Abstract
The whitefly Bemisia tabaci (Gennadius) is a species complex containing at least 35 morphologically indistinguishable cryptic species. Some members of the complex are pests of agricultural and horticultural crops in temperate and tropical regions. During the past 20 years, two species of the complex, Middle East-Asia Minor 1 (hereafter MEAM1) and Mediterranean (hereafter MED), which have been commonly referred to as the B and Q ‘biotypes’, have risen to international prominence due to their global invasions. In the middle-1990s, the MEAM1 species invaded China most probably with the import of infested plants and seedlings and has become a pest since the late 1990s. In 2003, the MED species of the B. tabaci complex was first recorded in China and it is now the dominant species in the Yangtze River Valley and eastern coastal areas. In this chapter, we first reviewed the invasion histories of MEAM1 and MED whiteflies in China and their negative effects. Then, the research progresses on behavior, biotic, environmental and molecular mechanisms of MEAM1 and MED whitefly invasions and replacement of native whitefly species were discussed. Finally, the strategies for whitefly management in China were summarized. These research efforts have provided solid foundation for future investigations on the molecular mechanisms of whitefly invasions and are expected to open important avenues for the discovery of novel strategies for whitefly management in China.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
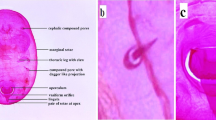
The whitefly Bemisia tabaci (Gennadius) is a phloem-feeding insect that lives predominantly in temperate and tropical regions (Byrne and Bellows 1991; Naranjo et al. 2010). It is highly polyphagous and attack more than 600 plant species including food, vegetable, fiber and ornamental host plants under field and greenhouse conditions (Brown 1995). Bemisia tabaci is now recognized as a species complex containing at least 35 cryptic species (Boykin and De Barro 2014; De Barro et al. 2011; Liu et al. 2012; Xu et al. 2010a). Some members of the complex are considerable pests of ornamental, vegetable and cotton production, causing damages directly through feeding and indirectly through the transmission of plant viruses (Fig. 8.1) (Naranjo et al. 2010). During the past 20 years, two species of the complex, Middle East-Asia Minor 1 (hereafter MEAM1) and Mediterranean (hereafter MED), which have been commonly referred to as B and Q whiteflies, have risen to international prominence due to their global invasions (Dalton 2006; De Barro et al. 2011; Liu et al. 2007). The invasive MEAM1 has been considered as one of the world’s top 100 invasive species according to the International Union for the Conservation of Nature and Natural Resources (IUCN) (http://www.issg.org) and are regulated species in a number of countries, e.g., Australia, Africa, China, EU, and United States. Similarly, the MED species has also begun to invade a large number of countries in Africa, Asia, Europe and America from the Mediterranean region.
Interestingly, despite MEAM1 and MED species of the B. tabaci complex are highly invasive, other members of the complex apparently lack the capacity to invade and establish in neighboring regions. It suggests that the invasive ability for MEAM1 and MED is actually uncommon. Therefore, unraveling the process and mechanism of whitefly invasions may be crucial to advance studies on their biology and introduce new methods to control them. In this review, we will outline the invasion processes and mechanisms of MEAM1 and MED whiteflies in China and then summarize the recent developments in the management of B. tabaci. These research efforts have provided valuable information for future investigations on the molecular mechanisms of whitefly invasions and are expected to open important avenues for the discovery of novel strategies for whitefly management.
2 The Invasion of B. tabaci in China
Although the invasions of B. tabaci have caused devastating consequences worldwide, the invasion history and processes of MEAM1 and MED have been poorly documented in most instances. The whitefly B. tabaci was first recorded in China in 1949. However, for several decades, this insect was not considered as a severe agricultural pest. In the middle-1990s, the MEAM1 species invaded China most probably with the import of infested plants and seedlings (Luo et al. 2002; Zhang 2000). Subsequently B. tabaci has gradually become a severe agricultural pest and significantly threatened the vegetable production in China since the late 1990s. The exotic MEAM1 whitefly species was found to be the dominant species in many agricultural production regions in 1990s (Luo et al. 2002). For example, among the 23 B. tabaci populations collected from 1999 to 2001 from 14 provinces, 17 populations were MEAM1. Interestingly, MEAM1 whiteflies mainly occur in regions near the coast or with convenient transportation while only six populations are other species distributed in the inland or mountainous regions (Qiu et al. 2003a). It was also found that during the invasion of B. tabaci MEAM1, a number of native whitefly species have been completely replaced in many regions, such as in Zhejiang Province, China during 2004–2006 (Liu et al. 2007).
In 2003, one population collected from poinsettia in Yunnan Province was identified as B. tabaci MED based on the mitochondrial cytochrome oxidase subunit I (mtCOI) gene (Chu et al. 2005). Later on, Chu et al. (2012) examined the whitefly populations collected from ten provinces and found that MED was only occasionally detected in Yunnan, Beijing and Henan and MEAM1 was still the dominant species in China. However, in the subsequent years, B. tabaci MED was detected in many regions in China and has replaced the previously well-established MEAM1. For instance, in 2001, among the 22 whitefly populations collected from 15 provinces, MED were found in 19 populations (Teng et al. 2010). Of the 14 samples collected from 2008 to 2009, nine populations belonged to MED, three were MEAM1, and two were a mixture of MED and MEAM1 (Wang et al. 2010b). Hu et al. (2011) found that during June 2009 to March 2010 among the 16 provinces in south and east China, 12 provinces had MED. Pan et al. (2012) found that, for the 55 populations collected from 18 provinces, 43 populations were composed of MED. These data demonstrate that though the exotic MEAM1 was predominant across China in the past, the MED is now the dominant species in the Yangtze River and eastern coastal areas, and MEAM1 is the predominant species only in the south and south eastern coastal areas.
3 Invasion Mechanisms
The population dynamics of B. tabaci is governed entirely by four processes – birth, death, immigration and emigration. Each of them is governed by a wide array of interacting biotic and abiotic factors that determine whitefly occurrence and abundance over time and space (Naranjo et al. 2010). During the last decade, a number of factors that contribute to the invasion and spread of MEAM1 and MED in China have been investigated, such as mating interactions (Liu et al. 2007), host plant adaptation (Xu et al. 2015a), insecticide resistance (Pan et al. 2015), virus-vector-host mutualism (Luan et al. 2013a) and the roles of endosymbionts (Bing et al. 2013; Himler et al. 2011). These studies suggest that the successful invasions of MEAM1 and MED in China is largely due to their intrinsic biological traits, such as high insecticide resistance, wide host range and strong reproduction capacity . These findings will help us to develop novel strategies to manage the invasion and spread of MEAM1 and MED in China.
3.1 Asymmetric Mating Interactions
Reproductive interference is one of the major factors mediating species exclusion among insects. Liu et al. (2007) found that asymmetric mating interactions between different cryptic species of B. tabaci species complex was a driving force contributing to widespread invasions of the MEAM1 species in China and Australia. The authors conducted long-term field surveys, caged population experiments, and detailed behavioral observations in Zhejiang, China, and Queensland, Australia, to investigate the invasion process and its underlying behavioral mechanisms. During invasion and displacement, they found that the increased frequency of copulation led to increased production of female progeny among invaders, as well as reduced copulation and female production in indigenous genetic groups. Such asymmetric mating interactions may be critical to determining the capacity of a haplodiploid invader and the consequences for its closely related indigenous organisms.
The cryptic species MEAM1 and MED of B. tabaci complex have invaded many parts of the world and often exhibit niche overlap and reproductive interference. However, contrasting patterns of competitive displacement between the two invaders have been observed. Sun et al. (2014) investigated the role of reproductive interference on species exclusion between MEAM1 and MED in China. In mixed cohorts of the two species, MEAM1 always excluded MED in a few generations when the initial proportion of MEAM1 was ⩾0.25. Importantly, as MEAM1 increased in relative abundance, MED populations became increasingly male-biased. All these results suggest that MEAM1 should have a stronger reproductive interference than MED, leading to reduced frequency of copulation and female progeny in MED. These findings not only reveal the importance of reproductive interference in the competitive interactions between the two invasive whiteflies, but also provide a valuable framework against which the effects of other factors mediating species exclusion can be explored (Sun et al. 2014).
