Abstract
Flies of the genus Bradysia (Diptera, Sciaridae) are considered as major insect pests of greenhouse-grown horticultural crops. The ability of hooked trichomes of the French bean Phaseolus vulgaris to impale and entrap herbivorous insects thus resulting in insect reduced longevity, reproduction and larval mortality is well known. The present study investigates under laboratory conditions the ability of hooked trichomes of bean leaves to entrap fungus gnats Bradysia paupera, in order to estimate the reduction of their population. We characterized the entrapment mechanism of hooked trichomes towards B. paupera using cryo-scanning electron microscopy, and evaluated the silicon distribution in hooked trichomes with the energy dispersive X-ray microanalysis. We evaluated the trapping efficiency of hooked trichomes in fertilized and unfertilized bean plants towards B. paupera, in comparison with insects feeding on the plant leaf such as black bean aphid Aphis fabae and young stages of the southern green stinkbug Nezara viridula. For B. paupera, we recorded about 30% of entrapped insects in unfertilized plants. Considering the number of entrapped insects in relation to the leaf surface, the percentage of entrapped insect was higher in unfertilized than in fertilized plants having lower density of hooked trichomes. The presence of P. vulgaris plants in greenhouses could represent a useful method in integrated pest management to reduce Bradysia spp. population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that Phaseolus plants display a wide variety of trichome types (Freytag 1955) encompassing specialized hooked trichomes evolved to anchor climbing vines of the plant (Haberlandt 1918; Juniper and Southwood 1986). Such trichomes revealed to be effective to perform a defensive function entrapping arthropods, as highlighted in pioneering studies examining the role of plant pubescence in genetic resistance to leafhoppers (Poos and Smith 1931). Among the first studies on this topic, Johnson (1953) studied the ability of hooked trichomes on the growing shoots of the French bean Phaseolus vulgaris L. (Fabales: Fabaceae) to entrap aphids (Aphis craccivora Koch, Hemiptera: Aphididae) resulting in reduced longevity and reproduction. Since these work, the ability to entrap arthropods by bean leaves was reported many times in different insect species (see Table 1). They encompass many herbivorous species belonging to different insect orders, such as Hemiptera, Diptera and Coleoptera, some entomophagous species belonging to Thysanoptera, Coleoptera and Hymenoptera and also bed bugs (Hemiptera: Cimicidae) (despite no direct relationship between bed bugs and bean plants).
Sciarid flies, commonly referred to as fungus gnats, are small representatives of Diptera usually common where decaying organic material is present. Some species, such as those of the genus Bradysia (Diptera: Sciaridae), initially considered as minor insect pests, are now considered major insect pests of greenhouse-grown horticultural crops and nurseries (Hamlen and Meads 1979; Chabannes et al. 2009; Cloyd 2015). The most common species are Bradysia coprophila Comstock and Bradysia impatiens Johannsen (Lindquist et al. 1985; Harris et al. 1996). Eggs are deposited below the surface of the soil, usually close to the stem of young plants. Larvae hatch and feed on young plant roots and occasionally on lower stem portions of a wide range of crops (Wilkinson and Daugherty 1970). Direct damage is caused to the plant together with indirect damage owing to pathogens infecting the damaged tissue. The larva feeds on fungi and decaying plant material (Kennedy 1974), but can also attack healthy plant roots and tunnel into stems of seedlings and young cuttings (Wilkinson and Daugherty 1970), and plant crowns (Cloyd 2015). Larvae can interfere with the ability of plants to uptake water and nutrients, causing wilting and stunted growth (Wilkinson and Daugherty 1970; Fawzy and Kelly 1982). Fungus gnats are especially a problem during propagation under excessively moist conditions, when plant cuttings or plugs are rooting. Adults are mainly a nuisance causing minimal direct plant damage, but as well as larvae may spread plant pathogens, such as the fungi Pythium and Thielaviopsis spp., which cause seedlings to ‘damp off’ (Jarvis et al. 1993).
Control of fungus gnats is realized mainly using chemical insecticides, but this is becoming problematic, owing to their toxicity and development of resistance within sciarid populations (Bartlett and Keil 1997). More sustainable approaches encompass integrated pest management systems where cultural and physical practices are used together with natural enemies (Chambers et al. 1993, Gouge and Hague 1995; Harris et al. 1995; reviewed by Cloyd 2015).
The present study investigates under laboratory conditions the entrapment of the adult fungus gnat Bradysia paupera Tuomikosky (Diptera: Sciaridae) by hooked trichomes on the leaf of the common bean P. vulgaris. To describe the trapping mechanism, observations in cryo-scanning electron microscope (cryo-SEM) have been performed. In order to better understand and characterize the biomechanics of hooked trichomes in entrapping insects, we performed a detailed EDX analysis of silicon distribution in these trichomes. To evaluate the trapping efficiency of hooked trichomes towards B. paupera in comparison with insects feeding on the plant leaf, such as the black bean aphid Aphis fabae Scopoli (Hemiptera: Aphididae) and young stages of the southern green stinkbug Nezara viridula L. (Hemiptera: Pentatomidae), trapping experiments have been carried out with the three herbivorous species under laboratory conditions. The experiments have been performed on nitrogen fertilized and unfertilized bean plants to evaluate any influence of nitrogen fertilization (extra nutrition) on the trichome entrapment ability.
