Abstract
Oak hybridization have important effects on the structure of herbivorous insect communities and associated natural enemies. We tested the effects of hybridization between Q. magnoliifolia and Q. resinosa on insect gallers trophic networks and their parasitoids. We characterized the genotypes of 35 individuals of Q. magnoliifolia, 30 of Q. resinosa, and 57 hybrids using eight nuclear microsatellite markers. We collected 6,798 galls from the oak hybrid complex distributed in 33 gall morphospecies on Q. magnoliifolia, 28 on Q. resinosa, and 42 on hybrid oaks. Galler-parasitoid networks were realized by 21 gall morphospecies and 21 parasitoid species for Q. magnoliifolia; 16 gall morphospecies and 30 parasitoid species for Q. resinosa; and 25 gall morphospecies and 23 parasitoid species for hybrids. Plant-galler networks were different among three oak groups, having the hybrid network higher values of diversity of interactions, nestedness and modularity and lower values of specialization than Q. magnoliifolia and Q. resinosa networks. Hybrid network of gallers and parasitoids had higher diversity of interactions, connectance and generality and lower modularity than Q. magnoliifolia and Q. resinosa networks. Hybrids are more vulnerable to insect galler incidence having low pressure by parasitoids, which allow more gall incidence in hybrid plants. Our study corroborated that hybridization generates changes in oak genetic composition influencing insect gallers trophic networks and their parasitoids. Our findings are also consistent with the rule of genetic similarity which suggest a relationship between plant genetics and the associated arthropod community, where genetically similar plants support similar arthropod communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the mechanisms that determine community organization is one of the main goals of ecology (Loreau et al. 2001; Cronin et al. 2020). Ecological interactions constitute one of these mechanisms, since the nature of species interactions and their persistence in time and space shape the structure and stability of communities (Elsadany et al. 2012; Fagundes et al. 2018). Species interactions can be represented as complex ecological networks, in which each species generally interacts with other species within and between trophic levels (Waser and Ollerton 2006; Tylianakis and Morris 2017). Therefore, ecological network theory provides an approach to understand multi-species interactions in complex systems by representing species as nodes and interactions as linkages, and then estimating different network metrics (Jordano et al. 2009).
During the last two decades, this approach has allowed a considerable increase in our understanding of the patterns related to the organization and stability of natural communities (Tylianakis and Morris 2017; Landi et al. 2018). Because quantitative trophic networks incorporate information about the frequency and abundance of interactions (Alhmedi et al. 2011), they provide a clear functional description of community structure (Delmas et al. 2019; Oliveira et al. 2019). Most studies of trophic networks have focused on mutualistic interactions, mainly pollination (Welti and Joern 2015; Losapio et al. 2019), seed dispersal (Donatti et al. 2011; Sebastian-Gonzalez et al. 2015), as well as mutualisms between plant and ants (Rico-Gray et al. 2012; Staab et al. 2017). However, fewer studies of trophic networks have focused on antagonistic interactions (Kaartinen and Roslin 2012; Oliveira et al. 2019). For example, recent evidences indicate that intraspecific genetic variation can affect the topology of trophic networks (Barbour et al. 2016), but little is known about the effects of plant hybridization on the structure of the trophic network of associated insects, especially those that include three trophic levels (Lau et al. 2016; Keith et al. 2017).
Hybridization is a natural phenomenon defined as the successful mating between individuals belonging to populations that differ in at least one heritable character (Martinsen et al. 2001). Hybridization frequently occurs in many plant groups and is considered an important evolutionary mechanism with a suite of possible outcomes, including the transference of adaptive variation between species and the production of novel adaptations (Carson and Dowling 2006; Cheng et al. 2011), and the modification of genetic variation and diversification patterns (Soltis and Soltis 2009). Plant hybridization can also have important consequences for associated organisms, since genetic differences among parental plant species and hybrids can determine variation in the composition and structure of their insect herbivore community, mutualists, predators and parasitoids (Lau et al. 2016; Pérez-López et al. 2016; Maldonado-López et al. 2018). For example, some studies show that intraspecific genetic variation in multiple plant traits can be related to variation in the community structure of diverse organisms, from microbes to vertebrates (Whitham et al. 2012; Keith et al. 2017). Therefore, trophic networks can provide a useful approach to quantify the consequences of plant hybridization on the community of associated insect herbivores and their natural enemies.
The genus Quercus (Fagaceae) is a highly diverse group of woody plants with temperate origin known for a high frequency of interspecific hybridization (González-Rodríguez et al. 2004; Cavender-Bares and Pahlich 2009). The genus includes 300–600 species distributed throughout the northern hemisphere (Jones 1986). Mexico is a main center of diversification with approximately 161 species, 109 of which are endemic (Valencia 2004; Hipp et al. 2018). Oak species harbor a great diversity of insect herbivores (Stone et al. 2002; Maldonado-López et al. 2018), including cynipid gall wasps (Hymenoptera: Cynipidae) (Pérez-López et al. 2016). More than 80% of the approximately 1000 species of cynipid gall wasps are associated with oak species (Stone et al. 2002). A recent multiple-taxonomic comparison of plant-galler interactions showed that cynipids are involved in complex networks of interactions with their host plants, with many species occurring over different species of oak trees (Araújo et al. 2019).
Gall induction represents a complex developmental process resulting from a stimulation of injected fluids by female wasps during oviposition, or by secretions of saliva by larvae during feeding (Dreger-Jauffret 1992). This chemical interaction generates hypertrophy (i.e., abnormal growth of cells) and hyperplasia (i.e., abnormal multiplication of cells) that lead to the abnormal structures of plant tissues called galls, where wasp insects spend most of their life cycle (Stone and Schönrogge 2003). Each wasp species induces a particular and distinct gall morphology, which is mainly controlled by the insect (Maldonado-López et al. 2016; Pérez-López et al. 2016; Coutinho et al. 2019). In general, cynipid gall wasps are highly specific to their host plants and can discriminate between closely related hosts (Evans et al. 2012). Besides, some studies have reported the presence of super hosts (i.e., plants that support several gall-inducing species) as result of historical and ecological traits (e.g., wide geographical distribution) (Mendonça 2007; Fagundes et al. 2020). Phylogenetic proximity and consequently genetic, phytochemical, and morphological similarity may lead some species of insect gallers to be able to use closely related host plant species (Coutinho et al. 2019). In the case of the cynipid-oak system, there is evidence that cynipid galler wasp species are able to colonize different host species (Araújo et al. 2019). These results lead to the expectation that hybridization in oaks may be an important factor to increase the sharing of cynipid gall wasps between species, as well as to enhance the colonization of insect gallers in new oak hosts.
