Abstract
This study was conducted to find the optimal cryopreservation conditions for efficiently preserving the genetic resources of the aquatic plant lotus (Nelumbo nucifera Gaertn). The seed viability differed depending on the type of vitrification treatment agent, and the longer the PVS2 treatment time, the lower the viability. Among the treatments, T1 (2M glycerol) and T7 (Loading solution + PVS2) were the treatments that showed viability rates similar to those of the control (92.1 ± 5.95%). When the seeds were stored for 12 weeks, the viability rate of the control dropped to 0%, but the treatments T1 and T7 were maintained unchanged. The change in the germination rate of seeds according to the storage period, the control was 0% after 4 weeks. In the case of the treatments, T1 showed a high germination rate in the 1 day (86.67 ± 6.67%), but after 12 weeks, a lower germination rate (54.67 ± 5.58%). On the other hand, T7 had a slightly lower initial germination rate of 73.33%, but it was maintained even after a prolonged storage period. The shoot growth of the cryopreserved sprouted seedlings was lower than that of the control, and there was no morphological difference, as T7 was the best among the treatments. As a result of RAPD analysis using seedling primers, no genetic variation was found. The above results are expected to be widely used not only for treatment but also for the conservation of genetic resources of other aquatic plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the efficient use of genetic resources, processes such as seed preservation or the development of propagation technology are required. In particular, the long-term preservation of genetic resources is essential. Traditional genetic resource conservation methods need a lot of land, labor, and management cost, and the preserved plants are exposed to environmental stress and the threat of pests (Barrientos-Priego et al. 1992). Also, the viability of seeds is determined by various factors such as storage method and drying (Seaton and Hailes 1989; Pritchard et al. 1999). These traditional methods have the disadvantage that they take a lot of time and effort, and, crucially, they cannot be preserved for a long time. To overcome these shortcomings, we are developing a method of preserving genetic resources in liquid nitrogen (LN, − 196 °C). Moreover, experiments are underway to extend the storage period using various species worldwide (Rajametov et al. 2014).
Many advanced countries have developed long-term storage techniques using plant tissues such as plant vegetative organs and seeds without any damage using liquid nitrogen (Ahn and Sakai 1994; Niino et al. 1992; Sakai and Kobayashi 1990; Stanwood and Bass 1981; Choi et al. 2000). The cryopreservation method stops the metabolic process and can be stored semi-permanently, minimizes human and material resources, and can eliminate the risk of genetic variation or disease or pests (Panis 2008; Kaviani 2011; Engelmann 2004, 2012; Benson 2008).
Cryopreservation methods are classified into vitrification, encapsulation, and droplet-vitrification methods (Sakai et al. 1990; Gonzalez-Arnao and Engelmann 2006; Pawlowska and Szewczyk-Taranek 2014; Zamecnik et al. 2021). Among them, the most commonly used vitrification method is freezing after treatment with a cryoprotectant, which is most commonly used because it is simple and inexpensive (Galdiano et al. 2012).
Aquatic plants typically grow in water or live underwater during one of their life cycles, even when they come out of the water. Around 1% (25,000 species) of angiosperm and 2% (200 species) of ferns are known as aquatic plants worldwide (Chamber et al. 2008). In Korea, there are about 200 species, such as Quillworts (Isoetes japonica A. Braun), Prickly water lily (Euryale ferox Salisb.), and Watershield (Brasenia schreberi J.F.Gmel.). It is in extreme extinction due to the reclamation and development of habitats, a transformation of rivers or wetlands, and the inflow of various pollutants into the aquatic ecosystem. However, no conservation and restoration measures have been taken (Phillips et al. 2016). Although it is possible to apply research or technology of terrestrial plants to aquatic plants, they have different ecological characteristics than terrestrial plants. Therefore, developing unique conservation and restoration technology unique to aquatic plants must be a very urgent task that cannot be postponed any longer.