Material and methods
Insects and plants
Adults of fungus gnats belonging to the species B. paupera used in this study were obtained from the growing medium of potted Vicia faba L. (Fabales: Fabaceae) plants, kept in the laboratory for aphid rearing.
Aphids belonging to the species of the black bean aphid A. fabae have been reared on V. faba young plants inside net cages (300 mm × 300 mm × 300 mm) (Vermandel, Hulst, The Netherlands) in a controlled-condition chamber (14 h photophase, temperature of 25 ± 1 °C; RH of 70 ± 10%). Only apterous adult aphids have been used for the experiments.
N. viridula bugs were collected in the field in June 2018 close to Bastia (Perugia, Umbria region, Italy) and reared in a controlled-condition chamber (14 h photophase, temperature of 25 ± 1 °C; RH of 70 ± 10%) inside clear plastic food containers (300 mm × 195 mm × 125 mm) with 5 cm diameter mesh-covered holes. All stages were fed with seeds, fruits and vegetative parts of their preferred food plants. In particular, sunflower seeds (Helianthus annuus L., Asterales: Asteraceae) and French beans (P. vulgaris) were used to feed the insects. Only N. viridula nymphs of the second stage have been used in the experiments.
Unfertilized and fertilized plants of the common bean P. vulgaris of the cultivar Bronco at the stage of 3–4 true fully expanded leaves have been used for the experiments. Two seeds were sown into a single plastic pot (9 cm × 9 cm × 13 cm) filled with a commercial soil (Gaia, Agrochimica Bolzano, Italia), and grown in a climate-controlled chamber (14 h photophase, temperature of 25 ± 1 °C; RH of 70 ± 10%) and a photosynthetic photon flux density (PPFD) of 400 µmol m−2 s−1. Plants were watered daily. Fertilized plants used in the experiment were fertilized 5 and 12 days after the emergence with an aqueous solution (2 g L−1) of urea (AL.FE., Mantova, Italy).
Cryo-scanning electron microscopy (cryo-SEM)
The shock-frozen samples of the P. vulgaris leaf with entrapped fungus gnats and the trichomes on the abaxial leaf side of fertilized and unfertilized plants were studied in a scanning electron microscope (SEM) Hitachi S-4800 (Hitachi High-Technologies Corp., Tokyo, Japan) equipped with a Gatan ALTO 2500 cryo-preparation system (Gatan Inc., Abingdon, UK). For details of sample preparation and mounting for cryo-SEM, see Gorb and Gorb (2009). Whole mounts of small leaf surface pieces with entrapped insects were sputter-coated in frozen conditions with gold–palladium (thickness 10 nm) and examined at 3 kV acceleration voltage and temperature of − 120 °C at the cryo-stage within the microscope.
Scanning electron microscopy with energy dispersive X-ray microanalysis (EDX)
Fully expanded leaf (3rd–4th) of P. vulgaris was cut into pieces, shortly washed with distilled and air-dried during 12 h and mounted on aluminium stubs by double-sided carbon tape. The elemental composition of hooked trichomes was determined without any coating on the samples using a Zeiss Gemini Ultra 55 Plus. Elementary analysis was carried out with the EDX Oxford x-act 10 mm2 Silicon Drift Detector accessory of the SEM instrument and AZtecOne software. Following parameters were applied: measurement time 113.7 s, accelerating voltage 20.00 kV, magnification × 300 to × 350, working distance 6.4 mm. The microanalysis was conducted on four different hooked trichomes located on the lamina of the abaxial leaf side of an unfertilized plant of P. vulgaris. For each trichome, 4 points (base, proximal-medial, distal-medial, tip) have been considered from the proximal to the distal portion of the trichome.
Behavioural observations
To highlight the fungus gnat trapping behaviour in contact with the leaf surface of P. vulgaris, observations have been carried under the stereomicroscope (Wild M420). One adult of B. paupera has been introduced inside a glass Petri dish (15 cm in diameter) containing a fully expanded bean leaflet with its ventral side upwards. The leaf petiole was inserted into a vial of water to maintain moisture. The fungus gnat behaviour has been observed from the first contact with the leaf surface until the entrapment. During the observations the following behaviours have been recorded using EthoWatcher® an event recorder software: walking behaviour on the leaf, resting on the leaf, entrapment. The following parameters have been analysed: time elapsed from the first contact with the leaf to the first entrapment, total time walking before each entrapment, total time resting before each entrapment, percentage of temporary entrapments, percentage of entrapped insects during walking or percentage of entrapped insects when the insect begins to move just after resting. A total of 14 insects have been observed.