3.2 Host Plant Adaptation
The capacity of the MEAM1 and MED species of B. tabaci species complex to invade has often been linked to their presumed wider host range than the indigenous competitors. To determine whether the invasive MEAM1 species and the indigenous Asia II 1 whitefly species, commonly referred to as the ‘ZHJ2 biotype’, differ in their ability to use different host plants, Xu et al. (2011) compared their development, survival and reproduction on eight crop species/cultivars that are commonly cultivated in Zhejiang, China. Of the eight host plants tested, MEAM1 performed substantially better than ZHJ2 on squash, tomato and tobacco; MEAM1 and ZHJ2 preformed equally well on cotton and sweet potato, while ZHJ2 performed better than MEAM1 on kidney bean and pepper. These results indicate that while MEAM1 generally has a wider host range than many indigenous B. tabaci, an indigenous B. tabaci can perform as well as or better on some host plants. This suggests that the differential capacity to use various host plants between whitefly species is important in mediating the process of invasions by an alien whitefly species. Sun et al. (2013a) also examined the effects of host plants on the competitive interactions between the two species in the laboratory. When cohorts with equal abundance of MEAM1 and MED were set up on different host plants, MEAM1 displaced MED on cabbage and tomato in five and seven generations, respectively, but MED displaced MEAM1 on pepper in two generations.
Despite its importance, few studies have addressed the molecular mechanisms of B. tabaci against major plant secondary defense compounds. Alon et al. (2012) compared the gene expression between B. tabaci adults on wild-type Nicotiana tabacum plants or transgenic plants constitutively activating the phenylpropanoid/flavonoids biosynthetic pathway. Both the SSH and cDNA microarray analyses indicated a complex interaction between B. tabaci and secondary defense metabolites produced by the phenylpropanoids/flavonoids pathway, such as expression of detoxification, immunity, oxidative stress and general stress related genes. However, the elevated transcriptional activity was not accompanied by reduction of whitefly reproductive performance, indicating high adaptability of B. tabaci to this large group of plant secondary defense metabolites (Alon et al. 2012). The same research group also investigated the effects of aliphatic and indolic glucosinolates on the expression of detoxification genes in MEAM1 and MED whiteflies (Elbaz et al. 2012). The result suggests that the two species use rather different strategies to cope with plant defense responses. While MEAM1 utilizes inducible defenses, MED invests significant resources in being always ‘ready’ for a challenge (Elbaz et al. 2012). Xu et al. (2015a) compared the performance of the invasive MEAM1 and the indigenous Asia II 3 whitefly species following host plant transfer from a suitable host (cotton) to an unsuitable host (tobacco) and analyzed their transcriptional responses. Transcriptional analysis showed that the patterns of gene regulation were very different with most of the genes up-regulated in MEAM1 but down-regulated in Asia II 3. Compared to the constitutive high expression of detoxification genes in MEAM1, most of the detoxification genes were down-regulated in Asia II 3. Enzymatic activities of P450, GST and esterase further verified that the detoxification of MEAM1 was much higher than that of Asia II 3. These results reveal obvious differences in response to host transfer in MEAM1 and Asia II 3.
3.3 Roles of Vector–Virus–Plant Interactions in Biological Invasions
Begomoviruses are a group of single stranded DNA viruses exclusively transmitted by the whitefly B. tabaci in a persistent, circulative manner (Czosnek and Ghanim 2002). During this process, virions are acquired by the stylet of whitefly vectors from the plant phloem, moving along the esophagus to the midgut, then crossing the gut epithelial cells to the hemocoel, circulating with the hemolymph and reaching the salivary glands, and finally were secreted with saliva (Gray et al. 2014). During the circulation, viruses have engaged host factors or unique strategies for replication, movement, transmission and pathogenesis (Wei et al. 2014). Meanwhile, the insect vectors have evolved immunologic surveillance system against viral invasions (Luan et al. 2011; Wang et al. 2016). Interactions among vector insects, plant viruses and host plants are complex and diverse. These interactions may affect the process of biological invasions and the displacement of indigenous species by invaders when the invasive and indigenous organisms occur with niche overlap. Jiu et al. (2007) compared the performance of the invasive MEAM1 and indigenous Asia II 3 whiteflies on healthy, TbCSV-infected and TYLCCNV-infected tobacco plants. Compared to its performance on healthy plants, the invasive MEAM1 increased its fecundity and longevity significantly when feeding on virus-infected plants. In contrast, the indigenous Asia II 3 species performed similarly on healthy and virus-infected plants. The indirect mutualism between the MEAM1 whitefly and these viruses via their host plants, and the apparent lack of such mutualism for the indigenous whitefly, may contribute to the ability of the MEAM1 whitefly to invade and displace indigenous whiteflies. Interestingly, transcriptional analyses of MEAM1 whiteflies feeding on TYLCCNV-infected and uninfected tobacco plants indicated that the genes involved in the oxidative phosphorylation pathway and detoxification enzyme were down-regulated in whiteflies feeding on virus-infected plants (Luan et al. 2013a). The reduced detoxification activity is likely to attenuate energy costs, thus, enhancing the performance of whiteflies on virus-infected plants (Luan et al. 2013a).
Zhang et al. (2012) found that TYLCCNV and betasatellite coinfection suppresses jasmonic acid defences in the plant. Impairing or enhancing defences mediated by jasmonic acid in the plant enhances or depresses the performance of the whitefly. The result indicate that TYLCCNV can benefit its whitefly vector indirectly, through suppression of jasmonic acid-mediated plant defense (Zhang et al. 2012). To reveal why begomovirus infection can benefit its whitefly vector, Luan et al. (2013b) used a next-generation sequencing technology to identify defense genes differentially regulated in whitefly-infested and/or virus-infected tobacco. The authors found that many of terpenoid synthesis genes were up-regulated in whitefly-infested plants. In contrast, in TYLCCNV-infected leaves, most terpenoid genes were unchanged and five genes were declined. Interestingly, in co-infested plants, most terpenoid genes were unchanged and only three terpenoid genes were up-regulated. This study demonstrates that virus infection can deplete the terpenoid-mediated plant defense against whiteflies, thereby favoring vector–virus mutualism (Luan et al. 2013b). Furthermore, Li et al. (2014b) found that βC1 of TYLCCNV can suppress plant terpene biosynthesis. βC1 directly interacts with the basic helix-loop-helix transcription factor MYC2 to compromise the activation of MYC2-regulated terpene synthase genes, thereby reducing whitefly resistance.
Shi et al. (2013) showed that TYLCV directly and indirectly modified the feeding behavior of B. tabaci in favor of MED rather than MEAM1. They further quantified the salicylic acid (SA) titers and relative gene expression of SA in tomato leaves that were infested with viruliferous or non-viruliferous MEAM1 and MED. The authors found that SA titer was always higher in leaves that were infested with viruliferous MEAM1 than with viruliferous MED, whereas the SA titer did not differ between leaves infested with non-viruliferous MEAM1 and MED. Their results also indicate MED may have a mutualistic relationship with TYLCV that results in the reduction of the plant’s defense response (Shi et al. 2013). Interestingly, He et al. (2015) found that when feeding on either cotton, a non-host of TYLCCNV, or uninfected tobacco, a host of TYLCCNV, virus-infection of the whiteflies impeded their feeding. Interestingly, when viruliferous whiteflies fed on virus-infected tobacco, their feeding activities were no longer negatively affected ; instead, the virus promoted whitefly behaviour related to rapid and effective sap ingestion.
3.4 Roles of Endosymbionts in B. tabaci Invasions
The life history traits of multicellular organisms are often influenced by interactions with symbiotic species. The whitefly B. tabaci harbors a primary symbiont “Candidatus Portiera aleyrodidarum”, which compensates for the deficient nutritional composition of its food sources and a variety of secondary symbionts. Interestingly, all of these secondary symbionts are found in co-localization with the primary symbiont within the same bacteriocytes, which should favor the evolution of strong interactions between symbionts. Recent study showed that Rickettsia sp. nr. bellii swept into a population of the invasive MEAM1 in just 6 years. Compared with uninfected whiteflies, Rickettsia-infected whiteflies produced more offspring, had higher survival to adulthood, developed faster, and produced a higher proportion of females (Himler et al. 2011). The Rickettsia thus functions as both mutualist and reproductive manipulator. The observed increased performance and sex-ratio bias of infected whiteflies are sufficient to explain the spread of Rickettsia across the southwestern United States.