On the other hand, natural enemies such as predators and parasitoids cause high mortality on cynipid gall wasp species associated with oak species (Schönrogge et al. 2006; Chust et al. 2007) acting as top-down forces regulating the structure of the cynipid community (Cooper and Rieske 2010). Particularly, parasitoids of cynipids (i.e., families Ichneumonidae, Braconidae, and Chalcidoidea) are responsible for 40–100% of mortality of cynipids in natural communities (Stone et al. 2002; Hayward and Stone 2005) and vary in their host specificity, with a large number of polyphagous species and a limited number of specialist parasitoids (Askew 1984). Some studies suggest that both specialist and generalist parasitoids differentially influence food webs (Schönrogge and Crawley 2000; Stone et al. 2002). In fact, specialist parasitoids have little influence on trophic webs because they attack few insect galler species, while generalist parasitoids can have larger effects as a result of both killing insect gallers and generating competition with other parasitoid species (Bailey et al. 2009, Lepais and Gerber 2011). Parasitoids of cynipid gall wasps and gall midges are also divided into early attacker and late attacker, based on the ontogenetic moment they colonize galls (Stone et al. 2002). However, little is known about tri-trophic interactions that include plants, galls, and parasitoids in temperate ecosystems, and even less in hybrid oak complexes.
In Mexico, the formation of hybrid zones between Quercus species with different degrees of relatedness (Pérez-López et al. 2016; Cuevas-Reyes et al. 2018; Maldonado-López et al. 2018) offers an excellent opportunity to analyze the effects of host-plant genetic variation on the trophic webs in natural communities. Therefore, the goal of this study was to examine the effects of hybridization in a complex of two Mexican white oaks, Q. magnoliifolia and Q. resinosa on the trophic networks of cynipid gall wasps and their parasitoids at a local scale in the Tequila Volcano, Mexico. We describe the structure of trophic networks using network measures of species diversity (number of species at upper and lower trophic level) and interactions (diversity of interactions, connectance, specialization, generality, and vulnerability) and arrangement of interactions (modularity and nestedness). The specific questions addressed were as follows: (i) Does the structure of trophic networks of cynipid gall wasps and their parasitoids differ between parental and hybrid plants in the hybrid oak complex Quercus magnoliifolia x Quercus resinosa? (ii) Does the specificity of parasitoids to cynipid gall wasp species differs in the trophic networks of parental and hybrid plants? We expect that due to the sharing of species and interactions between parental plants, hybrid individuals should form a network more diverse in species and interactions and less specialized (i.e., more connected, less modular, and more nested) than the networks of Quercus magnoliifolia and Quercus resinosa plants. Similarly, we hypothesized that the specificity of the parasitoids must be greater in the networks of parental plants than in the network of hybrid oaks.
Materials and methods
Study system
This study was conducted at the Tequila volcano, Jalisco state, Mexico (20°50’ N, 103°5’ W). In this volcano, Quercus magnoliifolia occurs between 1400 and 1800 m a.s.l. (Pérez-López et al. 2016). This is a tree which can reach up to 25 m in height, with obovate leaves, lustrous and almost glabrous on the adaxial surface, while the abaxial surface is tomentose with glabrescent petioles (Arizaga et al. 2009). Quercus resinosa is a tree growing up to 10 m and it is distributed from 1700 to 2100 m of elevation (Pérez-López et al. 2016). The leaf shape is obovate, rugose on the adaxial surface, and tomentose on the abaxial surface (Arizaga et al. 2009). A hybrid zone has been reported previously to occur between these two oak species, with hybrids being present at the whole altitudinal gradient but being more abundant between 1600 and 1800 m (Albarrán-Lara et al. 2010, 2019; Pérez-López et al. 2016). Previous morphometric analyses have shown that the two species are clearly differentiated and that hybrid individuals have an overall intermediate leaf shape (Albarrán-Lara et al., 2010; 2019).
Sampling design
Galls were collected from 50 individuals of the Q. magnoliifolia x Q. resinosa complex located between 1400 and 1500 m a.s.l. at the Tequila Volcano, 50 individuals between 1600 and 1800 m, and 50 individuals between 1900 and 2100 m. At each of these altitudinal intervals, Q. magnoliifolia, hybrids, and Q. resinosa trees are, respectively, more abundant (Albarrán-Lara et al. 2010; Pérez-López et al. 2016). The genetic assignment of each individual tree into genotypic classes (i.e., pure parental of the two species and hybrids) was performed using eight nuclear microsatellites that previously have been shown to have enough discriminatory power (see Albarrán-Lara et al. 2010; Pérez-López et al. 2016).
To collect galls, a systematic stratified sampling design was used by collecting three branches of similar length at each level of the crown of each tree: lower, medium, and upper (Maldonado-López et al. 2015). Each individual tree was permanently marked with aluminum tags. Samplings were performed monthly from July to February in two consecutive years (2011–2012 and 2012–2013). In each sampling year, we collected different branches from each tree marked of the Q. magnoliifolia x Q. resinosa complex. Cynipid species display alternation of generations in the same year, where the sexual generation occurs in the spring and the asexual generation in the summer and autumn (Stone et al. 2002; Maldonado-López et al. 2016). In the Tequila volcano, the dry season where the oaks lose their leaves is from March to June (i.e., spring), while the oaks maintain their leaves during July to February (i.e., summer and autumn) (Hernández-Calderón et al. 2013). Therefore, our sampling only included the asexual generation of the cynipid galls. In the field, all leaf and branch galls were separated and placed in plastic containers covered with tulle mesh and transported to the laboratory to wait for the emergence of the adult gall inducers and parasitoids. In the laboratory, leaf and branch galls were reared independently at a temperature of 20–23 °C in plastic cups covered with microfabric to allow air circulation. The insects that emerged from the galls were stored in 70% alcohol for taxonomic identification (i.e., parasitoid insects) using the taxonomic keys of Graham (1969), Borror et al. (1976), Graham and Gijswijt (1998 and Gibson et al. (1997). Galls were assigned to morphospecies according to their external morphology (Gagné 1994; Pérez-López et al. 2016). The use of morphospecies is a reliable approach to estimate the diversity and structure of insect galler communities, since usually each species of insect induces a gall with a unique morphology (Araújo et al. 2013; Pérez-López et al. 2016). However, whenever possible, the adult insect gallers were kept in ethanol as vouchers.
Comparing communities of insect gallers and parasitoid cynipids between oaks
To test for differences in communities of insect gallers and cynipid parasitoids among the three groups of oaks (Q. resinosa, Q. magnoliifolia, and hybrids), we performed a Non-Parametric Multivariate Analysis of Variance (NPMANOVA) based on an abundance matrix of insect gallers and parasitoids found in oaks. In addition, we estimated the Jaccard similarity index, using the EstimateSWin820 program (Colwell 2009), to compare the similarity of species composition between the three oak categories for both insect gallers and parasitoids. The richness of the parasitoid species present in each of the three oak groups with galls was obtained by rarefaction curves using the program EstimateS 9.1.0 (Colwell 2013). Because the number of plants with galls in the three oak groups was different, the richness species value was standardized (Gotelli and Colwell 2001). The scale of the independent variable (X) was represented by the accumulated number of plants with galls.