Trapping assays
Entrapped insects on the two leaf sides
To verify the ability to entrap fungus gnats adults by the two sides (adaxial and abaxial) of P. vulgaris leaf, the number of B. paupera adults emerged from the growing medium of potted P. vulgaris plants and entrapped in the leaves was evaluated. In particular the number of entrapped insects on 50 fully expanded leaflets of similar dimensions randomly chosen from 10 plants kept in a controlled condition chamber (14 h photophase, temperature of 25 ± 1 °C; RH of 70 ± 10%) was considered. Two replicates have been performed during 10 days.
Trapping efficiency towards B. paupera. in comparison with other insects in fertilized and unfertilized plants
30 adults of B. paupera were collected using a mouth aspirator from the net cages with potted V. faba plants. 50 apterous adult aphids were gently collected with a brush from the same plants. 20 nymphs of N. viridula of the second stage were collected using a brush from the rearing plastic containers. Each group of insects was transferred separately at the base of the two three- to four-leafed P. vulgaris plants inside a net cage (300 mm × 300 mm × 300 mm) (Vermandel, Hulst, The Netherlands) kept in a controlled-condition chamber (14 h photophase, temperature of 25 ± 1 °C; RH of 70 ± 10%). After 24 h, the number of entrapped insects by the two sides of all the leaves of the two plants in each net cage has been counted with the help of a stereomicroscope. To calculate the trapping efficiency (mean percentage of entrapped insects relative to the total number of released insects), insects on both leaf sides (pooled together) were counted. Moreover, to calculate the number of entrapped insects in relation to the leaf surface area, a digital scan of all the leaves of each plant was taken and measured using the open source image processing program ImageJ (Schneider et al. 2012).
Two sets of experiments have been performed: one with fertilized and one with unfertilized plants. Considering the simultaneous availability of plants in the same development stage and of the different species of insects, the number of replicates performed was: for Sciaridae, four with unfertilized plants and three with fertilized ones, for aphids—ten and eight replicates, respectively, and for N. viridula nymphs—five and five replicates, respectively.
Statistical analysis
The silicon content in the different portions of hooked trichomes was compared using the one-way ANOVA followed by the HSD Tukey’s post-hoc test for multiple comparisons between means (Statistica 6.0, Statsoft Inc. 2001).
To evaluate the different ability to entrap B. paupera adults on the two leaf sides of P. vulgaris, the mean number of insects entrapped on the adaxial vs. abaxial leaf side was compared using the Student t test for dependent samples.
To evaluate the trapping efficiency of hooked trichomes towards A. fabae and B. paupera in fertilized and unfertilized plants, both the mean percentage of entrapped insects relative to the total number of released insects and the number of entrapped insects in relation to the leaf surface area were analysed with two-way ANOVA considering the fertilization and the insect species as main factors.
The number of leaves, the leaf area and the number of hooked trichomes per unit leaf area (density) in unfertilized and fertilized plants were analysed with the Student t test for independent samples. Before the analysis, all the data were subjected to Box–Cox transformations, in order to reduce data heteroscedasticity (Sokal and Rohlf 1998).
Results
Hooked trichome characterization
In P. vulgaris, alternate, compound leaves are divided into three dentate or ovate leaflets. Both leaf surfaces (adaxial and abaxial) bear three types of trichomes: two non-glandular and one glandular (Fig. 1a, b). Non-glandular trichomes of the first type are uniseriate, elongated, cone-shaped, tapered trichomes with sharp tips and prominent multicellular sockets. Being inclined and pointed to one preferred directions, these numerous trichomes form a regular and dense anisotropic coverage on the adaxial leaf side. On the abaxial surface, these trichomes are nearly perpendicular and appear in a very small number only on veins. The other type is represented by typical hooked trichomes having small multicellular sockets and very sharp tips pointed to different directions (Fig. 1c, d). Small clavate glandular trichomes, having very short stalks and relatively large accumbent glandular heads, occur on veins on both leaf sides, however, in a higher number on the adaxial surface (for more details see Salerno et al. 2018).
Cryo-SEM micrographs of the adaxial (a) and abaxial (b) leaf surface of Phaseolus vulgaris.a, b General views showing the glandular (GT) and the non-glandular trichomes, represented by cone-shaped trichomes (arrow heads) and hooked trichomes (arrows). Note the long hooked trichomes on the vein (V); c detail of a hooked trichome with its basal (B), middle (M) and sharpened hooked distal (D) parts; d Hooked trichomes on a vein (V) showing the socket (SO)
In hooked trichomes, we distinguished basal, middle and hooked distal parts (Fig. 1c). The density and distribution of hooked trichomes differ between the adaxial and abaxial sides of the leaf (adaxial: 0.83 ± 0.02 mm−2 abaxial 19 ± 3.06 mm−2, from Salerno et al. 2018) (Fig. 1a, b). On the abaxial side, the non glandular hooked trichomes on the veins appear longer (height: 100.4 ± 4.3 μm, N = 25) than those on the rest of the leaf surface (height: 60.2 ± 2.1 μm, N = 23) (t = 9.27; df = 46: p < 0.0001) (Fig. 1b, d).