Bing et al. (2013) tested five of the six S-endosymbiont lineages (excluding Fritschea) from 340 whitely individuals representing six putative species from China. Hamiltonella was detected only in the two exotic invaders, MEAM1 and MED. Rickettsia was absent in Asia II 1 and MED, scarce in Asia II 3 (13%), but abundant in Asia II 7 (63.2%), China 1 (84.7%) and MEAM1 (100%). Wolbachia, Cardinium and Arsenophonus were absent in the invasive MEAM1 and MED but mostly abundant in the native putative species. Furthermore, phylogenetic analyses revealed that some S-endosymbionts have several clades and different B. tabaci putative species can harbor different clades of a given S-endosymbiont, demonstrating further the complexity of S-endosymbionts in B. tabaci. These results demonstrate the variation and diversity of S-endosymbionts in different putative species of B. tabaci, especially between invasive and native whiteflies.
Owing to the importance of endosymbionts in whitefly biology, a number of studies have been carried out to reveal the function of whitefly endosymbionts. In order to gain insight into the metabolic role of each symbiont, Rao et al. (2015) analyzed the genome sequences of the primary symbiont Portiera and of the secondary symbiont Hamiltonella in MED. Sequencing results shows that the genome of Portiera is highly reduced (357 kb). It has kept a number of genes encoding most essential amino-acids and carotenoids, but lacks almost all the genes involved in the synthesis of vitamins and cofactors. Interestingly, Hamiltonella can not only provide vitamins and cofactors, but also complete the missing steps of some of the pathways of Portiera. The data suggests that Portiera and Hamiltonella are not only complementary but could also be mutually dependent to provide a full complement of nutrients to their hosts. On the other hand, genome analysis revealed that another secondary symbiont Rickettsia was unable to synthesize amino acids required for complementing the whitefly nutrition (Zhu et al. 2016). Parallel genomic and transcriptomic analysis further revealed that the host genome contributes multiple metabolic reactions that complement or duplicate Portiera function, and that Hamiltonella may contribute multiple cofactors and one essential amino acid, lysine. These results shows that bacteria with genomic decay enable host acquisition of complex metabolic pathways by multiple independent horizontal gene transfers from exogenous bacteria (Luan et al. 2015).
3.5 Roles of Pesticides Application in B. tabaci Invasions
At present, more than 50 insecticides have been employed to control the growth of B. tabaci populations and viral transmission (Horowitz et al. 2011). However, due to the rapidly rising resistance to insecticides, utilizing chemical agents to control B. tabaci is facing ever-increasing difficulties (Dennehy et al. 2010; Wang et al. 2009). During the last 20 years, a number of studies have been carried out to reveal the molecular mechanisms of whitefly resistance to insecticides. Pyriproxyfen is one of the major insecticides used to control the whitefly, however, whitefly resistance to pyriproxyfen has been observed in many regions (Crowder et al. 2007). To investigate the molecular basis underlying this resistance, a cDNA microarray was used to monitor changes in gene expression in a resistant B. tabaci population (Ghanim and Kontsedalov 2007). Functional analysis showed that many of the up-regulated ESTs were associated with resistance and xenobiotic detoxification, protein, lipid and carbohydrate metabolism and JH-associated processes (Ghanim and Kontsedalov 2007). Yang et al. (2013b) analyzed the differences between resistant and susceptible stains at both transcriptional and translational levels. Among the 1338 differentially expressed genes, 118 were putatively linked to insecticide resistance . The same research group also compared gene expression in the egg, nymph and adult stages of a thiamethoxam-resistant strain with a susceptible strain using a custom whitefly microarray (Yang et al. 2013a). Gene ontology and bioinformatic analyses revealed that at all life stages many of the DEGs encoded enzymes were involved in metabolic processes and/or metabolism of xenobiotics. In addition, several ATP-binding cassette transporters were highly over-expressed at the adult stage of the TH-R strain and may play a role in resistance by active efflux (Yang et al. 2013a).
The wide application of insecticides in China may be the key factor driving the rapid displacement of MEAM1 by MED. Under laboratory conditions, the MEAM1 can displace MED without the selection of insecticides (Wu et al. 2010). However, field surveys in China after 2003 indicate that in many regions MED has been replacing the earlier invader MEAM1. Sun et al. (2013a) conducted laboratory experiments and field sampling to examine the effects of insecticide application on the competitive interactions between MEAM1 and MED. In the laboratory, MEAM1 displaced MED in five generations on cotton when initial populations of the two species were equal and no insecticide was applied. In contrast, MED displaced MEAM1 in seven and two generations, respectively, when imidacloprid was applied. Field sampling indicated that in a single season MED displaced MEAM1 on crops heavily sprayed with neonicotinoid insecticides but the relative abundance of the two species changed little on crops without insecticide spray. As field populations of MED have lower susceptibility than those of MEAM1 to commonly used insecticides, insecticide application seems to have played a major role in shifting the species competitive interaction in favor of MED in the field. Later on, Pan et al. (2015) confirmed that the rapid replacement of the MEAM1 by MED throughout China is because MED is more tolerant of insecticides. The field monitoring also revealed that the insecticide resistance of MED generally is higher than MEAM1. For instance, Luo et al. (2010) found that MEAM1 remained largely susceptible to acetamiprid, imidacloprid, and thiamethoxam, whereas MED expressed 20–170 folds resistance to these insecticides. These studies strongly support the hypothesis that insecticide use reverses the MEAM1-MED competition in China and allows MED to displace MEAM1 in different regions.
3.6 Adaptation of B. tabaci to Heat Stress
In nature, whiteflies are continuously exposed to abiotic stresses. The great adaptability of MEAM1 to harsh temperature conditions plays a major role in its rapid invasions and spread. Results of the comparative studies demonstrated that MEAM1 whiteflies possess greater tolerance to harsh temperatures than the greenhouse whitefly T. vaporariorum and some of the native whitefly species (Cui et al. 2008; Gao et al. 2015). The MEAM1 adults showed greater ability to adapt to higher temperature than T. vaporariorum under laboratory conditions. The thermal thresholds for survival of MEAM1 and T. vaporariorum were 45 °C and 43 °C, respectively. Fecundity of the MEAM1 was not significantly affected after 1 h heat shock, whereas that of T. vaporariorum was decreased (Cui et al. 2008). Mahadav et al. (2009) compared the expression patterns of MEAM1 and MED under 25 °C and 40 °C heat stress using microarray. The authors found that compared to the treatment of MED, exposure of MEAM1 to heat stress was accompanied by rapid alteration of gene expression. These differences might be due to better adaptation of one species over another and might eventually lead to change of MEAM1 and MED distribution (Mahadav et al. 2009). To reveal why females are more heat resistant than males, Lu and Wan (2008) identified the DEGs in male and female whiteflies, respectively. The authors found that difference of heat-resistance under heat-shock condition was associated with DEGs between B. tabaci sexes.
3.7 Genetic Base of B. tabaci Invasions
The recent advancement in genomic technologies offers great opportunities for a better understanding of the complex mechanisms underlying whitefly invasions (Edwards and Papanicolaou 2012). With the development of high performance sequencing technology, transcript profiling techniques allow the simultaneous examination of thousands of genes, and can be utilized to study changes in gene expression (Gibbons et al. 2009). In 2010, a new short read sequencing technology (Illumina) was employed to analyze the transcriptome of the MED. A total of 168,900 distinct sequences were assembled. Based on similarity search with known proteins, 27,290 sequences with a cut-off E-value of 10−5 were identified (Wang et al. 2010a). In 2011, Wang et al. (2011) re-sequenced the transcriptome of the invasive MEAM1 whitefly using Illumina and compared it with the MED transcriptome. The comparison revealed that the level of sequence divergence in coding region was 0.83%, strongly supporting to the previous proposition that MEAM1 and MED whiteflies are two species. This study further showed that 24 genes, which have evolved in response to positive selection, were involved in metabolism and insecticide resistance. These genes might contribute to the divergence of the two whitefly species (Wang et al. 2011). To reveal the possible mechanism of whitefly invasions, 52,535 transcriptome sequences were identified from the native Asia II 3 species (Wang et al. 2012). Comparison of the sequence divergence between the transcriptomes of Asia II 3 and the invasive species MEAM1 and MED indicated that the overall divergence of coding sequences between the orthologous gene pairs of Asia II 3 and MEAM1, and that between Asia II 3 and MED, was 1.73 and 1.84%, respectively, much higher than that between MEAM1 and MED (0.83%). The data also demonstrated that the most divergent gene classes between the native and invasive species were related to cytochrome P450, glutathione metabolism and oxidative phosphorylation, which seemed relevant to the invasion, displacement and speciation of the species in the B. tabaci complex (Wang et al. 2012).
