin EL, Domingos GM, Bentivenha JP, Canassa VF, Lourenção AL (2022) Antibiosis and antixenosis resistance of collard genotypes to Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae). Int J Trop Insect Sci 42:1783–1793. https://doi.org/10.1007/s42690-021-00705-2
Barbosa LD, Marubayashi JM, De Marchi BR, Yuki VA, Pavan MA, Moriones E, Navas-Castillo J, Krause‐Sakate R (2014) Indigenous american species of the Bemisia tabaci complex are still widespread in the Americas. Pest Manag Sci 70:1440–1445. https://doi.org/10.1002/ps.3731
Barreto SS, Hallwass M, Aquino OM, Inoue-Nagata AK (2013) A study of weeds as potential inoculum sources for a tomato-infecting begomovirus in central Brazil. Phytopathology 103:436–444. https://doi.org/10.1094/PHYTO-07-12-0174-R
Barroso AAM, Ferreira PSH, Martins D (2019) Growth and development of Ipomoea weeds. Planta Daninha 37:e019186421. https://doi.org/10.1590/S0100-83582019370100034
Bernays EA, Chapman RF (1994) Chemicals in plants. In: Bernays EA, Chapman RF (eds) Host-plant selection by phytophagous insects. Chapman & Hall, New York, pp 14–60
Bezerra MA, De Oliveira MR, Vasconcelos SD (2004) Does the presence of weeds affect Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) infestation on tomato plants in a semi-arid agro-ecosystem? Neotrop Entomol 33:769–775. https://doi.org/10.1590/S1519-566X2004000600015
Bonato O, Lurette A, Vidal C, Fargues J (2007) Modelling temperature-dependent bionomics of Bemisia tabaci (Q‐biotype). Physiol Entomol 32:50–55. https://doi.org/10.1111/j.1365-3032.2006.00540.x
Brown JK (1994) Current status of Bemisia tabaci as a plant pest and virus vector in agroecosystems worldwide. FAO Plant Prot Bull 42:3–32
Buckner JS, Freeman TP, Ruud RL, Chu CC, Henneberry TJ (2002) Characterization and functions of the whitefly egg pedicel. Arch Insect Biochem Physiol 49:22–33. https://doi.org/10.1002/arch.10006
Cheek S, Macdonald O (1994) Statutory controls to prevent the establishment of Bemisia tabaci in the United Kingdom. Pestic Sci 42:135–142
Chen L, Yuan YA, Rongjiang W, Fengming Y, Dunxiao H, Zhili Z (2002) The use of mitochondrial cytochrome oxidase I (mtCO I) gene sequences for the identification of biotypes of Bemisia tabaci (Gennadius) in China. Acta Entomol Sin 45:759–763
Chu CC, Henneberry TJ, Cohen AC (1995) Bemisia argentifolii (Homoptera: Aleyrodidae): host preference and factors affecting oviposition and feeding site preference. Environ Entomol 24:354–360. https://doi.org/10.1093/ee/24.2.354
Costa HS, Brown JK, Byrne DN (1991) Host plant selection by the whitefly, Bemisia tabaci (Gennadius),(Hom., Aleyrodidae) under greenhouse conditions. J Appl Entomol 112:146–152. https://doi.org/10.1111/j.1439-0418.1991.tb01040.x
Cruz PL, Baldin EL (2017) Performance of Bemisia tabaci biotype B on soybean genotypes. Neotrop Entomol 46:210–215. https://doi.org/10.1007/s13744-016-0445-3
Cruz PL, Baldin EL, Castro MJP (2014) Characterization of antibiosis to the silverleaf whitefly Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) in cowpea entries. J Pest Sci 87:639–645. https://doi.org/10.1007/s13744-016-0445-3
De Barro PJ, Scott KD, Graham GC, Lange CL, Schutze MK (2003) Isolation and characterization of microsatellite loci in Bemisia tabaci. Mol Ecol 3:40–43. https://doi.org/10.1046/j.1471-8286.2003.00344.x
De Barro PJ, Liu SS, Boykin LM, Dinsdale AB (2011) Bemisia tabaci: a statement of species status. Annu Rev Entomol 56:1–9. https://doi.org/10.1146/annurev-ento-112408-085504
Dinsdale A, Cook L, Riginos C, Buckley YM, De Barro P (2010) Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann Entomol Soc Am 103:196–208. https://doi.org/10.1603/AN09061
Ellsworth PC, Martinez-Carrillo JL (2001) IPM for Bemisia tabaci: a case study from North America. Crop Prot 20:853–869. https://doi.org/10.1016/S0261-2194(01)00116-8
Fariña AE, Rezende JA, Wintermantel WM (2019) Expanding knowledge of the host range of tomato chlorosis virus and host plant preference of Bemisia tabaci MEAM1. Plant Dis 103:1132–1137. https://doi.org/10.1094/PDIS-11-18-1941-RE
Gachoka KK, Obeng-Ofori D, Danquah EY (2005) Host suitability of two ghanaian biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) on five common tropical weeds. Int J Trop Insect Sci 25:236–244. https://doi.org/10.1079/IJT200583