EDX analyses gave an overview of the elemental composition of hooked trichomes (Fig. 2a). The elements carbon (C) (54.5 ± 2.8%), oxygen (O) (35.5 ± 2.4%) and silicon (Si) (6.1 ± 0.8%) had the highest counts in the spectrum, followed by potassium (K) (2.1 ± 0.5%), chlorine (Cl) (0.9 ± 0.4%), phosphorus (P) (0.2 ± 0.1%), sulphur (S) (0.2 ± 0.1%) and magnesium (Mg) (0.2 ± 0.04%). EDX line scan spectra and dot mappings of silicon showed that the silicon content changed from the proximal to the distal portion of the trichome (Fig. 2b, c). In particular, the amount of silicon (atomic percentage) was significantly higher in the hooked distal part of the trichomes than in its basal part, while in the middle part (basal-medial and distal-medial portions), it was intermediate (F = 4.05; df = 3, 10; p = 0.0401) (Fig. 2b–d).
SEM image and EDX spectra of hooked trichomes of the Phaseolus vulgaris abaxial leaf surface. a, Example of EDX spectrum of the elemental composition of a hooked trichome; b, SEM image indicating the areas where the EDX spectra of hooked trichomes have been performed (B, basal, BM, basal-medial, DM, distal-medial and D, hooked distal part) and corresponding (d) dot mapping of silicon; c, Atomic percentage related to the total of the detected elements of the silicon content in the different parts of hooked trichomes (cfr b). Points (means ± SEM) with different letters are significantly different at p < 0.05, one-way ANOVA, HSD Tukey test
Entrapment of B. paupera by P. vulgaris leaves
In our assay testing the different ability to entrap fungus gnats adults by the two sides of P. vulgaris leaf, we could observe that the number of entrapped flies on the abaxial leaf side was significantly higher than that on the adaxial side (Fig. 3a–c). On the abaxial side, adults of B. paupera were entrapped mainly on veins where hooked trichomes are larger (Fig. 4a). Insects were entrapped mainly at the level of fore, middle and hindlegs (Fig. 4), sometimes in regions of intersegmental membranes (Fig. 4b, f). The trichomes can entrap insects by hooking them (Fig. 4b, c, f) or by impaling them with their hooked sharpened distal portion (Fig. 4d–f), especially at the level of pretarsus (Fig. 4e). Occasionally, the basal and medial portions of the trichome appeared twisted (Fig. 4f).
Different ability to entrap Bradysia paupera adults by the two sides (adaxial and abaxial) of Phaseolus vulgaris leaf. Note in b and c the different amount of entrapped insects (arrows) on the adaxial (b) and abaxial (c) sides of the leaf. Columns in a indicate the means ± SEM. Different letters in a indicate significant difference at p < 0.05, Student’s t-test for dependent samples
Cryo-SEM micrographs of the abaxial leaf surface of Phaseolus vulgaris with entrapped adults of Bradysia paupera. a Adult entrapped at the level of the legs on a vein; b, c details of an entrapped insect showing the trichomes interlocking with the insect legs; d detail of an entrapped insect showing a trichome (arrow) impaling the insect leg; e detail of an entrapped insect showing a trichome (arrow) impaling the insect leg at the level of the pretarsus (P pretarsus, T tarsus); f detail of an entrapped insect showing a trichome impaling the insect leg at the site of the intersegmental membrane (arrow). Note the twisted basal-medial portion of the trichome (arrow head)
In the observations under stereomicroscope to highlight the fungus gnat trapping behaviour in contact with the leaf surface of P. vulgaris, the time elapsed from the first contact of the insect with the leaf to its first entrapment was 275.8 ± 54.2 s (mean ± SE), the total time spent walking before each entrapment was 123.6 ± 40.4 s (mean ± SE), the total time spent resting before each entrapment was 160.5 ± 46.9 s (mean ± SE), the percentage of temporary entrapments out of the total entrapments was 78.6%, the percentage of insects entrapped during walking was 21.4%, and the percentage of insects entrapped when they tried to move after resting was 78.6%.