Wang et al. (2013) further examined the transcriptional difference between the two invasive whitefly species, MEAM1 and MED, and one indigenous whitefly species Asia II 3. The results showed that 2422 genes between MEAM1 and MED; 3073 genes between MEAM1 and Asia II 3; and 3644 genes between MED and Asia II 3 were differentially expressed. Carbohydrate, amino acid and glycerolipid metabolisms were more active in MEAM1 and MED than in Asia II 3. Furthermore, the majority of genes involved in basic metabolism and detoxification were expressed at a higher level in MEAM1 and MED than in Asia II 3, which might be responsible for their higher resistance to insecticides and environmental stresses (Wang et al. 2013). Sequencing and comparison of the gut transcriptomes of MEAM1 and MED revealed that many genes related to detoxification were expressed at an elevated level in the gut of MED compared to MEAM1, which might be responsible for the MED’s higher resistance to insecticides and environmental stresses (Ye et al. 2014). Xu et al. (2015a) compared the transcriptional responses of MEAM1 and Asia II 3 whitefly species following host plant transfer from a suitable host (cotton) to an unsuitable host (tobacco). Transcriptional analysis showed that compared to the constitutive high expression of detoxification genes in MEAM1, most of the detoxification genes were down-regulated in Asia II 3. Enzymatic activities of P450, GST and esterase further verified that the detoxification of MEAM1 was much higher than that of Asia II 3. These results reveal obvious differences in responses of MEAM1 and Asia II 3 to host transfer.
4 Management of B. tabaci
4.1 Monitoring and Treatment Decisions
By collecting information of several factors (e.g., longitude, latitude, altitude, average annual rainfall, average annual temperature and its range, average temperature in January and July, annual highest and lowest temperature) at whitefly occurred spots in China and analyzing with Maxent prediction model, Ren et al. (2011) reported that South and East China, as well as the southern part of North China were suitable for B. tabaci distribution. Using life table data of B. tabaci and 10 years’ climate information in different regions in China, Ren et al. (2011) warned that B. tabaci may have 11–15 generations in Guangdong, Guangxi and Hainan provinces, 7–15 generations in Shandong, Henan and Chongqing areas, and one generation in Tibet and Qinhai provinces. Based on this result, a monitoring and early warning platform has been established (http://www.ipm.ioz.ac.cn/them_gefeng/fenshi/index.as).
The use of yellow sticky cards (24 cm × 20 cm) is an important method of monitoring whitefly populations in the wild. Five yellow sticky cards were put in every 667 m2 field using 5-points or “Z”-shaped methods, and replaced with new ones every 10 days. The upper edge of the card was kept at an equal level to or slightly above the plants. If the number of adult whitefly captured on the cards reached 0.25–0.5 adult/cm2, biological control measures should be taken; while it reached 3–4 adult/cm2, chemical control measures should be taken to suppress the outbreak of B. tabaci (Ren et al. 2014). Sampling via leaf-turn method was also adopted. For Solanaceous and Cucurbitaceae plants, the third and fourth leaf from the top was sampled; while for vegetables such as cabbage and Chinese kale, the young leaves in plant heart part were sampled. If the number of adult whitefly reached 0.5–2 adult/leaf, biological control measures should be taken; while it reached 5–6 adult/leaf, environmental friendly insecticides should be sprayed (Tian et al. 2015). Such action threshold has been defined as the level of pest populations at which control should be implemented to avoid significant damages to crops (Dik and Albajes 1999), while an “economic injury level” (EIL) requires more rigorous criteria employing economic consideration. Related to market and environmental conditions in China, an economic injury level of 18 adult B. tabaci per cucumber plant at the four-leaf stage was determined (Shen et al. 2005); for greenhouse tomato, a level of 13.6 adults per 100 leaves was established (Cao and Cao 2011); while for cabbage, an economic threshold of 6.0–6.8 adults per plant in the seedling stage and 2.1–3.8 adults per plant in the rosette stage were determined (Wang 2007).
4.2 Cultural Control
Cultural control is to improve crop system to make the environment less favorable to pest reproduction, dispersal, survival and damage, while more suitable to natural enemies. Cultural control options for B. tabaci include the use of physical barriers or other barriers to prevent the pest from reaching the crop, adjusting planting dates, planting in low infestation areas, rotation with non-susceptible crops, destroying crop residues and selecting resistant crops or cultivars (Ren et al. 2001).
Planting Dates
One way to reduce whitefly infestations is to adjust planting dates to avoid the heaviest insect migration periods or crop overlap. Early planting in spring or delayed planting in fall can be an effective way to avoid whiteflies, since they reproduce more rapidly under hot and dry conditions. Highly susceptible crops such as cucurbits, crucifers and tomatoes should avoid to be planted when whitefly migration is expected.
Crop Termination
Whitefly infestations most often occur in fields where damages ever occurred. Susceptible crops should not be planted near infestation sources. Godfrey et al. (2008) suggested that cotton should be planted at least one-half mile upwind from other key host crops, such as melons, tomatoes, from key ornamental plants, and from key weed species that harbor B. tabaci. Infected plants should be removed and destroyed, and susceptible crops should not be grown continuously. It is reported that B. tabaci can continue to increase up to 6 weeks after final cotton irrigation even following defoliation since red eye pupa were able to continue development to the adult stage on cotton leaves that abscised and fell from the plants (Nuessly et al. 1994). Thus crop residues, which can harbor whitefly and virus inoculum, should be rapidly and completely destroyed after the final harvest.
Water and Fertility Management
Water and fertility management play important roles as cultural control methods in whitefly management. Overuse of nitrogen fertilizer will greatly increase whitefly numbers and honeydew production thus exacerbate damages from B. tabaci infestations (Bi et al. 2001, 2005). Higher populations of B. tabaci were observed on water-stressed cotton as compared to well-watered plants (Flint et al. 1996). However, B. tabaci feeding on well-watered plants produced more honeydew and sugars per gram of honeydew than on water stressed cotton (Henneberry et al. 2002).
4.3 Host Plant Resistance
Planting varieties that are resistant to B. tabaci and associated viruses (e.g., tomato yellow leaf curl virus, TYLCV) is a preferred solution for minimizing damages caused by whitefly at the early stage. The Mi-1 gene, present in many varieties of cultivated tomato (Solanum lycopersicum L.) and introduced into this plant from its wild relative, S. peruvianum, regulates resistance to B. tabaci, aphid Macrosiphum euphorbiae and root-knot nematodes Meloidogyne spp. (Roberts and Thomason 1986; Rossi et al. 1998; Nombela et al. 2001). However, tomato varieties bearing Mi-1 gene has not been widely used in China yet. A number of studies on different plant varieties resistant to this pest have been carried out in China during the last decade. Studies have compared the biotic potential of whiteflies in different cultivated plants such as tomato, cucumber, beans, eggplant, peanut, squash and pepper in China (Ren et al. 2001; Xu et al. 2010b; Sun et al. 2013b; Wu 2013; Ji 2015).
4.4 Physical Control
Fine-mesh nylon screen (normally pore diameter 0.125 mm) can be used in greenhouse production to reduce the potential for infestation of greenhouse pests including whitefly, flower thrips, aphid and leafminer. Under field conditions, several types of barriers can reduce whitefly problems, including reflective mulches that tend to repel whiteflies, oil-coated yellow mulches that act as a trap for whiteflies, floating row covers that exclude whiteflies during the vegetative growth of crops, as well as barrier and trap crops (Ren et al. 2001; Tian et al. 2015).