Trophic networks
Quantitative trophic networks (based on abundance) were constructed for the three oak groups (Q. magnoliifolia, Q. resinosa, and hybrids) according to the genetic assignment analysis as the first trophic level, insect galler species as the second trophic level, and the parasitoid species as the third trophic level. Metrics describing the topology of the networks were calculated for each genotypic class (Q. magnoliifolia, Q. resinosa, and hybrids) in two bipartite networks. The first network analyzed the interactions among the individuals in each oak genotypic class with gall morphospecies, and the second network analyzed the interactions among the gall morphospecies and parasitoids. Interactions in the bipartite networks were quantified considering the total abundance of gall morphospecies and their parasitoids in the community, respectively.
To characterize the trophic structure of the networks, we used the following network descriptors: diversity, connectance, specialization, vulnerability, generality, modularity, and nestedness. We calculated the diversity index of interactions based on the Shannon index (Kaartinen and Roslin 2012). Connectance was calculated as the proportion of possible interactions that are realized in the network (Dormann et al. 2009). Network specialization was obtained using the index H2, which can take values from 0 (absolute generalization) to 1 (absolute specialization). To determine the average number of oak categories attacked (i.e., parental plants and hybrid plants) by each insect galler species and the average number of gall morphospecies attacked by each parasitoid species, we used the generality metric (Jordano 1987). Similarly, to evaluate the average number of insect gallers that attack each oak individual and the average number of parasitoids that attack each gall morphospecies, we used the vulnerability measure (Jordano, 1987). We calculated the modularity index in order to identify, within a trophic network, the presence of species groups/modules of a particular trophic level that interact more frequently with another group of species from another trophic level (Marquitti et al. 2014). This index takes values from 0 to 1, with higher values indicating the existence of modules or semi-independent sets of interactors within the general network (Dormann et al. 2009). In each subnetwork, the distribution of species interactions was determined by calculating the nesting index using the NODF metric based on overlap and decreasing fill (Almeida-Neto et al. 2008) that identifies the species with the highest number of interactions at each trophic level (i.e., hyper-connected species, generalist-generalist, generalist-specialist and specialist-specialist interactions) (Almeida-Neto et al. 2008).
In order to test the significance of network descriptors obtained for the different bipartite networks we used 500 simulated networks generated by null models (Dormann and Strauss 2014). Null models were generated using the quasiswap algorithm (Dormann et al. 2009). All network analyses were performed in the BIPARTITE package (Dormann et al. 2008) on R software (ver. 2.8.1). Visual representations of network structure were constructed with Pajek 3.10 (Batagelj and Mrvar 1998).
Finally, in each network, the species were categorized as generalist species nucleus (i.e., generalist species with many interactions) and peripheral species (i.e., selective species with few interactions) to assess the replacement of species between the insect gallers and parasitoids within each subnetwork in each host oak category (Dáttilo et al. 2014).
Results
General results
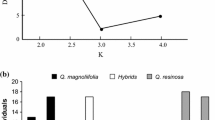
According to the genetic analyses, 35 oak individuals were assigned to Q. magnoliifolia, 30 to Q. resinosa, and 57 were genetically intermediate (hybrids) (see Pérez-López et al. 2016). We had failures in the amplification of some microsatellite loci of 28 of the oak individuals collected that were therefore not assigned to a category.
In total, we collected 6,798 cynipid galls from the hybrid complex Q. magnoliifolia x Q. resinosa. Of these, we identified a total of 33 gall morphospecies on Q. magnoliifolia, 28 on Q. resinosa, and 42 on hybrid oaks. Additionally, we reared over 2700 parasitoids belonging to 45 different species, 19 genera and seven families (Eurytomidae, Eulophidae, Ormyridae, Torymidae, Pteromalidae, Eupelmidae, and Encyrtidae).
We found a total of 20 parasitoid species associated with galls of Q. magnoliifolia, 30 with galls of Q. resinosa, and 22 with galls of hybrids oaks. The rarefaction curve suggested that we reached a high level of completeness with a standard sample size of 30 plants. Moreover, the rarefaction curves also suggested that the richness of gall parasitoid species was higher in Q. resinosa than in hybrids and Q. magnoliifolia (Fig. 1).
The results of NPMANOVA indicated that communities of insect gallers change among oaks plants (F = 3.645, P < 0.001), with the post hoc pairwise comparisons showing that insect galler communities varied significantly among all oak categories (P < 0.05). The communities of insect galler parasitoids also change among oak categories (F = 4.1, P < 0.001). However, results of post hoc pairwise NPMANOVA showed that the communities of insect gallers varied significantly only between Q. magnoliifolia and Q. resinosa (P < 0.001), and between hybrids oaks and Q. resinosa (P = 0.007).
The Jaccard similarity index showed that Q. resinosa individuals had a greater similarity of insect galler species (51%) and parasitoids (56%) with the hybrids. While the Q. magnoliifolia individuals had a similarity of 46% in insect galler species and 36% in parasitoid species with the hybrids. The similarity in the composition of insect galler species and parasitoids between Q. magnoliifolia and Q. resinosa were 19% and 50%, respectively.
Plant–insect galler networks
The plant–insect galler networks were composed by 35 oak individuals and 33 insect galler species for Q. magnoliifolia, 30 oaks individuals and 28 insect galler species for Q. resinosa and 57 oak individuals and 42 insect galler species for hybrids. The network of Q. magnoliifolia had five exclusive species of insect gallers (15.2%), while Q. resinosa registered only one exclusive insect galler species (3.6%). For hybrid oaks there were seven exclusive insect galler species (16.6%).
Topological descriptors ranged greatly between Q. magnoliifolia, Q. resinosa, and hybrid networks (Table 1). The hybrid network had the highest diversity of interactions (5.583) when compared to the Q. magnoliifolia (5.021) and Q. resinosa (4.457) networks. Similarly, the hybrid network was more nested (30.468) than the Q. magnoliifolia (26.636) and Q. resinosa (29.159) networks. Network modularity also was higher for the hybrid network (0.510) than for the other two networks (0.372 and 0.429 for Q. magnoliifolia and Q. resinosa networks, respectively). On the other hand, specialization was lower for the hybrid network (0.000) than for the other networks (Q. magnoliifolia = 0.466 and Q. resinosa = 0.438). The connectance was higher for the Q. resinosa (16.4%) than for the Q. magnoliifolia (14.5%) and the hybrid (10.8%) networks. Both generality and vulnerability were higher for the hybrid network (14.879 and 7.045, respectively) than for the networks of Q. magnoliifolia (9.576 and 5.932, respectively) and Q. resinosa (7.080 and 4.778, respectively).