Trapping efficiency of hooked trichomes towards B. paupera in comparison with aphids and green stink bugs in fertilized and unfertilized P. vulgaris plants
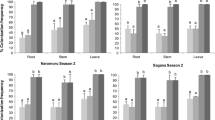
In the assay aiming to evaluate the trapping efficiency (mean percentage of entrapped insects relative to the total number of released insects) of hooked trichomes towards the fungus gnat B. paupera in comparison with insects feeding on the plant leaf, such as the apterous forms of the black bean aphid A. fabae and young stages of the southern green stinkbug N. viridula we recorded about 30% entrapped fungus gnats, about 65% entrapped aphids and no entrapped nymphs of N. viridula in the case of unfertilized plants. In the case of fertilized plants, we recorded about 20% entrapped B. paupera, about 60% entrapped aphids and, likewise in unfertilized plants, no entrapped nymphs of N. viridula (Fig. 5a). The trapping efficiency varied significantly depending on the insect species (F = 68.65; df = 1, 28; p < 0.0001), but not on the plant fertilization (F = 3.40; df = 1, 28; p = 0.0760). The interaction effect between the insect species and the plant fertilization was not statistically different (F = 0.41; df = 1, 28; p = 0.5260). In particular, the trapping efficiency was higher for aphids than for B. paupera and was similar in fertilized vs. unfertilized plants for both insects (Fig. 5a). Considering the number of entrapped insects in relation to the leaf surface area, the trapping efficiency varied significantly depending on both the insect species (F = 97.48; df = 1, 28; p < 0.0001) and the plant fertilization (F = 24.54; df = 1, 28; p < 0.0001) (Fig. 5b). In particular, the trapping efficiency was higher for aphids than for B. paupera and was higher in unfertilized than in fertilized plants (Fig. 5b). There was no interaction between the insect species and the plant fertilization (F = 0.01; df = 1, 28; p = 0.9285). The unfertilized and the fertilized plants were characterized by a similar number of leaves (fertilized: 13.19 ± 0.80; unfertilized: 12.27 ± 0.89; t = 0.79, df = 29, p = 0.4341), but by a significantly different area of the leaf surface (fertilized: 4482.82 ± 398.78 mm2; unfertilized: 2706.52 ± 250.96 mm2; t = 3.91; df = 29; p = 0.0005) and significantly different density of hooked trichomes (fertilized: 12.11 ± 0.42 mm−2; unfertilized: 19.51 ± 1.57 mm−2; t = 4.55, df = 8, p = 0.0019).
Trapping efficiency of hooked trichomes towards Aphis fabae and Bradysia paupera in fertilized and unfertilized plants. a Trapping efficiency (mean percentage of entrapped insects relative to the total number of released insects); b number of entrapped insects in relation to the leaf surface area. Columns indicate the means ± SEM. Asterisk (*) means significantly different at p < 0.05 and ns means not significantly different, 2-way ANOVA. Nymphs of Nezara viridula were not trapped by the bean leaves
Discussion
The results of this study performed under controlled conditions clearly demonstrate that hooked trichomes of the French bean P. vulgaris can effectively entrap the fungus gnat B. paupera, leading insects to death, thus reducing their population number. Indeed, in unfertilized plants we recorded a percentage of about 30% of entrapped fungus gnats. Although this percentage is lower than that recorded for aphids (60%), is anyway noteworthy if we consider that in similar entrapment experiments with leafhoppers, which feed on and oviposit in vegetative plant parts, capture mortalities ranged from 0 to 36.8% depending on the plant cultivar (Pillemer and Tingey 1976). The percentage of entrapped fungus gnats appear high also in consideration that they are winged and consequently can potentially spend less time walking on the leaf plant surface compared with the apterous stages of aphids that we used in the experiments. In the present study, all entrapped sciarids on the leaves were dead or going to die from starvation and/or dehydration. Considering the amount of dead sciarids on the bottom of the cages (data not reported), we presume that even those specimens that were able to escape had reduced their fitness due to injuries or energy expended during escape attempts from hooked trichomes, as previously reported for other species entrapped on P. vulgaris leaves (Johnson 1953; Pillemer and Tingey 1976; Xing et al. 2017). Our observations reveal that the entrapment of B. paupera occurs mainly when the insect starts to move after a period of resting, or when walking across hooked trichomes as highlighted for other insects (Gilbert 1971; Levin 1973; Eisner et al. 1998; Riddick and Simmons 2014). The potential effect of anisotropic leaf surface in affecting B. paupera locomotion on P. vulgaris leaves owing to obstacles provided by trichomes due to their orientation (Vermeij 2015) can be excluded because the sharp tips of hooked trichomes point to different directions and the cone-shaped non-glandular trichomes of the first type on the abaxial side of the leaf of P. vulgaris (the leaf side able to entrap a higher number of sciarids) do not show specific orientation.
The entrapment sites for hooked trichomes on the fungus gnat body involve mainly fore, middle and hindlegs sometimes in the region of joints, where the cuticle of intersegmental membranes is particularly soft. The trichomes can entrap sciarids hooking them or impaling them with their hooked sharpened distal portion, especially at the level of pretarsus, as also recorded in aphids (Johnson 1953) or in the leafminer fly Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) which, obviously differently from sciarids, can be entrapped also at the level of mouthparts or ovipositor as described in detail by Xing et al. (2017). The efficiency of the entrapment mechanism of hooked trichomes is linked to their composition and stiffness along their length. Indeed, the high amount of silicon in the hooked distal part of the trichome observed in our EDX analysis (in agreement with data reported by Szyndler et al. (2013)) makes the very tip of the hook particularly stiff. The lower amount of silicon in the basal portion of the trichome makes it more flexible. The basal and medial portions of the trichome apparently are much softer. This basal region serves as a micro-joint, able to twist without breaking, as frequently observed in our Cryo-SEM observations. The combination of stronger stiffness at the tip and higher flexibility at the base provides stronger piercing ability of the tip and higher hook adaptability to complex insect geometry and in turn stronger ability to interlock with insect surfaces. The effects in the resisting of insect movements trying to escape from the trichomes is similar to that observed in the fruit hooks of Galium aparine L. (Gentianales: Rubiaceae) plants by Gorb et al. (2002), where the flexible base of the hooks could be considered a joint promoting attachment of the hooks when force is applied from different directions.