Yellow sticky cards were also used to trap whitefly adults, especially in greenhouse condition. It is suggested that yellow sticky card (24 cm × 20 cm) could trap whitefly efficiently when placed vertically among plants and one card per 3–5 m, kept the upper edge of the card at an equal level or slightly above the plants (Qiu and Ren 2006; Chen et al. 2012).
4.5 Biological Control
In China, a number of natural enemies of B. tabaci have been recorded including 56 species of parasitoids, mainly belonging to the genera Eretmocerus and Encarsia; 54 species of arthropod predators, dominated by lady beetles and lacewings in Coleoptera and Neuroptera; and seven species of entomopathogenic fungi (Li et al. 2011). More than ten species of parasitoids and predators are commercially available in China now (Table 8.1).
Parasitoids
Encarsia bimaculata and Eretmocerus sp. nr. furuhashii are the two dominate species parasitizing B. tabaci in South China, while the population of Encarsia formosa is abundant in north China in protected fields, and Eretmocerus hayati is abundant in Xinjiang province, located in northwest China, on cotton in fields (Li et al. 2011; Abuduhani et al. 2013; Zhang et al. 2015).
Among the four Aphelinid species which are commercially available in China (Table 8.1), Encarsia sophia (formerly known as En. transvena) is a solitary, arrhenotokous, heteronomous autoparasitoid. For this species, fertilized eggs are laid in whitefly nymphs and developed into female progeny, while the unfertilized eggs are laid externally on immature parasitoids inside the whitefly host, either on conspecific species or on heterospecific primary parasitoids, and developed into male progeny (Hunter and Kelly 1998). It is originated from Pakistan, and currently present across northern and southern China (Yang et al. 2012; Li et al. 2011). Er. hayati is a primary, solitary parasitoid which oviposits externally under the nymphal host (Yang and Wan 2011). After eclosion, the first instar larva penetrates the host from underneath and develops internally. Both of these two parasitoids attack all nymphal stages (N1-N4) of B. tabaci, while En. sophia prefer old instar and Er. hayati prefer young ones (Yang and Wan 2011). Although Er. hayati is capable of high parasitism on B. tabaci, its field population often fluctuates because of the severe consumption of hosts by adults (Yang et al. 2012; Xu et al. 2015b). The combined release of the autoparasitoid En. sophia will stabilize the effect of Er. hayati (Xu et al. 2015b, 2016; Huang et al. 2016).
Predators
The lady beetle Axinoscymnus cardilobus is a dominant predator of B. tabaci in south China, while Harmonia axyridis and Propylea japonica are dominant predators species in north China (Zhang et al. 2007a, b; Li et al. 2011). H. axyridis is a generalist predator extensively employed as a biological control agent in China (Koch 2003). This ladybird species exhibits significantly lower selectivity for B. tabaci and a reduced reproduction rate when fed on B. tabaci (Tan et al. 2016). However, its generalist character could be helpful in controlling secondary pests. Besides, when it was released in combination with whitefly parasitoids, En. sophia and En. formosa, H. axyridis showed a significant preference for non-parasitized nymphs as prey; and both parasitoid species parasitized more B. tabaci as compared to the wasps were released either alone or mixed with the other parasitoid (Tan et al. 2016). This combination release of predator and parasitoids enhanced whitefly control. Li et al. (2014a) also reported an enhanced control effects on B. tabaci by combined release of Orius sauteri , a generalist predatory stink bug, with En. formosa.
Entomopathogenic Fungi
Several species of entomopathogenic fungi active against B. tabaci are commercially available in China, including Beauveria bassiana , Aschersonia aleyrodis , Verticillium lecanii , and Isaria fumosorosea . B. bassiana could significantly decrease the survival rate of B. tabaci nymphs while increase developmental time and pre-oviposition period (Xia et al. 2013). A. aleyrodis could parasitize on 1–3 instar nymphs of B. tabaci , and had no contradicted effect on whitefly parasitoid Eretmocerus sp. (Qiu et al. 2003b). I. fumosorosea infected all immature stages and adults of B. tabaci (Tian et al. 2014). V. lecanii showed deterrent activity to B. tabaci adults and contact toxicity to B. tabaci nymphs (Wang et al. 2006). However, V. lecanii required high humidity to be effective, this shortage could be made up by using V. lecanii toxic (Hong et al. 2011). The deterrent and anti-feeding activity of V. lecanii toxic-VIII on B. tabaci adults was tested as 41% and 23%, respectively (Hong et al. 2011). Restricted by environmental factors such as temperature and humility, the effect of entomopathogenic fungi in B. tabaci management was slow and not very stable. Recent researches revealed that when I. fumosorosea combined with thiamethoxam or imidacloprid, and B. bassiana combined with non-ionic surfactants, a synergic effect on B. tabaci was observed (Mascarin et al. 2014; Zou et al. 2014).
4.6 Chemical Control
Even the system of organic crops production inclines to employ increasing biological control measures, chemical control is indispensable when B. tabaci occurs in high density and quick eliminating effect is required. However, B. tabaci can develop resistance not only to conventional insecticides in China, but also to a number of new insecticides, such as pyriproxyfen , acetamiprid, imidacloprid, and thiamethoxam, especially MED (Crowder et al. 2007; Luo et al. 2010). Besides, thiamethoxam was highly toxic to Serangium japonicum, a predator ladybeetle, regardless of exposure routes (e.g., residue contact, egg-dip, and systemic treatment) (Yao et al. 2015). Therefore, thiamethoxam should be used with caution in IPM of B. tabaci. Some environmental friendly insecticides, avermectin, spinetoram, sulfozaflor and cyantraniliprole, are recommended to be applied in B. tabaci management (Keyimu et al. 2014; Xie et al. 2014).
5 Concluding Remarks
Even though many field surveys have been conducted to monitor the spread of B. tabaci during the last 15 years, our understanding of the invasion processes of MEAM1 and MED in China remain largely observational and anecdotal. This is mainly due to the lack of efficient methods to easily distinguish different whitefly species in the field and the presence of the B. tabaci species complex . Nevertheless, the current surveys have clearly showed that while MEAM1 was dominate near 2000, MED has replaced MEAM1 in many regions of China now. During the last decade, we have identified multiple factors that contribute to the invasions of B. tabaci into new regions and habitats, such as mating interactions, host plant adaptation, insecticide resistance, virus-vector-host mutualism and the roles of endosymbionts. Those studies obviously suggest that the successful invasions of MEAM1 and MED in China are largely due to their intrinsic traits, such as high insecticide resistance, wide host range and strong reproduction capacity . More comparative studies on the biological characteristics between invasive and indigenous species may provide better understanding of the mechanism underlying invasions and displacement. It is also crucial to identify the key factors that contribute to the unique features of MEAM1 and MED. The obtained knowledge will help us to develop new strategies to control the spread of invasive B. tabaci in China. At this stage , Chinese farmers mainly rely on insecticides as immediate solutions to B. tabaci problems. However, the use of insecticides impairs the benefits of consumers and regulators concerned with food safety and environmental protection . This conflict leads to interest and development in alternative control measures. In response, Integrated Pest Management (IPM) has been the main paradigm in modern pest control. In China, IPM has been developed in the last decade with a combination of tactics including cultural control methods, resistant plant varieties, physical control such as mechanical screen and yellow sticky cards, biological control by natural enemies, and the judicious use of insecticides based on sampling and economic thresholds. In the production of some high-value crops, the B. tabaci IPM has been tested to be effective and been gradually adopted in organic farms. However, for most farmers, considering the IPM cost and knowledge access, chemical measures are still the prior choice. Thus, the wide demonstration and dissemination of B. tabaci IPM in end-users to improve the implement of environmental friendly measures to control B. tabaci needs extensive devotion from regulators and researchers.