Null model comparisons showed that the observed values of diversity of interactions, connectance, nestedness, modularity, generality, and vulnerability of the oak hybrids network were higher than expected by chance (Table 1). On the other hand, the hybrid network had a lower value of specialization than expected by null models. For both magnoliifolia and Q. resinosa networks, null model values indicated less diverse, connected, nested, modular, general and vulnerable networks than expected by chance. The networks of the two parental oak species had higher modularity and specialization than null model expectation.
Insect galler-parasitoid networks
Interactions of insect gallers and parasitoids were realized by 21 gall morphospecies and 21 parasitoid species (with four exclusive parasitoid species = 19.0%) for Q. magnoliifolia; 25 insect gallers species and 23 parasitoid species (six exclusive parasitoids = 26.0%) for hybrids, and 16 insect gallers species and 30 parasitoid species (10 exclusive = 33.3%) for Q. resinosa.
The insect galler-parasitoid network of Q. magnoliifolia was structured by a nucleus of 11 generalist species of insect gallers (33.3%) that were interconnected with more than three different parasitoid species, and eight peripheral insect galler species that were parasitized by only one parasitoid species (24.0%) (Fig. 2). Of this generalist species nucleus of insect galler species, four were shared with the networks of hybrids and Q. resinosa (see Fig. 2, 3, 4). In the network of Q. resinosa, we detected six nucleus generalist insect galler species that were parasitized by at least three different parasitoid species (20.0%) (Fig. 3). We also observed 11 nucleus species of parasitoids (36.7%), eight peripheral parasitoid species that parasitized only one insect galler species (26.6%), and three parasitoid species associated with two insect galler species (10.0%) on Q. resinosa oaks (Fig. 3). For hybrid oaks, we detected 10 nucleus generalist species of galler insect species that were parasitized by at least three different parasitoid species (23.8%) (Fig. 4). We also observed 10 nucleus species of parasitoids (43.4%), eight peripheral parasitoid species that parasitized only one insect galler species (34.8%), and three parasitoid species associated with two insect galler species (13.0%) (Fig. 4).
Quantitative trophic network of Q. magnoliifolia. Line thickness represents the number of interactions (based on abundance). Insect galler morphospecies: Gray triangles: generalist species nucleus of insect gallers. White triangles: Insect gallers parasitized by a single parasitoid species. Black triangles: non-parasitized insect gallers. White triangles with lines: exclusive insect gallers to Q. magnoliifolia. Parasitoids: Gray squares: generalist species nucleus of parasitoids, White squares: Species that parasitized a single insect galler species (hyper-specialists). Black squares: Species that parasitized two insect galler species (specialists). White squares with lines: exclusive parasitoids to Q. magnoliifolia
Quantitative trophic network of Q. resinosa. Line thickness represents the number of interactions (based on abundance). Insect galler morphospecies: Gray triangles: generalist species nucleus of insect gallers. White triangles: Insect gallers parasitized by a single parasitoid species. Black triangles: non-parasitized insect gallers. White triangles with lines: exclusive insect gallers to Q. resinosa. Parasitoids: Gray squares: generalist species nucleus of parasitoids, White squares: Species that parasitized a single insect galler species (hyper-specialists). Black squares: Species that parasitized two insect galler species (specialists). White squares with lines: exclusive parasitoids to Q. resinosa
Quantitative trophic network of hybrids. Line thickness represents the number of interactions (based on abundance). Insect galler morphospecies: Gray triangles: generalist species nucleus of insect gallers. White triangles: Insect gallers parasitized by a single parasitoid species. Black triangles: non-parasitized insect gallers. White triangles with lines: exclusive insect gallers to hybrids. Parasitoids: Gray squares: generalist species nucleus of parasitoids, White squares: Species that parasitized a single insect galler species (hyper-specialists). Black squares: Species that parasitized two insect galler species (specialists). White squares with lines: exclusive parasitoids to hybrids
Insect galler-parasitoid networks of Q. magnoliifolia, Q. resinosa, and hybrid oaks differed in the network descriptors (Table 2). The hybrid network had higher diversity (0.136), connectance (13.6%), and generality (5.855) than Q. magnoliifolia (3.324, 10.4%, and 3.315, respectively) and Q. resinosa (4.901, 10.3%, and 3.743, respectively) networks. On the other hand, the hybrid network had lower modularity (0.385) compared to Q. magnoliifolia (0.627) and Q. resinosa (0.462) networks. The value of specialization was high for Q. magnoliifolia (0.685) and low for hybrid (0.487) and Q. resinosa (0.479) networks. On the other hand, values of nestedness and vulnerability were higher for the Q. resinosa network (34.207 and 8.108, respectively) than for hybrid (20.902 and 3.863) and Q. magnoliifolia (16.154 and 2.525). Compared to null models, observed values of diversity of interactions, connectance, nestedness, generality, and vulnerability were lower than expected by chance for Q. magnoliifolia, Q. resinosa, and hybrid networks (Table 2). On the other hand, all insect galler-parasitoid networks have higher values of modularity and specialization than expected by null models.
Discussion
Most of the cynipid gall wasps are associated with the genus Quercus and include more than 1400 described species (Ronquist and Liljeblad 2001), representing the largest evolutionary group of the Hymenoptera (Cynipoidea), which is dominated by parasitoids. Although a general consensus has been reached that galls are plant tissues induced by insects that provide shelter, food of high nutritional quality, protection from both, environmental fluctuations and natural enemies such as parasitoids (Stone and Schönrogge 2003; Ronquist et al. 2015), little is known about the effects of plant hybridization on community assemblages of antagonistic trophic networks of insect gallers and their parasitoids (Pérez- Nakamura et al. 2010; López et al. 2016. Our findings are relevant to understand the ecology and evolution of cynipid galler wasp assemblages on oak hybrid complexes, as well as the processes and mechanisms that shape and maintain insect diversity in the tree canopy of temperate species (Cuevas-Reyes et al. 2018).
In our study, according to genetic analyses, we identified three different oak groups that included individuals of Q. magnoliifolia, Q. resinosa, and hybrids. Regarding our fist research question, our results showed that insect galler communities, as well as parasitoid communities associated with them, were different among the three oak groups, suggesting that oak hybridization influences the tri-trophic networks in this oak hybrid complex at the Tequila Volcano. Particularly, plant–insect galler networks showed differences in the topological descriptors between Q. magnoliifolia, Q. resinosa, and hybrid networks, with the hybrid network having the highest diversity of interactions, as well as a greater nestedness and modularity than Q. resinosa and Q. magnoliifolia networks (see Table 1). A similar pattern was observed for the generality and vulnerability, these descriptors were higher for hybrid networks than for Q. magnoliifolia and Q. resinosa networks. These results suggest that hybrid oaks are more vulnerable to the occurrence of insect gallers (Pérez-López et al. 2016). This could be related to changes in genetic structure by the effect of the hybridization phenomenon (Rieseberg and Ellstrand 1993; Whitham et al. 1999; Cuevas-Reyes et al. 2018). Conversely, specialization (H2 index) was lower for hybrid networks in comparison with Q. resinosa and Q. magnoliifolia networks. This result indicates that different gall morphotypes are widely distributed among hybrid individuals, whereas, in parental plants, there is probably a segregation of morphotypes between different individuals.