Our assays aiming to evaluate the trapping efficiency of hooked trichomes towards B. paupera, in comparison with insects feeding on the plant leaf, such as aphids and stinkbugs, revealed that P. vulgaris hooked trichomes did not entrap the southern green stinkbug nymphs of the second stage. In this regard, it is noteworthy to mention that, in a previous study (Salerno et al. 2018), cryo-SEM investigation of pulvilli of N. viridula adults after pulling on the abaxial leaf surface of P. vulgaris, showed clearly that these trichomes are able to penetrate deeply inside the ventral surface of pulvilli, which are composed mainly of resilin (Rebora et al. 2018), and are able to damage them under some loading conditions (Salerno et al. 2018). The damage caused by hooked trichomes to the pulvilli of the adult N. viridula was confirmed in the latter study by the reduction in traction force of this insect on the reference glass surface after walking on P. vulgaris leaves (Salerno et al. 2018). As far as the nymphs are concerned, probably their pulvilli are damaged by hooked trichomes, likewise in adults, when forced to pull on P. vulgaris leaves, but insect dimension in relation to the plant trichome characteristics make the hooks ineffective in entrapping them when freely walking over the trichomes. Other possible explanations could be a quick locomotion on the leaves helping the nymphs to avoid self-impaling in consideration that some time is required to pierce the tarsi (Bustamante et al. 2017) or careful placement of tarsi to avoid trichomes documented for bryocorine mirid bugs, who are adapted to pubescent plant surfaces (Voigt et al. 2007; Voigt and Gorb 2010). Further investigations are necessary to clarify these hypotheses.
Previous studies showed that capture efficiency of P. vulgaris trichomes during the plant’s vegetative stage was considerably higher than in the fruiting and cotyledon stage and the abaxial surface of the leaf was more effective in trapping flies than other parts of the plant (Xing et al. 2017). The capture efficiency of hooked trichomes is determined by their density, which depends on the plant cultivar (Johnson 1953; Pillemer and Tingey 1976, 1978). In agreement with this, our experiments revealed that the abaxial side of the leaf, where hooked trichome density is higher (Salerno et al. 2018), was more efficient in entrapping B. paupera than the leaf adaxial surface. Moreover, since the number of trichomes is fixed early during plant development (Pillemer and Tingey 1976) and the densities of hooked trichomes decrease as leaves expand (Stenglein et al. 2004), we could observe that the trapping efficiency (considering the number of entrapped insects in relation to the leaf surface) varied significantly depending on the plant nitrogen fertilization.
In conclusion, currently, insecticides and biological control agents are being used to reduce fungus gnat populations in greenhouse production systems. Together with these methods, alternative management strategies such as cultural, physical, and sanitation ones are also implemented by greenhouse producers (Chabannes et al. 2009; Cloyd 2015). Our data show that the entrapment by P. vulgaris hooked trichomes has a noteworth impact on the population of B. paupera at the adult stage. For this reason, planting P. vulgaris plants could be used as intercropping practice to improve the sciarid management and control systems in greenhouses. In consideration that also economically important entomophagous arthropods are hampered and trapped by the bean plant surface (Riddick and Simmons 2014), the use of P. vulgaris plants as intercropping practice could be particularly useful in reducing fungus gnats population in seedling greenhouses before biocontrol agent release. Moreover, in consideration that P. vulgaris hosts a large number of insect pests, its effective role as intercropping practice in greenhouses should be tested in field studies in order to reduce any possible “side effect” linked to its introduction, which could change depending upon plant species grown and a variety of other environmental factors.