References
Abuduhani R, Abulimiti M, Jamali, R, Zhang YN, Li J, Ma DY (2013) Population dynamics and spatial distribution of Bemisia tabaci and dominat parasitic wasp Eretmocerus sp. from the field of jujubi intercropping with cotton. Xinjiang Agric Sci 50(10): 1842–1849 (in Chinese, English abstract)
Alon M, Elbaz M, Ben-Zvi MM, Feldmesser E, Vainstein A, Morin S (2012) Insights into the transcriptomics of polyphagy: Bemisia tabaci adaptability to phenylpropanoids involves coordinated expression of defense and metabolic genes. Insect Biochem Mol Biol 42:251–263
Bi JL, Ballmer GR, Hendrix DL, Hennneberry TJ, Toscano NC (2001) Effect of cotton nitrogen fertilization on Bemisia argentifolii populations and honeydew production. Entomol Exp Appl 99:25–36
Bi JL, Lin DM, Li KS, Toscano NC (2005) Impact of cotton planting date and nitrogen fertilization on Bemisia argentifolii populations. Insect Sci 12:31–36
Bing XL, Ruan YM, Rao Q, Wang XW, Liu SS (2013) Diversity of secondary endosymbionts among different putative species of the whitefly Bemisia tabaci. Insect Sci 20:194–206
Boykin LM, De Barro PJ (2014) A practical guide to identifying members of the Bemisia tabaci species complex and other morphologically identical species. Front Ecol Evol. doi:10.3389/fevo.2014.00045
Brown JK (1995) The sweet potato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex. Annu Rev Entomol 40:511–534
Byrne DN, Bellows TS (1991) Whitefly biology. Annu Rev Entomol 36:431–457
Cao ZG, Cao L (2011) The action threshold of Bemisia tabaci on protected tomato. Shanghai Agric Technol 6:122 (in Chinese, English abstract)
Chen D, Zhang YH, Reziwanguli, Miriguli, Yang M, Pan WP, Ma DY (2012) Control effects of Yellow Sticky Trap (YST) to Bemisia tabaci in the greenhouses in Turpan Area. Xinjiang Agric Sci 49(2):255–260 (in Chinese, English abstract)
Chu D, Zhang YJ, Cong B, Xu BY, Wu QJ (2005) Identification for Yunnan Q-biotype Bemisia tabaci population. Chin Bull Entomol 42:54–56
Chu D, Li X, Zhang Y (2012) Microsatellite analyses reveal the sources and genetic diversity of the first-introduced Q-biotype population and the well-established B-biotype populations of Bemisia tabaci in China. Acta Entomol Sin 55:1377–1386
Crowder DW, Dennehy TJ, Ellers-Kirk C, Yafuso LC, Ellsworth PC, Tabashnik BE, Carriere Y (2007) Field evaluation of resistance to pyriproxyfen in Bemisia tabaci (B biotype). J Econ Entomol 100:1650–1656
Cui XH, Wan FH, Xie M, Liu TX (2008) Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci Biotype B. J Insect Sci 8:1–10
Czosnek H, Ghanim M (2002) The circulative pathway of begomoviruses in the whitefly vector Bemisia tabaci – insights from studies with Tomato yellow leaf curl virus. Ann Appl Biol 140:215–231
Dalton R (2006) Whitefly infestations: the christmas invasion. Nature 443:898–900
De Barro PJ, Liu SS, Boykin LM, Dinsdale AB (2011) Bemisia tabaci: a statement of species status. Annu Rev Entomol 56:1–19
Dennehy TJ, Degain BA, Harpold VS, Zaborac M, Morin S, Fabrick JA, Nichols RL, Brown JK, Byrne FJ, Li X (2010) Extraordinary resistance to insecticides reveals exotic Q biotype of Bemisia tabaci in the New World. J Econ Entomol 103:2174–2186
Dik AJ, Albajes R (1999) Principals of epidemiology, population biology, damage relationships, and integrated control of diseases and pests. In: Albajes R, Lodovica-Gullino M, van Lenteren JC, Elad Y (eds) Integrated Pest and Disease Management in Greenhouse Crops. Springer, Amsterdam, pp 69–81
Edwards O, Papanicolaou A (2012) A roadmap for whitefly genomics research: lessons from previous insect genome projects. J Integr Agric 11:269–280
Elbaz M, Halon E, Malka O, Malitsky S, Blum E, Aharoni A, Morin S (2012) Asymmetric adaptation to indolic and aliphatic glucosinolates in the B and Q sibling species of Bemisia tabaci (Hemiptera: Aleyrodidae). Mol Ecol 21:4533–4546
Flint HM, Naranjo SE, Leggett JE, Henneberry TJ (1996) Cotton water stress, arthropod dynamics, and management of Bemisia tabaci (Homoptera: Aleyrodidae). J Econ Entomol 89:1288–1300
Gao XL, Li JM, Xu HX, Yan GH, Jiu M, Liu SS, Wang XW (2015) Cloning of a putative extracellular Cu/Zn superoxide dismutase and functional differences of superoxide dismutases in invasive and indigenous whiteflies. Insect Sci 22:52–64
Ghanim M, Kontsedalov S (2007) Gene expression in pyriproxyfen-resistant Bemisia tabaci Q biotype. Pest Manag Sci 63:776–783
Gibbons JG, Janson EM, Hittinger CT, Johnston M, Abbot P, Rokas A (2009) Benchmarking next-generation transcriptome sequencing for functional and evolutionary genomics. Mol Biol Evol 26:2731–2744
Godfrey LD, Goodell PB, Natwick ET, Haviland DR (2008) Insects and mites. In: UC IPM Pest Management Guidelines: cotton. UC ANR Publication 3444. http://www.ipm.ucdavis.edu/PMG/r114300311.html
Gray S, Cilia M, Ghanim M (2014) Circulative,“nonpropagative” virus transmission: an orchestra of virus-, insect-, and plant-derived instruments. Adv Virus Res 89:141–199
He WB, Li J, Liu SS (2015) Differential profiles of direct and indirect modification of vector feeding behaviour by a plant virus. Sci Rep 5:7682
Henneberry TJ, Jech LJ, De La Torre TM (2002) Effects of cotton plant water stress on Bemisia tabaci strain B (Homoptera: Aleyrodidae) honeydew production. Southwest Entomol 27:117–133
Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, Chiel E, Duckworth VE, Dennehy TJ, Zchori-Fein E, Hunter MS (2011) Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332:254–256
Hong HJ, Yang YH, Wang LD (2011) Repellence and feeding deterrence of Verticillium lecanii toxic-VII against Bemisia tabaci. Chin J Appl Entomol 48(1):60–64 (in Chinese, with English abstract)
Horowitz A, Antignus Y, Gerling D (2011) Management of Bemisia tabaci Whiteflies. Springer, Dordrecht
Hu J, De Barro P, Zhao H, Wang J, Nardi F, Liu SS (2011) An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PLoS One 6:e16061
Huang YX, Yang NW, Qin Y, An F, Li ZH, Wan FH (2016) Enhanced stability in host–parasitoid interactions with autoparasitism and parasitoid migration. J Theor Biol 393:43–50
Hunter MS, Kelly SE (1998) Hyperparasitism by an exoticautoparasitoid: secondary host selection and the window of vulnerability of conspecific and native heterospecific hosts. Entomol Exp Appl 89:249–259
Ji XZ (2015) Oviposition and feeding choice of the tabacco whitefly, Bemisia tabaci (Gennadius) on different cucumber strains. Chinese Agricultural University, Master thesis (in Chinese, English abstract)
Jiu M, Zhou XP, Tong L, Xu J, Yang X, Wan FH, Liu SS (2007) Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS One 2:e182
Keyimu M, Xu HX, Xian JH, Li J, Wang HQ, Hu AZ, Ma DY (2014) Control effect evaluation of five environmental friendly insecticides against Bemisia tabaci MEAM1 cryptic species. Xinjiang Agric Sci 51(6):1137–1142 (in Chinese, with English abstract)
Koch RL (2003) The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J Insect Sci 3:32
Li SJ, Xue X, Ahmed MZ, Ren SX, Du YZ, Wu JH, Cuthbertson AGS, Qiu BL (2011) Host plants and natural enemies of Bemisia tabaci (Hemiptera: Aleyrodidae) in China. Insect Sci 18:101–112