Hybrid individuals represent a wide range of resources and conditions that can be exploited by their associated fauna (Fritz et al. 1999). This is due to increased resources, besides the high ecological and evolutionary activity that characterizes hybrid zones, as they may be generating new habitats for associated organisms (Tovar-Sánchez and Oyama 2006). Hybridization often results in new characteristics of the host plant, such as changes in foliar morphology (González-Rodríguez et al. 2004; Cuevas-Reyes et al. 2018), phenology (Hunter et al. 1997), architecture (Bangert et al. 2005), as well as secondary chemistry (Cheng et al. 2011). These characteristics may be associated with the preferences of insect herbivores, their development and distribution (Fritz et al. 1999; Hochwender and Fritz 2004; Bailey et al. 2009).
Contrasting patterns with regard to the effect of hybridization on plants and their influence on the insect herbivore diversity have been found in several studies, ranging from higher, intermediate, lower, or even no difference in the composition of herbivores in hybrid plants (Fritz 1996; Floate et al. 2016; Pérez-López et al. 2016; Cuevas-Reyes et al. 2018). To explain these findings, a theory of the trophic level interactions has been proposed, predicting that the genotype of the plant can affect the susceptibility of herbivores to their natural enemies, because genetic recombination by hybridization effect can generate the rupture or dominance of the inheritable characters associated with the defense and/or establishment of herbivores in the host plant through different trophic levels (Fritz 1992; Fritz et al. 1996).
Networks composed by host plants and insect gallers tend to be highly specialized due to a large incidence of specialist insect species (Araújo and Maia 2021). Previous studies suggest that networks of cynipids tend to be more connected than networks of other groups of galling insects, due to the high level of overlapping interactions of insect gallers over Quercus species (Araújo et al. 2019). However, our results show that the structure of the interactions of cynipids and oaks is not homogeneous, but varies between different species and oak hybrids. On the other hand, the results of the connections of insect galler-parasitoid networks are within the range of previous studies on antagonistic networks, ranging from 10 to 16% of average interactions present in each subnet (Hirao and Murakami 2008; Kaartinen and Roslin 2012). Paniagua et al. (2009) suggest that greater connection is given by the number of parasitoid species associated with each gall-inducer (vulnerability) and not by the amplitude of the range of hosts (generality) presented by each parasitoid.
Regarding our second question, the interactions observed in the hybrid individuals between the insect gallers and parasitoids were more diverse, presenting greater generality in both oak-insect gallers and insect gallers-parasitoids subnetworks; while vulnerability was greater in hybrid plants only in the oak-insect galler subnetworks, and in the insect galling-parasitoid subnetwork the vulnerability was greater in Q. resinosa. These results point out that hybrid oaks are more vulnerable to the incidence of insect gallers, but in turn the insect gallers present in hybrids have lower pressure from natural enemies (parasitoids), which allow for the incidence of galls to be higher in hybrids. It has been well documented that parasitoid insects play a very important role in the trophic networks, because they influence density and population dynamics of their hosts (Hassell 2000).
In addition, insect gallers can differentiate between hybrid host plants from individuals of the parental species in hybrid zones of Quercus (Pérez-López et al. 2016), Salix (Hochwender and Fritz 2004), and Populus (Evans et al. 2012) as result of the recognition capacity of certain secondary metabolites associate to the host plants as well as the stimulation of injected fluids by female wasps during oviposition, or by secretions of saliva by larvae during feeding (Fritz et al. 2003). Other effects of these secondary metabolites are to act as feed deterrents for both herbivores (Lill and Marquis 2001) and natural enemies (Chaplin-Kramer et al. 2011). These results suggest that hybridization generates changes in the genetic structure of plants, which leads to fauna associated (i.e., insect gallers and their parasitoids) with such plant species to respond to inheritable plant characteristics (Crutsinger et al. 2006; Pérez-López et al. 2016) generating differences in the assembly of arthropods (Whitham et al. 2006; Underwood et al. 2009; Schädler et al. 2010), which confers a degree of resistance or susceptibility for herbivores (e.g., Fritz and Price, 1988; Dungey et al. 2000). These ideas agree with our study due the plant-galler networks of hybrids that were composed by 57 oak individuals and 42 insect galler species had the highest number of exclusive species of gall insects (16.6%), the highest diversity of interactions (5.583), nesting (30.468), and modularity (0.510) in comparison with Q, magnoliifolia and Q. resinosa networks.
With regard to the morphospecies of generalist nucleus insect gallers, differential patterns were observed between subnetworks. For the case of the oak-insect galler subnetworks, we find 13 to 15 generalist nucleus galls, of which nine are present in the subnetworks of the three species of oaks. This pattern of interaction where species of insect gallers categorized as a generalist nucleus co-occur with insect gallers that have fewer interactions with oak individuals, results in a nested topology, which indicates that the interactions recorded for oaks with a low incidence of insect gallers are a cohesive subset of the interactions found in oaks with a higher incidence of insect gallers. Therefore, these insect gallers are not a group of species that interacts with a specific group of oak individuals. Likewise, this nested topology allows the persistence of species of insect gallers minimizing the effect of inter-specific competition (Bastolla et al. 2009). While, in the insect galler-parasitoid subnetworks, the nested topology presents it only with the subnet of Q. resinosa individuals. The Jaccard similarity index showed that individuals of Q. resinosa and hybrids had the greatest similarity in the composition of insect gallers by 51% and parasitoids by 56%. This pattern could be related to those genetically more similar individuals, as it has been documented that plants with similar genotypes have a similar arthropod composition (Bangert 2006; Whitham et al. 2006; Floate et al. 2016).
Our study is one of the first to demonstrate how hybridization affects the structure of tri-trophic interactions between plants, insect gallers, and parasitoids show that plant hybridization generates differential patterns about the diversity of interactions between parental and hybrid plants with insect gallers and their parasitoids, where hybrid plants endure a greater diversity of interactions associated with the increase of genetic diversity. We postulate that hybrid plants are more vulnerable to the incidence of insect gallers and have low pressure by natural enemies (parasitoids), which allow higher occurrence of insect gallers on hybrid plants. In addition, is possible that the rupture or dominance of inheritable characteristics associated to the defense and/or establishment of insect herbivores on the host plant, gives insect gallers the ability to discriminate between hybrid and progenitor host plants, which can confer different degrees of resistance or susceptibility against insect gallers affecting upper trophic levels, such as parasitoids. This fact was confirmed, because the three-way network of hybrid individuals presented greater generatability of insect gallers and a lower vulnerability of parasitoids.
Data availability
Data will be made available upon request.