References
Bartlett GR, Keil CBO (1997) Identification and characterization of a permethrin resistance mechanism in populations of the fungus gnat Lycoriella mali (Fitch) (Diptera: Sciaridae). Pest Biochem Physiol 58:173–181
Biddinger DJ (1993) Toxicity, stage specificity, and sublethal effects of abamectin and several classes of insect growth regulators to Platynota idaeusalis (Lepidoptera: Tortricidae) and Stethorus punctum (Coleoptera: Coccinellidae). PhD. Dissertation, The Pennsylvania State University
Bogdandy S (1927) Ausrottung von Bettwanzen mit Bohnenblättern Naturwiss 15:474
Bustamante J, Panzarino JF, Rupert TJ, Loudon C (2017) Forces to pierce cuticle of tarsi and material properties determined by nanoindentation: the Achilles’ heel of bed bugs. Biol Open 6:1541–1551
Chabannes M, Hatt G, Thèbaud G, Bedford ID, Lamb C (2009) Establishment of an in vitro sciarid fly larvae assay to study plant resistance. Ann Appl Biol 155:293–296
Chambers RJ, Wright EM, Lind RJ (1993) Biological control of glasshouse sciarid flies (Bradysia spp.) with the predatory mite, Hypoaspis miles, on cyclamen and poinsettia. Biol Sci Technol 3:285–293
Cloyd RA (2015) Ecology of Fungus Gnats (Bradysia spp.) in greenhouse production systems associated with disease-interactions and alternative management strategies. Insects 6:325–332
Eisner T, Eisner M, Hoebeke ER (1998) When defense backfires: detrimental effect of a plant’s protective trichomes on an insect beneficial to the plant. Proc Natl Acad Sci USA 95:4410–4414
de Fluiter HJ, Ankersmit GW (1948) Gegevens betreffende de aantasting van bonen (Phaseolus vulgaris L.) door de zwarte bonenluis (Aphis (Dralis) fabae Scop.). Tijdsch Plantenziekten 54:1–13
Fawzi TH, Kelly WC (1982) Cavity spot of carrots caused by feeding of fungus gnat larvae. J Am Soc Hortic Sci 107:1177–1181
Freytag GF (1955) Variation of the common bean (Phaseolus vulgaris L.) in Central America. Ph.D. thesis. Washington Univ., St. Louis
Gepp VJ (1977) Hindrance of arthropods by trichomes of bean plants (Phaseolus vulgaris L.). Anz Schädlkd Pflanzenschutz Umweltschutz 50:8–12
Gouge DH, Hague NGM (1995) Glasshouse control of fungus gnats, Bradysia paupera, on fuchsias by Steinernema feltiae. Fund Appl Nematol 18:77–80
Gilbert LE (1971) Butterfly-plant coevolution: has Passiflora adenopoda won the selectional race with Heliconiiine bufferflies? Science 172:585–586
Gorb EV, Gorb SN (2009) Functional surfaces in the pitcher of the carnivorous plant Nepenthes alata: a cryo-SEM approach. In: Gorb SN (ed) Functional surfaces in biology—adhesion related phenomena, vol 2. Springer, Heidelberg, pp 205–238
Gorb EV (2002) Popov VL Gorb SN (2002) Natural hook-and-loop fasteners: anatomy, mechanical properties, and attachment force of the jointed hooks of the Galium aparine fruit. In: Brebbia CA, Sucharov LJ, Pascolo P (eds) Design and nature comparing design in nature with science and engineering. WIT Press, Southampton, pp 151–160
Haberlandt G (1918) Physiologische Pflanzenanatomie, 5th edn. Engelmann, Leipzig
Hamlen RA, Mead FW (1979) Fungus gnat larval control in greenhouse plant production. J Econ Entomol 72:269–271
Harris MA, Gardner WA, Oetting RD (1996) A review of the scientific literature on fungus gnats (Diptera: Sciaridae) in the genus Bradysia. J Entomol Sci 31:252–276
Harris MA, Oetting RD, Gardner WA (1995) Use of entomopathogenic nematodes and a new monitoring technique for control of fungus gnats, Bradysia coprophila (Diptera: Sciaridae), in floriculture. Biol Control 5:412–418
Hely PC (1945) Fruit flies (Strumeta tryonii) trapped by bean leaves. Agric Gaz NSW 56:22–23
Jarvis WR, Shipp JL, Gardiner RB (1993) Transmission of Pythium aphanidermatum to greenhouse cucumber by the fungus gnat Bradysia impatiens (Diptera: Sciaridae). Ann Appl Biol 122:23–29
Johnson B (1953) The injurious effects of the hooked epidermal hairs of French beans (Phaseolus vulgaris L.) on Aphis craccivora Koch. Bull Entomol Res 44:779–788
Juniper BE, Southwood TRE (1986) Insects and the plant surface. Edward Arnold Publishers Ltd., London
Kavousi A, Chi H, Talebi K, Bandani A, Ashouri A, Naveh VH (2009) Demographic traits of Tetranychus urticae (Acari: Tetranychidae) on leaf discs and whole leaves. J Econ Entomol 102:595–601
Kennedy MK (1974) Survival and development of Bradysia impatiens (Diptera: Sciaridae) on fungal and non-fungal food sources. Ann Entomol Soc Am 67:745–749
Levin DA (1973) The role of trichomes in plant defense. Q Rev Biol 48:3–15
Lindquist RK, Faber WR, Casey ML (1985) Effect of various soilless root media and insecticides on fungus gnats. Hortic Sci 20:358–360