Gilbertson RL, Rojas M, Natwick E (2011) Development of integrated pest management (IPM) strategies for whitefly (Bemisia tabaci)-transmissible geminiviruses. In: Thompson WMO (ed) The whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) interaction with geminivirus-infected host plants. Springer, Dordrecht, pp 323–356
Gilbertson RL, Batuman O, Webster CG, Adkins S (2015) Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu Rev Virol 9:67–93. https://doi.org/10.1146/annurev-virology-031413-085410
Inoue-Nagata AK, Lima MF, Gilbertson RL (2016) A review of geminivirus diseases in vegetables and other crops in Brazil: current status and approaches for management. Hortic Bras 34:8–18. https://doi.org/10.1590/S0102-053620160000100002
Jiao X, Xie W, Wang S, Wu Q, Zhou L, Pan H, Liu B, Zhang Y (2012) Host preference and nymph performance of B and Q putative species of Bemisia tabaci on three host plants. J Pest Sci 85:423–430. https://doi.org/10.1007/s10340-012-0441-2
Jones DR (2003) Plant viruses transmitted by whiteflies. Eur J Plant Pathol 109:195–219
Kissmann KG, Groth D (1999) Plantas infestantes e nocivas. BASF, São Paulo
Lima A, Lara FM (2004) Resistência de genótipos de soja à mosca branca Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae). Neotrop Entomol 33:71–75
Lin K, Wu K, Zhang Y, Guo Y (2007) Overwintering and population dynamics of Bemisia tabaci biotype B in greenhouse during the spring in northern China. J Crop Prot 26:1831–1838. https://doi.org/10.1016/j.cropro.2007.04.002
Lorenzi H (2008) Plantas daninhas do Brasil: terrestres, aquáticas, parasitas e tóxicas. Plantarum, Nova Odessa
Lorenzi H (2014) Manual de identificação de plantas daninhas: plantio direto e convencional. Plantarum, Nova Odessa
Lourenção AL, Krause-Sakate R, Valle GE (2015) Mosca-branca, Bemisia tabaci (Genn.) biótipo B. In: Vilela EF, Zucchi RA (eds) Pragas introduzidas no Brasil, insetos e ácaros. FEALQ, Piracicaba, pp 682–707
Martin JH, Mound LA (2007) An annotated check list of the world’s whiteflies (Insecta: Hemiptera: Aleyrodidae). Zootaxa 1492:1–84. https://doi.org/10.11646/zootaxa.1492.1.1
Naranjo SE, Ellsworth PC (2017) Methodology for developing life tables for sessile insects in the field using the whitefly, Bemisia tabaci, in cotton as a model system. J Vis Exp 129:e56150. https://doi.org/10.3791/56150-v
Naranjo SE, Castle SJ, Barro PJD, Liu SS (2010) Population dynamics, demography, dispersal and spread of Bemisia tabaci. In: Stansly PA, Naranjo SE (eds) Bemisia: Bionomics and management of a global pest. Springer, Dordrecht, pp 185–226
Nauen R, Ghanim M, Ishaaya I (2014) Whitefly special issue organized in two parts. Pest Manag Sci 10:1438–1439. https://doi.org/10.1002/ps.3870
Navas-Castillo J, Fiallo-Olivé E, Sánchez-Campos S (2011) Emerging virus diseases transmitted by whiteflies. Annu Rev Phytopathol 49:219–248. https://doi.org/10.1146/annurev-phyto-072910-095235
Novaes NS, Lourenção AL, Bentivenha JP, Baldin EL, Melo AM (2020) Characterization and potential mechanisms of resistance of cucumber genotypes to Bemisia tabaci (Hemiptera: Aleyrodidae). Phytoparasitica 48:643–657. https://doi.org/10.1007/s12600-020-00826-3
Oliveira MR, Henneberry TE, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 20:709–723. https://doi.org/10.1016/S0261-2194(01)00108-9
Pantoja KF, Rocha KC, Melo AM, Marubayashi JM, Baldin EL, Bentivenha JP, Gioria R, Kobori RF, Pavan MA, Krause-Sakate R (2018) Identification of Capsicum accessions tolerant to Tomato severe rugose virus and resistant to Bemisia tabaci Middle East-Asia Minor 1 (MEAM1). Trop Plant Pathol 43:138–145. https://doi.org/10.1007/s40858-018-0212-6
Perring TM (2001) The Bemisia tabaci species complex. Crop Prot 20:725–737. https://doi.org/10.1016/S0261-2194(01)00109-0
Polston JE, De Barro P, Boykin LM (2014) Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest Manag Sci 70:1547–1552. https://doi.org/10.1002/ps.3738
Quintela ED, Abreu AG, Lima JF, Mascarin GM, Santos JB, Brown JK (2016) Reproduction of the whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) B biotype in maize fields (Zea mays L.) in Brazil. Pest Manag Sci 72:2181–2187. https://doi.org/10.1002/ps.4259
Santos TL, Baldin EL, Ribeiro LP, Souza CM, Soares MC, Fanela TL, Lourenção AL (2021) Resistance sources and antixenotic factors in brazilian bean genotypes against Bemisia tabaci. Neotrop Entomol 50:129–144. https://doi.org/10.1007/s13744-020-00821-7