References
Albarrán-Lara AL, Mendoza-Cuenca L, Valencia-Avalos S, Gonzalez-Rodriguez A, Oyama K (2010) Leaf fluctuating asymmetry increases with hybridization and introgression between Quercus magnoliifolia and Quercus resinosa (Fagaceae) through an altitudinal gradient in Mexico. Int J Plant Sci 171:310–322
Albarrán-Lara AL, Petit RJ, Kremer A, Caron H, Peñaloza-Ramírez JM, Gugger PF, Oyama K (2019) Low genetic differentiation between two morphologically and ecologically distinct giant-leaved Mexican oaks. Plant Syst Evol 305:89–101
Alhmedi A, Haubruge E, D’Hoedt S, Francis F (2011) Quantitative food webs of herbivore and related beneficial community in non-crop and crop habitats. Biol Control 58:103–112
Almeida-Neto M, Guimaraes P, JrPR G, Loyola RD, Ulrich W (2008) A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117:1227–1239
Araújo WS, Maia VC (2021) Topological structure of a tritrophic network composed of host plants, gall-inducing insects and parasitoids in a restinga area in Brazil. Entomol Sci. https://doi.org/10.1111/ens.12468
Araújo WS, Scareli-Santos C, Guilherme FAG, Cuevas-Reyes P (2013) Comparing galling insect richness among Neotropical savannas: effects of plant richness, vegetation structure and super-host presence. Biodivers Conserv 22:1083–1094
Araújo WS, Freitas ÉVDD, Kollár J, Pessoa RO, Corgosinho PHC, Valério HM, Borges MAZ (2019) Host specialization in plant-galling interactions: contrasting mites and insects. Diversity 11:180
Arizaga S, Martínez-Cruz J, Salcedo-Cabrales M, Bello-González MA (2009) Aspectos generales de los encinos. In: Arizaga S, Cruz JM, Cabrales MS, González MAB (eds) Manual de la biodiversidad de encinos michoacanos. Secretaría de Medio Ambiente y Recursos Naturales (Semarnat). Instituto Nacional de Ecología (INESemarnat), México, pp 12–141
Askew RR (1984) The biology of gall wasps. In: Ananthakrishnan TN (ed) Biology of gall insects, pp 223–271
Bailey JK, Schweitzer JA, Ubeda F, Koricheva J, LeRoy CJ, Madritch MD, Rehill BJ, Bangert RK, Fischer DG, Allan GJ, Whitham TG (2009) From genes to ecosystems: a synthesis of the effects of plant genetic factors across levels of organization. Philos Trans R Soc Lond, B 364:1607–1616
Bangert RK, Turek RJ, Martinsen GD, Wimp GM, Bailey JK, Whitham TG (2005) Benefits of conservation of plant genetic diversity on arthropod diversity. Conserv Biol 19:379–390
Bangert RK, Turek RJ, Rehill B, Wimp GM, Schweitzer JA, Allan GJ, Whitham TG (2006) A genetic similarity rule determines arthropod community structure. Mol Ecol 15:1379–1391
Barbour MA, Fortuna MA, Bascompte J, Nicholson JR, Julkunen-Tiitto R, Jules ES, Crutsinger GM (2016) Genetic specificity of a plant–insect food web: implications for linking genetic variation to network complexity. Proc NatL Acad Sci 11:2128–2133
Bastolla U, Fortuna MA, Pascual-García A, Ferrera A, Luque B, Bascompte J (2009) The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 458:1018–1020
Batagelj V, Mrvar A (1998) Pajek-program for large network analysis. Connections 21:47–57
Borror D, DeLong DM, Charles A (1976) An introduction to the study of insects, 4th edn. Holt, Rinehart & Winston, New York
Carson EW, Dowling TE (2006) Influence of hydrogeographic history and hybridization on the distribution of genetic variation in the pupfishes Cyprinodon atrorus and C. bifasciatus. Mol Ecol 15:667–679
Cavender-Bares J, Pahlich A (2009) Molecular, morphological, and ecological niche differentiation of sympatric sister oak species, Quercus virginiana and Q. geminata (Fagaceae). Am J Bot 96:1690–1702
Chaplin-Kramer R, Kliebenstein DJ, Chiem A, Morrill E, Mills NJ, Kremen C (2011) Chemically mediated tritrophic interactions: opposing effects of glucosinolates on a specialist herbivore and its predators. J Appl Ecol 48:880–887
Cheng D, Vrieling K, Klinkhamer PGL (2011) The effect of hybridization on secondary metabolites and herbivore resistance: implications for the evolution of chemical diversity in plants. Phytochemistry Rev 10:107–117
Chust G, Garbin L, Pujade-Villar JULI (2007) Gall wasps and their parasitoids in cork oak fragmented forests. Ecol Entomol 32:82–91
Colwell RK (2009). EstimateS: statistical estimation of species richness and shared species from simples, version 8.0. http://purl.oclc.org/estimates
Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Version 9. User's Guide and application published at: http://purl.oclc.org/estimates
Cooper WR, Rieske LK (2010) Gall structure affects ecological associations of Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae). Environ Entomol 39:787–797
Coutinho RD, Cuevas-Reyes P, Fernandes GW, Fagundes M (2019) Community structure of gall-inducing insects associated with a tropical shrub: regional, local and individual patterns. Trop Ecol 60(74):82
Cronin JT, Melika G, Abrahamson WG (2020) Time-since fire and cynipid gall wasp assemblages on oaks. Biodiversity Conserv 29:1177–1203
Crutsinger GM, Collins MD, Fordyce JA, Gompert Z, Nice CC, Sanders NJ (2006) Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313:966–968
Cuevas-Reyes P, Canché-Delgado A, Maldonado-López Y, Fernandes GW, Oyama K, González-Rodríguez A (2018) Patterns of herbivory and leaf morphology in two Mexican hybrid oak complexes: importance of fluctuating asymmetry as indicator of environmental stress in hybrid plants. Ecol Indic 90:164–170
Dáttilo W, Díaz-Castelazo C, Rico-Gray V (2014) Ant dominance hierarchy determines the nested pattern in ant-plant networks. Biol J Linn Soc 113:405–414
de Graham MWR, V, Gijswijt MJ, (1998) Revision of the European species of Torymus Dalman (s. lat.) (Hymenoptera: Torymidae). Zoologische Verhandelingen, Leiden 317:1–202
Delmas E, Besson M, Brice MH, Burkle LA, Dalla-Riva GV, Fortin MJ (2019) Analyzing ecological networks of species interactions. Biol Rev 94:16–36
Donatti CI, Guimaraes PR, Galetti M, Pizo MA, Marquitti FMD, Dirzo R (2011) Analysis of a hyper-diverse seed dispersal network: modularity and underlying mechanisms. Ecol Lett 14:773–781
Dormann CF, Strauss R (2014) A method for detecting modules in quantitative bipartite networks. Methods Ecol Evol 5:90–98
Dormann CF, Gruber B, Fründ J (2008) Introducing the bipartite package: analysing ecological networks. Interaction 1:2413793