Li S, Lao SB, Wang S, Guo XJ, Zhang F (2014a) Control effect of Orius sauteri collaborated with Encarsia formosa on Bemisia tabaci in the greenhouse. J Environ Entomol 36(6):978–982 (in Chinese, with English abstract)
Li R, Weldegergis BT, Li J, Jung C, Qu J, Sun Y, Qian H, Tee C, van Loon JJ, Dicke M, Chua NH, Liu SS, Ye J (2014b) Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell 26:4991–5008
Liu SS, De Barro PJ, Xu J, Luan JB, Zang LS, Ruan YM, Wan FH (2007) Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 318:1769–1772
Liu SS, Colvin J, De Barro P (2012) Species concepts as applied to the whitefly Bemisia tabaci systematics: how many species are there? J Integr Agric 11:176–186
Lu ZC, Wan FH (2008) Differential gene expression in whitefly (Bemisia tabaci) B-biotype females and males under heat-shock condition. Comp Biochem Physiol Part D Genomics Proteomics 3:257–262
Luan JB, Li JM, Varela N, Wang YL, Li FF, Bao YY, Zhang CX, Liu SS, Wang XW (2011) Global analysis of the transcriptional response of whitefly to Tomato yellow leaf curl China virus reveals the relationship of coevolved adaptations. J Virol 85:3330–3340
Luan JB, Wang YL, Wang J, Wang XW, Liu SS (2013a) Detoxification activity and energy cost is attenuated in whiteflies feeding on Tomato yellow leaf curl China virus-infected tobacco plants. Insect Mol Biol 22:597–607
Luan JB, Yao DM, Zhang T, Walling LL, Yang M, Wang YJ, Liu SS (2013b) Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol Lett 16:390–398
Luan JB, Chen W, Hasegawa DK, Simmons AM, Wintermantel WM, Ling KS, Fei Z, Liu SS, Douglas AE (2015) Metabolic coevolution in the bacterial symbiosis of whiteflies and related plant sap-feeding insects. Genome Biol Evol 7:2635–2647
Luo C, Yao Y, Wang R (2002) The use of mitochondrial cytochrome oxidase I (mtCO I) gene sequences for the identification of biotypes of Bemisia tabaci (Gennadius) in China. Acta Entomol Sin 45:759–763
Luo C, Jones C, Devine G, Zhang F, Denholm I (2010) Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China. Crop Prot 29:429–434
Mahadav A, Kontsedalov S, Czosnek H, Ghanim M (2009) Thermotolerance and gene expression following heat stress in the whitefly Bemisia tabaci B and Q biotypes. Insect Biochem Mol Biol 39:668–676
Mascarin GM, Kobori NN, Quintela ED, Arthurs SP, Delalibera I (2014) Toxicity of non-ionic surfactants and interactions with fungal entomopathogens toward Bemisia tabaci biotype B. Biocontrol 59(1):111–123
Naranjo SE, Castle SJ, De Barro PJ, Liu SS (2010) Population dynamics, demograpgy, dispersal and spread of Bemisia tabaci. In Bemisia: Bionomics and Management of a Global Pest, pp 185–226. Springer
Nombela G, Beitia F, Muniz M (2001) A differential interaction study of Bemisia tabaci Q-biotype on commercial tomato varieties with or without the Mi resistance gene, and comparative host responses with the B-biotype. Entomol Exp Appl 98:339–344
Nuessly GS, Meyerdirk DE, Coudriet DL, Henneberry TJ (1994) The effect of short season cotton production schedules on Bemisia tabaci (Gennadius). Southwest Entomol 19:209–217
Pan H, Chu D, Yan W, Su Q, Liu B, Wang S, Wu Q, Xie W, Jiao X, Li R, Yang N, Yang X, Xu B, Brown JK, Zhou X, Zhang Y (2012) Rapid spread of tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS One 7:e34817
Pan H, Preisser E, Chu D, Wang S, Wu Q, Carriére Y, Zhou X, Y Z (2015) Insecticides promote viral outbreaks by altering herbivore competition. Ecol Appl 25: 1585–1595
Qiu BL, Ren SX (2006) Using yellow sticky traps to inspect population dynamics of Bemisia tabaci and its parasitoids. Chin Bull Entomol 43(1):53–56. in Chinese, English abstract
Qiu BL, Ren SX, Wen S, Mandour N (2003a) Biotype identification of the populations of Bemisia tabaci (Homoptera: Aleyrodidae) in China using RAPD-PCR. Acta Entomol Sin 46:605–608
Qiu BL, Ren SX, Xiao Y, Mandour NS (2003b) Effectiveness of Eretmocerus sp. and Aschersonia aleyrodis in controlling Bemisia tabaci pooulations. Chin J Appl Ecol 14(12):2251–2254 (in Chinese, with English abstract)
Rao Q, Rollat-Farnier PA, Zhu DT, Santos-Garcia D, Silva FJ, Moya A, Latorre A, Klein CC, Vavre F, Sagot MF, Liu SS, Mouton L, Wang XW (2015) Genome reduction and potential metabolic complementation of the dual endosymbionts in the whitefly Bemisia tabaci. BMC Genomics 16:226
Ren SX, Wang ZZ, Qiu BL, Xiao Y (2001) The pest status of Bemisia tabaci in China and non-chemical control strategies. Entomol Sin 8(3):279–288
Ren SX, Qiu BL, Ge F, Zhang YJ, Du YZ, Chen XX, Guo JY, Lin KJ, Peng ZQ, Yao SL, Hu YH, Wang LD, Zhang WQ (2011) Research progress of the monitoring, forecast and sustainable management of whitefly pests in China. Chin J Appl Entomol 48(1):7–15 (in Chinese, English abstract)
Ren SX, Liu TX, Du YZ, Peng ZQ, Qiu BL, Ge F (2014) Systematic investigation and monitoring of whitefly pests in a vegetable ecosystem. Chin J Appl Entomol 51(3):859–862 (in Chinese, English abstract)
Roberts PA, Thomason IJ (1986) Variability in reproduction of isolates of Meloidogyne incognita and M. javanica on resistant tomato genotypes. Plant Dis 70:547–551
Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VM (1998) The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci U S A 95:9750–9754
Shen BB, Ren SX, Musa PH, Chen C (2005) A study on economic threshold of Bemisia tabaci. Acta Agric Univ Jiangxiensis, 27(2):234–237 (in Chinese, English abstract)
Shi X, Pan H, Xie W, Wu Q, Wang S, Liu Y, Fang Y, Chen G, Gao X, Zhang Y (2013) Plant virus differentially alters the plant’s defense response to its closely related vectors. PLoS One 8:e83520
Sun D, Liu Y, Qin L, Xu J, Li F, Liu S (2013a) Competitive displacement between two invasive whiteflies: insecticide application and host plant effects. Bull Entomol Res 103:344–353
Sun HL, Zhou FQ, You XY, Hu RL, Lv M, Wu L, Zhu SD (2013b) The selectivity of Q-biotype Bemisia tabaci for twenty varities of eggplang, Solanum melongena. Plant Prot 39(2):67–71 (in Chinese, English abstract)
Sun D, Li J, Liu Y, Crowder D, Liu S (2014) Effects of reproductive interference on the competitive displacement between two invasive whiteflies. Bull Entomol Res 104:334–346
Tan XL, Hu NN, Zhang F, Ramirez-Romero R, Desneus N, Wang S, Ge F (2016) Mixed release of two parasitoids and a polyphagous ladybird as a potential strategy to control the tobacco whitefly Bemisia tabaci. Sci Rep-UK 6:28245
Teng X, Wan F, Chu D (2010) Bemisia tabaci biotype Q dominates other biotypes across China. Fla Entomol 93:363–368
Tian J, Hao C, Liang L, Ma RY (2014) Effects of temperature and relative humidity on conidial germination of Isaria fumosorosea (Hypocreales: Cordycipitaceae) IF-1106 and pathogenicity of the fungus against Bemisia tabaci (Homoptera: Aleyrodidae). Mycosystema 33(3):668–679
Tian XQ, Li N, Liu XC, Xu YJ, Zhang SZ (2015) The occurrence and integrated pest management of Bemisia tabaci on vegetables. Shaanxi J Agric Sci 61(1):123–126 (in Chinese, English abstract)