SAS Institute (2001) SAS/STAT: users guide. p. SAS Institute, Cary, North Carolina, USA, p 502
Shah MM, Liu TX (2013) Feeding experience of Bemisia tabaci (Hemiptera: Aleyrodidae) affects their performance on different host plants. PLoS ONE 10:e77368. https://doi.org/10.1371/journal.pone.0077368
Silva AK, Santos CD, Nascimento AK (2010) Transmissão de begomovirus de plantas daninhas para tomateiros pela mosca-branca. Planta Daninha 28:507–514
Silva JP, Baldin EL, Souza ES, Lourenção AL (2012) Assessing Bemisia tabaci (Genn.) Biotype B resistance in soybean genotypes: antixenosis and antibiosis. Chil J Agric Res 72:516. https://doi.org/10.4067/S0718-58392012000400009
Simmons AM, Harrison HF, LING KS (2008) Forty-nine new host plant species for Bemisia tabaci (Hemiptera: Aleyrodidae). Entomol Sci 11:385–390. https://doi.org/10.1111/j.1479-8298.2008.00288.x
Smith CM (2005) Plant resistance to arthropods: molecular and conventional approaches. Springer Netherlands, Dordrecht
Sottoriva LD, Lourenção AL, Colombo CA (2014) Performance of Bemisia tabaci (Genn.) Biotype B (Hemiptera: Aleyrodidae) on weeds. Neotrop Entomol 43:574–581. https://doi.org/10.1007/s13744-014-0238-5
Tamai MA, Martins MC, Lopes PVL (2006) Perda de produtividade em cultivares de soja causada pela mosca-branca no cerrado baiano. Comunicado Técnico 21, Fundação BA. 7p
Tay WT, Evans GA, Boykin LM, De Barro PJ (2012) Will the real Bemisia tabaci please stand up? PLoS ONE 7:e50550. https://doi.org/10.1371/journal.pone.0050550
Varma A, Mandal B, Singh MK (2011) Global emergence and spread of whitefly (Bemisia tabaci) transmitted geminiviruses. In: Thompson WMO (ed) The whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) interaction with geminivirus-infected host plants. Springer, Dordrecht, pp 205–292
Vilela EF, Zucchi RA (eds) Pragas introduzidas no Brasil, insetos e ácaros. FEALQ, Piracicaba, pp 682–707
Villas Bôas GL, França FH, Macedo N (2002) Potencial biótico da mosca-branca Bemisia argentifolii a diferentes plantas hospedeiras. Hortic Bras 20:71–79. https://doi.org/10.1590/S0102-05362002000100014
Walker GP, Perring TM, Freeman TP (2010) Life history, functional anatomy, feeding and mating behavior. In: Stansly PA, Naranjo SE (eds) Bemisia: bionomics and management of a global pest. Springer, New York, pp 109–160
Wang X, Yang N (2017) The whitefly Bemisia tabaci (Gennadius). In: Wan F, Jiang M, Zhan A (eds) Biological invasions and its management in China: volume 1. Springer, Dordrecht, pp 159–182
Xia WQ, Wang XR, Liang Y, Liu SS, Wang XW (2017) Transcriptome analyses suggest a novel hypothesis for whitefly adaptation to tobacco. Sci Rep 7:12102. https://doi.org/10.1038/s41598-017-12387-3
Xia J, Guo Z, Yang Z, Han H, Wang S, Xu H, Yang X, Yang F, Wu Q, Xie W, Zhou X (2021) Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 184:1693–1705. https://doi.org/10.1016/j.cell.2021.02.014
Zalom FG, Castañé C, Gabarra R (1995) Selection of some winter spring vegetable crop hosts by silverleaf whitefly. J Econ Entomol 88:70–76. https://doi.org/10.1093/jee/88.1.70
Acknowledgements
We thank the National Council for Scientific and Technological Development (CNPq) for granting a master’s scholarship to the first author (process no. 131658/2020-4) and the productivity scholarship in research to the fifth author (process no. 306947/2018-8), and the São Paulo Research Foundation (FAPESP) for partially funding this research (process no. 2021/03987-7). We also thank the Coordination for the Improvement of Higher Education Personnel - Brasil (CAPES) - Finance Code 001. This study is part of master’s thesis of Matheus Gerage Sacilotto.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
M. G. Sacilotto, F. S. F. Souza, E. L. L. Baldin, C. A. Carbonari and A. L. Lourenção declare that they have no competing interests.
Additional information
Communicated by Hongbo Jiang.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sacilotto, M.G., de Souza, F.S.F., Baldin, E.L.L. et al. Comparison of the performance of Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae) on weed and cultivated plant species. Arthropod-Plant Interactions 18, 55–63 (2024). https://doi.org/10.1007/s11829-023-09994-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-023-09994-5