Dormann CF, Fründ J, Blüthgen N, Gruber B (2009) Indices, graphs and null models: analyzing bipartite ecological networks. Open J Ecol 2:7–24
Dreger-Jauffret F (1992) Diversity of gall-inducing insects and their galls. In: Shorthouse JD, Rohfritsch O (eds) Biology of insect-induced galls. Oxford University Press, Oxford, pp 8–33
Dungey HS, Potts BM, Whitham TG, Li HF (2000) Plant genetics affects arthropod community richness and composition: evidence from a synthetic eucalypt hybrid population. Evolution 54:1938–1946
Elsadany AEA, El-Metwally HA, Elabbasy EM, Agiza HN (2012) Chaos and bifurcation of a nonlinear discrete prey-predator system. Comput Ecol Softw 2:169
Evans LM, Allan GJ, Whitham TG (2012) Populus hybrid hosts drive divergence in the herbivorous mite, Aceria parapopuli: implications for conservation of plant hybrid zones as essential habitat. Conserv Genet 13:1601–1609
Fagundes M, Xavier RCF, Faria ML, Cuevas-Reyes P, Lopes LG, Reis-Junior R (2018) Plant phenological asynchrony and community structure of gall-inducing insects in a super-host tropical tree species. Ecol Evol 8:10687–10697
Fagundes M, Santos ÉMS, Duarte K, Santos L, Vieira J, Oliveira C, Silva PS (2020) Diversity of gall-inducing insect associated with a superhost plant species: plant architecture, resource availability and interspecific interactions. Biodivers J Biol Divers 21:3
Floate KD, Godbout J, Lau MK, Isabel N, Whitham TG (2016) Plant–herbivore interactions in a trispecific hybrid swarm of Populus: assessing support for hypotheses of hybrid bridges, evolutionary novelty and genetic similarity. New Phytol 209:832–844
Fritz RS, Price PW (1988) Genetic variation among plants and insect community structure: willows and sawflies. Ecology 9:845–856
Fritz RS, Roche BM, Brunsfeld SJ, Orians CM (1996) Interspecific and temporal variation in herbivore responses to hybrid willows. Oecologia 108:121–129
Fritz RS, Moulia C, Newcombe G (1999) Resistance of hybrid plants and animals to herbivores, pathogens, and parasites. Annu Rev Ecol Syst 30:565–591
Fritz RS, Hochwender CG, Brunsfeld SJ, Roche BM (2003) Genetic architecture of susceptibility to herbivores in hybrid willows. J Evol Biol 6:1115–1126
Fritz RS (1992) Community Structure and Species Interactions of Phytophagous Insects on Resistant and Susceptible Host Plants. In: Fritz RS, Simms EL (eds) Plant resistance to herbivores and pathogens: ecology, evolution and genetics. The University of Chicago Press, pp 240
Gagné RJ (1994) The gall midges of the Neotropical region. Cornell University Press, New York
Gibson GAP, Huber JT, Woolley JB (1997) Annotated keys to the genera of the Nearctic Chalcidoidea (Hymenoptera). In: Gibson GAP, Huber JT, Woolley JB (eds) NRC Research Press, Ottawa, Ontario, Canada
González-Rodríguez A, Arias DM, Valencia S, Oyama K (2004) Morphological and RAPD analysis of hybridization between Quercus affinis and Q. laurina (Fagaceae), two mexican red oaks. Ame J Bot 91:401–409
Gotelli N, Colwell RK (2001) Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett. https://doi.org/10.1046/j.1461-0248.2001.00230.x
Graham MWR de V (1969) The Pteromalidae of north-western Europe (Hymenoptera: Chalcidoidea). Bulletin of the British Museum (Natural History) (Entomology) Supplement 16
Hassell MP (2000) Host parasitoid population dynamics. J Anim Ecol 69:543–566
Hayward A, Stone GN (2005) Oak gall wasp communities: evolution and ecology. Basic Appl Ecol 6:435–443
Hernández-Calderón E, González-Rodríguez A, Méndez-Alonzo R, Vega-Peña E, Oyama K (2013) Contrasting leaf phenology in two white oaks, Quercus magnoliifolia and Quercus resinosa, along an altitudinal gradient in Mexico. Can J for Res 43(2):208–213
Hipp AL, Manos PS, González-Rodríguez A, Hahn M, Kaproth M, McVay JD, Cavender-Bares J (2018) Sympatric parallel diversification of major oak clades in the Americas and the origins of Mexican species diversity. New Phytol 217:439–452
Hirao T, Murakami M (2008) Quantitative food webs of lepidopteran leafminers and their parasitoids in a Japanese deciduous forest. Ecol Res 23:159–168
Hochwender CG, Fritz RS (2004) Plant genetic differences influence herbivore community structure: evidence from a hybrid willow system. Oecologia 38:547–557
Hunter MD, Varley GC, Gradwell GR (1997) Estimating the relative roles of topdown and bottom-up forces on insect herbivore populations: a classic study revisited. Proc Natl Acad Sci 94:9176–9181
Jones JH (1986) Evolution of the Fagaceae: the implications of foliar features. Ann Missouri Bot Gard 73:228–275
Jordano P (1987) Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution. Ame Nat 129:657–677
Jordano P, Vázquez D, Bascompte J (2009) Redes complejas de interacciones mutualistas planta-animal. In: Medel R, Aizen MA, Zamora R (eds) Ecología y evolución de interacciones planta-animal. Universitaria S.A Press, Santiago de Chile, pp 17–41
Kaartinen R, Roslin T (2012) High temporal consistency in quantitative food web structure in the face of extreme species turnover. Oikos 121:1771–1782
Keith AR, Bailey JK, Lau MK, Whitham TG (2017) Genetics-based interactions of foundation species affect community diversity, stability and network structure. Proc R Soc Lond, B 284:20162703
Landi P, Minoarivelo HO, Brännström A, Hui C, Dieckmann U (2018) Complexity and stability of ecological networks: a review of the theory. Popul Ecol 60:319–345
Lau MK, Keith AR, Borrett SR, Shuster SM, Whitham TG (2016) Genotypic variation in foundation species generates network structure that may drive community dynamics and evolution. Ecology 97:733–742
Lepais O, Gerber S (2011) Reproductive patterns shape introgression dynamics and species succession within the European white oak species complex. Int J Org Evol 65:156–170
Lill JT, Marquis RJ (2001) The effects of leaf quality on herbivore performance and attack from natural enemies. Oecologia 126:418–428
Loreau MS, Naeem P, Inchausti J, Bengtsson JP, Grime A, Hector DU, Hooper MA, Huston D, Raffaelli B, Schmid D, Tilman D, Wardle DA (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–808
Losapio G, Fortuna MA, Bascompte J, Schmid B, Michalet R, Neumeyer R, Castro L, Cerretti P, Germann C, Haenni JP, Klopfstein S, Ortiz-Sanchez FJ, Pont AC, Rousse P, Schmid J, Sommaggio D, Schöb C (2019) Plant interactions shape pollination networks via nonadditive effects. Ecology 100:e02619