Wang H (2007) Study on the ecology and physiology traits of Bemisia tabaci on three brassica vegetables and abutilon and the management strategy. Yangzhou University, Master thesis (in Chinese, English abstract)
Wang LD, Huang J, Liu B (2006) Assessment of the control effectiveness of insecticidal toxins from Verticillium lecanii on the population of Bemisia tabaci (Gennadius) in greenhouse Assessment of the control effectiveness of insecticidal toxins from Verticillium lecanii on the population of Bemisia tabaci (Gennadius) in greenhouse. Acta Ecol Sin 26(2):391–398
Wang Z, Yao M, Wu Y (2009) Cross-resistance, inheritance and biochemical mechanisms of imidacloprid resistance in B-biotype Bemisia tabaci. Pest Manag Sci 65:1189–1194
Wang XW, Luan JB, Li JM, Bao YY, Zhang CX, Liu SS (2010a) De novo characterization of a whitefly transcriptome and analysis of its gene expression during development. BMC Genomics 11:400
Wang Z, Yan H, Yang Y, Wu Y (2010b) Biotype and insecticide resistance status of the whitefly Bemisia tabaci from China. Pest Manag Sci 66:1360–1366
Wang XW, Luan JB, Li JM, Su YL, Xia J, Liu SS (2011) Transcriptome analysis and comparison reveal divergence between two invasive whitefly cryptic species. BMC Genomics 12:458
Wang XW, Zhao QY, Luan JB, Wang YJ, Yan GH, Liu SS (2012) Analysis of a native whitefly transcriptome and its sequence divergence with two invasive whitefly species. BMC Genomics 13:529
Wang YL, Wang YJ, Luan JB, Yan GH, Liu SS, Wang XW (2013) Analysis of the transcriptional differences between indigenous and invasive whiteflies reveals possible mechanisms of whitefly invasion. PLoS One 8:e62176
Wang LL, Wang XR, Wei XM, Huang H, Wu JX, Chen XX, Liu SS, Wang XW (2016) The autophagy pathway participates in resistance to Tomato yellow leaf curl virus infection in whiteflies. Autophagy 12(9):1560–1574
Wei J, Zhao JJ, Zhang T, Li FF, Ghanim M, Zhou XP, Ye GY, Liu SS, Wang XW (2014) Specific cells in the primary salivary glands of the whitefly Bemisia tabaci control retention and transmission of begomoviruses. J Virol 88:13460–13468
Wu L (2013) Studies on resistance of different pepper varieties to Bemisia tabaci (Gennadius) and its physiological and biochemical mechanism. Yangzhou University, Master thesis (in Chinese, English abstract)
Wu S, Wang Z, Wu Y (2010) Competition between the B and Q biotypes of Bemisia tabaci and its relevance to insecticide resistance. Chin Bull Entomol 47:1118–1121
Xia J, Zhang CR, Zhang S, Li FF, Feng MG, Wang XW, Liu SS (2013) Analysis of whitefly transcriptional responses to Beauveria bassiana infection reveals new insights into insect-fungus interactions. PLoS One 8(7):e68185
Xie W, Liu Y, Wang S, Wu Q, Pan H, Yang X, Guo L, Zhang Y (2014) Sensitivity of Bemisia tabaci (Hemiptera: Aleyrodidae) to several new insecticides in china: effects of insecticide type and whitefly species, strain, and stage. J Insect Sci 14:261
Xu J, De Barro PJ, Liu SS (2010a) Reproductive incompatibility among genetic groups of Bemisia tabaci supports the proposition that the whitefly is a cryptic species complex. Bull Entomol Res 100:359–366
Xu R, Li W, Zhang LF, Lin YH, Qi B, Xing H (2010b) A study on the inheritance of resistance to whitefly in Soybean. Sci Agric Sin 43(1):80–86 (in Chinese, English abstract)
Xu J, Lin KK, Liu SS (2011) Performance on different host plants of an alien and an indigenous Bemisia tabaci from China. J Appl Entomol 135(10):771–779
Xu HX, Hong Y, Zhang MZ, Wang YL, Liu SS, Wang XW (2015a) Transcriptional responses of invasive and indigenous whiteflies to different host plants reveal their disparate capacity of adaptation. Sci Rep 5:10774
Xu HY, Yang NW, Wan FH (2015b) Field cage evaluation of interspecific interaction of two aphelinid parasitoids and biological control effect on Bemisia tabaci (Hemiptera: Aleyrodidae) Middle East-Asia Minor 1. Entomol Sci 18:237–244
Xu HY, Yang NW, Duan M, Wan FH (2016) Functional response, host stage preference and interference of two whitefly parasitoids. Insect Sci 23:134–144
Yang NW, Wan FH (2011) Host suitability of different instars of Bemisia tabaci biotype B for the parasitoid Eretmocerus hayati. Biol Control 59:313–317
Yang NW, Ji LL, Lövei GL, Wan FH (2012) Shifting preference between oviposition vs. host-feeding under changing host densities in two Aphelinid parasitoids. PLoS One 7:e41189
Yang N, Xie W, Jones CM, Bass C, Jiao X, Yang X, Liu B, Li R, Zhang Y (2013a) Transcriptome profiling of the whitefly Bemisia tabaci reveals stage-specific gene expression signatures for thiamethoxam resistance. Insect Mol Biol 22:485–496
Yang N, Xie W, Yang X, Wang S, Wu Q, Li R, Pan H, Liu B, Shi X, Fang Y, Xu B, Zhou X, Zhang Y (2013b) Transcriptomic and proteomic responses of sweetpotato whitefly, Bemisia tabaci, to thiamethoxam. PLoS One 8:e61820
Yang NW, Zang LS, Wang S, Guo JY, Xu HX, Zhang F, Wan FH (2014) Biological pest management by predators and parasitoids in the greenhouse vegetables in China. Biol Control 68:92–102
Yao FL, Zheng Y, Zhao JW, Desneux N, He YX, Weng QY (2015) Lethal and sublethal effects of thiamethoxam on the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae) through different exposure routes. Chemosphere 128:49–55
Ye XD, Su YL, Zhao QY, Xia WQ, Liu SS, Wang XW (2014) Transcriptomic analyses reveal the adaptive features and biological differences of guts from two invasive whitefly species. BMC Genomics 15:370
Zhang ZL (2000) Some thoughts to the outbreaks of tobacco whitefly. Beijing Agri Sci:1–3
Zhang GF, Lü ZC, Wan FH (2007a) Detection of Bemisia tabaci remains in predator guts using a sequencecharacterized amplified region marker. Entomol Exp Appl 123:81–90
Zhang GF, Lü ZC, Wan FH, Lövei GL (2007b) Real-time PCR quantification of Bemisia tabaci (Homoptera: Aleyrodidae) B-biotype remains in predator guts. Mol Ecol Notes 6:947–954
Zhang T, Luan JB, Qi JF, Huang CJ, Li M, Zhou XP, Liu SS (2012) Begomovirus- whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Mol Ecol 21:1294–1304
Zhang YB, Yang NW, Zhang GF, Wang JJ, Wan FH (2015) Identification of naturally occurring Eretmocerus parasitoids by CO I sequence analysis and crossing test. J Environ Entomol 37(2):281–292 (in Chinese, English abstract)
Zhu DT, Xia WQ, Rao Q, Liu SS, Ghanim M, Wang XW (2016) Sequencing and comparison of the Rickettsia genomes from the whitefly Bemisia tabaci Middle East Asia Minor I. Insect Sci 23:531–542
Zou CH, Li L, Dong TY, Zhang BW, Hu QB (2014) Joint action of the entomopathogenic fungus Isaria fumosorosea and four chemical insecticides against the whitefly Bemisia tabaci. Biocontrol Sci Techn 24(3):315–324
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Wang, X., Yang, N. (2017). The Whitefly Bemisia tabaci (Gennadius). In: Wan, F., Jiang, M., Zhan, A. (eds) Biological Invasions and Its Management in China. Invading Nature - Springer Series in Invasion Ecology, vol 11. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-0948-2_8
Download citation
DOI: https://doi.org/10.1007/978-94-024-0948-2_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-0946-8
Online ISBN: 978-94-024-0948-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)