McKinney KB (1938) Physical characteristics of the foliage of beans and tomatoes that tend to control some small insects. J Econ Entomol 31:630–631
Pillemer EA, Tingey WM (1976) Hooked trichomes: a physical plant barrier to a major agricultural pest. Science 193:482–484
Pillemer EA, Tingey WM (1978) Hooked trichomes and resistance of Phaseolus vulgaris to Empoasca fabae (Harris). Entomol Exp Appl 24:83–94
Poos FW, Smith FF (1931) A comparison of oviposition and nymphal development of Empoasca fabae (Harris) on different host plants. J Econ Ent 24:361–371
Putman WL (1955) Bionomics of Stethorus punctillum Weise (Coleoptera: Coccinellidae) in Ontario. Can Entomol 87:9–33
Quiring DT, Timmins PR, Park SJ (1992) Effect of variations in hooked trichome density of Phaseolus vulgaris on longevity of Liriomyza tifolii (Diptera: Agromyzidae) adults. Environ Entomol 21:1357–1361
Rebora M, Michels J, Salerno G, Heepe L, Gorb EV, Gorb SN (2018) Tarsal attachment devices of the southern green stink bug Nezara viridula (Heteroptera: Pentatomidae). J Morphol 279(5):660–672
Ricci C, Cappelletti G (1988) Relationship between some morphological structures and locomotion of Clitostethus arcuata Rossi (Coleoptera: Coccinellidae), a whitefly predator. Frustula Entomol 11:195–202
Richardson HH (1943) The action of bean leaves against the bedbug. J Econ Entomol 36:543–545
Riddick EW, Simmons AM (2014) Do plant trichomes cause more harm than good to predatory insects? Pest Manag Sci 70:1655–1665
Riddick EW, Wu Z (2011) Lima bean–lady beetle interactions: hooked trichomes affect survival of Stethorus punctillum larvae. Biocontrol 56:55–63
Rogers DJ (1979) Host plant resistance of Ophyiomyia phaseoli (Diptera: Agromyzidae) in Phaseolus vulgaris. J Aust Entomol Soc 18:245–250
Salerno G, Rebora M, Gorb EV, Gorb SN (2018) Attachment ability of the polyphagous bug Nezara viridula (Heteroptera: Pentatomidae) to different host plant surfaces. Sci Rep 8:10975
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Sengonca C, Gerlach S (1984) Einfluss der Blattoberfläche auf die Wirksamkeit des räuberischen thrips, Scolothrips longicornis (Thysan: Thripidae). Entomophaga 29:55–61
Shah MA (1982) The influence of plant surfaces on the searching behaviour of coccinellid larvae. Entomol Exp Appl 31:377–380
Sokal RR, Rohlf FJ (1998) Biometry. New York, W.E, Freeman and Company
StatSoft Inc (2001) Statistica (Data Analysis Software System), Version 6. StatSoft Italia S.r.l, Italy
Stenglein SA, Arambarri MA, Vizgarra ON, Balatti PA (2004) Micromorphological variability of leaf epidermis in Mesoamerican common bean (Phaseolus vulgaris, Leguminosae). Aust J Bot 52:73–80
Szyndler MW, Haynes KF, Potter MF, Corn RM, Loudon C (2013) Entrapment of bed bugs by leaf trichomes inspires microfabrication of biomimetic surfaces. J R Soc Interface 10:20130174
Trouvelot B, Thenard J (1931) Remarques sur les éléments des végétaux contributant à limiter ou à empecher la pullulation du Leptinotarsa decemlineata sur de nombreuses espèces ou races végétales. Rev Path Vég Ent agric 18:277–285
Vermeij GJ (2015) Plants that lead: do some surface features direct enemy traffic on leaves and stems? Biol J Linn Soc 116:288–294
Voigt D, Gorb SN (2010) Egg attachment of the asparagus beetle Crioceris asparagi to the crystalline waxy surface of Asparagus officinalis. Proc R Soc B 277:895–903
Voigt D, Gorb E, Gorb S (2007) Plant surface–bug interactions: Dicyphus errans stalking along trichomes. Arthropod Plant Interact 1:221–243
Walters PJ (1974) A method for culturing Stethorus spp. (Coleoptera: Coccinellidae) on Tetranychus urticae (Koch) (Acarina: Tetranychidae). J Austral Entomol Soc 13:245–246
Wilkinson JD, Daugherty DM (1970) The biology and immature stages of Bradysia impatiens (Diptera: Sciaridae). Ann Entomol Soc Am 63:656–660
Xing Z, Liu Y, Cai W, Huang X, Wu S, Lei Z (2017) Efficiency of trichome-based plant defense in Phaseolus vulgaris depends on insect behavior, plant ontogeny, and structure. Front Plant Sci 8:2006
Acknowledgements
We are grateful to Leonardo Giontella, Federica Pietrelli and Chiara Rossetti for their help in the entrapping experiments. This study was funded by the European Cooperation in Science and Technology, ENBA COST Action CA15216, STSM Grant (ECOST-STSM-CA15216-41582).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Dagmar Voigt and Heikki Hokkanen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rebora, M., Salerno, G., Piersanti, S. et al. Entrapment of Bradysia paupera (Diptera: Sciaridae) by Phaseolus vulgaris (Fabaceae) plant leaf. Arthropod-Plant Interactions 14, 499–509 (2020). https://doi.org/10.1007/s11829-020-09760-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-020-09760-x