Maldonado-López Y, Cuevas-Reyes P, Oyama K (2016) Diversity of gall wasps (Hymenoptera: Cynipidae) associated with oak trees (Fagaceae: Quercus) in a fragmented landscape in Mexico. ArthropodPlant Interact 10:29–39
Maldonado-López Y, Vaca-Sánchez MS, González-Rodríguez A, Oyama K, López-Barbosa E, Fagundes M, Cuevas-Reyes P (2018) Hybridization increases canopy arthropod diversity in the Quercus affinis × Quercus laurina complex. J Insect Conserv 22:781–793
Marquitti FMD, JrPR G, Pires MM, Bittencourt LF (2014) MODULAR: software for the autonomous computation of modularity in large network sets. Ecography 37:221–224
Martinsen GD, Whitham TG, Turek RJ, Keim P (2001) Hybrid populations selectively filter gene introgression between species. Evolution 55:1325–1335
Mendonça JM (2007) Plant diversity and galling arthropod diversity searching for taxonomic patterns in an animal plant interaction in the neotropics. Boletin de la Sociedad Argentina de Botanica, Buenos Aires, pp 347–357
Nakamura M, Asanuma M, Hiura T (2010) Differential effects of host plant hybridization on herbivore community structure and grazing pressure on forest canopies. Oikos 119:1445–1452
Oliveira JBBS, Maurício LF, Borges MAZ, Fagundes M, Araújo WS (2019) Comparing the plant–herbivore network topology of different insect guilds in Neotropical savannas. Ecol Entomol 45:406–415
Paniagua MR, Medianero E, Lewis OT (2009) Structure and vertical stratification of plant galler–parasitoid food webs in two tropical forests. Ecol Entomol 34:310–320
Pérez-López G, González-Rodríguez A, Oyama K, Cuevas-Reyes P (2016) Effects of plant hybridization on the structure and composition of a highly rich community of cynipid gall wasps: the case of the oak hybrid complex Quercus magnoliifolia x Quercus resinosa in Mexico. Biodiversity Conserv 25:633–651
Rico-Gray V, Díaz-Castelazo C, Ramírez-Hernández A, Guimarães PR, Holland JN (2012) Abiotic factors shape temporal variation in the structure of an ant-plant network. Arthropod Plant Interact 6:289–295
Riesberg L, Ellstrand NC (1993) What can molecular and morphological markers tell us about plant hybridization? Crit Rev Plant Sci 12:213–241
Ronquist F, Liljeblad J (2001) Evolution of the gall wasp–host plant association. Evolution 55:2503–2522
Ronquist F, Nieves-Aldrey JL, Buffington ML, Liu Z, Liljeblad J, Nylander JAA (2015) Phylogeny, evolution and classification of gall wasps: The plot thickens. PLoS ONE 10:e0123301
Schädler M, Brandl R, Kempel A (2010) Host plant genotype determines bottom-up effects in an aphid-parasitoid-predator system. Entomol Exp Appl 135:162–169
Schönrogge K, Crawley MJ (2000) Quantitative webs as a means of assessing the impact of alien insects. J Anim Ecol 69:841–868
Schönrogge K, Moriya S, Melika G, Randle Z, Begg T, Aebi A, Stone GN (2006) Early parasitoid recruitment in invading cynipid galls. Galling arthropods and their associates. Springer, Tokyo, pp 91–101
Sebastian-Gonzalez E, Dalsgaard B, Sandel B, Guimaraes PR (2015) Macroecological trends in nestedness and modularity of seed-dispersal networks: human impact matters. Global Ecol Biogeogr 24:293–303
Soltis PS, Soltis DE (2009) The role of hybridization in plant speciation. Ann Rev Plant Biol 60:561–588
Staab M, Fornoff F, Klein AM, Blüthgen N (2017) Ants at plant wounds: a little-known trophic interaction with evolutionary implications for ant-plant interactions. Am Nat 190:442–450
Stone GN, Schönrogge K (2003) The adaptive significance of insect gall morphology. Trends Ecol Evol 18:512–522
Stone GN, Schönrogge K, Atkinson RJ, Bellido D, Pujade-Villar J (2002) The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annu Rev Entomol 47:633–668
Tovar-Sánchez E, Oyama K (2006) Effect of hybridization of the Quercus crassifolia x Quercus crassipes complex on the community structure of endophagous insects. Oecologia 147:702–713
Tylianakis JM, Morris RJ (2017) Ecological networks across environmental gradients. Annu Rev Ecol Evol Syst 48:25–48
Underwood EC, Viers JH, Klausmeyer KR, Cox RL, Shaw MR (2009) Threats and biodiversity in the mediterranean biome. Divers Distrib 15:188–197
Valencia AS (2004) Diversidad del género Quercus (Fagaceae) en México. Bot Sci 75:33–53
Waser NM, Ollerton J (2006) Plant-pollinator interactions: from specialization to generalization. University of Chicago Press, Chicago, IL
Welti EAR, Joern A (2015) Structure of trophic and mutualistic networks across broad environmental gradients. Ecol Evol 5:326–334
Whitham TG, Martinsen GD, Floate KD, Dungey HS, Potts BM, Keim P (1999) Plant hybrid zones affect biodiversity: tools for a genetic-based understanding of community structure. Ecology 80:416–428
Whitham TG, Bailey JK, Schweitzer JA, Shuster SM, Bangert RK, LeRoy CJ, Wooley SC (2006) A framework for community and ecosystem genetics: from genes to ecosystems. Nat Rev Genet 7:510–523
Whitham TG, Gehring CA, Lamit LJ, Wojtowicz T, Evans LM, Keith AR, Smith DS (2012) Community specificity: life and afterlife effects of genes. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2012.01.005
Acknowledgements
The study was funded by Coordination of Scientific Research (UMSNH) project 005. This project was supported by CONACYT Project CB105755 and CONACYT PDC2016-Project-3053.
Author information
Authors and Affiliations
Contributions
YML, KO, and PCR: planned and designed the research. GPL MAZ, and PCR: conducted fieldwork. AGR, GPL, and KO: performed laboratory work. YML, WSA, MF, CDC, KSE, MAZ: conducted analyzed data. YML, WSA, AGR, KO, MF, KSE, MAZ, and PCR: The first draft of the manuscript was written. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Additional information
Handling Editor: Makoto Tokuda.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maldonado-López, Y., de Araújo, W.S., González-Rodríguez, A. et al. Quantitative trophic networks of insect gallers and their parasitoids in the hybrid oak complex Quercus magnoliifolia x Quercus resinosa. Arthropod-Plant Interactions 16, 631–643 (2022). https://doi.org/10.1007/s11829-022-09927-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-022-09927-8