The lotus (Nelumbo nucifera Gaertn.) is one of the most beautiful ornamental aquatic native species endowed with unique biological and nutritional properties. The seeds of this species are long-lived plants. Lotus is an important model system for investigating longevity. In addition, the lotus flower is rich in nutrients and has high medicinal value and is of scientific interest in various aspects such as genome sequencing (Gowthami et al. 2021).
Few studies on the cryopreservation of aquatic plants, especially the cryopreservation of seeds. Kim and Oh (2009) reported a regenerative growth rate of 28.3% by cryopreserving a cell suspension cultured with embryonic cells of Ranunculus kazusensis, an aquatic plant. However, there was no report on plant regeneration after cryopreservation.
The application of the cryopreservation method of plants is not active because the sensitivity of plant tissues and cells to cryogenic temperatures is diverse. Therefore, it is essential to set conditions suitable for the characteristics of each plant species. First, can cryopreserve aquatic plants with very different characteristics from terrestrial plant? Second, is the cryopreservation method suitable for the cryopreservation of aquatic plants suitable for the vitrification method, which is widely used for the cryopreservation of terrestrial plants? Third, is it possible to obtain germinated plants from cryopreserved seeds? Fourth, it is necessary to investigate whether their morphology and genetic variation will occur. Therefore, this study investigated the germination efficiency of aquatic plant, Nelumbo nucifera Gaertn. seeds under cryogenic temperature optimum conditions and cryopreserved seeds and the growth and genetic variation of germinated plants.
Materials and methods
Plant material
Lotus (Nelumbo nucifera Gaertn) seeds were obtained in the plant seed conservation laboratory at the Korea National Arboretum (Fig. 1). Seeds were stored in a refrigerator at 4 ℃ with a humidity of 80% or more until use for experiments. The lotus seeds and plant identification used in the experiment were identified by Hyunsik Moon, a professor at the Department of Forest Environment and Resources, Gyeongsang National University, who majored in plant taxonomy and used them in the experiment. In addition, the seeds and plants were prepared as voucher specimens and stored in the specimen room of the Department of Environment and Forestry Science, Gyeongsang National University.
The surface of the seed was sterilized by immersion in ethanol 70% for 1 min and sodium hypochlorite 1.0% for 1 min. The seeds were rinsed three times with distilled water and used in the study.
Experimental design—loading and vitrification
Sterilized seeds were treated with loading solution (LS) (Table 1). LS contains 2M glycerol and 0.4M sucrose; glycerol and sucrose were used alone or in combination. LS was incubated at 25 °C for 20 min.
Afterward, the seeds were treated with various plant vitrification solutions (Table 1). Seeds treated in LS solution were treated with Plant vitrification solution 2 (PVS2) (30% w/v glycerol + 15% w/v ethylene glycol + 15% w/v dimethyl sulfoxide (DMSO) + 13.7% w/v sucrose) at different temperatures and treatment times. The treatment time of the vitrification solution was 10–30 min at 0 °C. However, T4 (PVS2 10 min at °C), T8 (PVS2 20 min at 0 °C), and T12 (PVS2 30 min at 0 °C) The vitrification treatment was not performed in each treatments, and the cryopreservation efficiency according to the loading and vitrification treatment was analyzed using 30 seeds in each treatments.
In this study, 15 treatments were designed based on these combinations, and the viability of seeds according to these treatments or treatment time was investigated. C1 was stored at room temperature without treatment, and C2 was stored in liquid nitrogen without treatment. T1 and T2 are glycerol and sucrose alone, and T3 is LS treated at 25 °C for 20 min. T4 was treated with PVS2 alone for 10 min at 0 °C without loading solution treatment, and T5-T7 was pretreated with glycerol and sucrose alone or LS at 25 °C for 20 min and then treated with PVS2 solution at 0 °C for 10 min. T8 was treated with PVS2 at 0 °C for 20 min without loading solution treatment, and T9-T11 was treated with glycerol and sucrose as a single solution or LS at 25 °C for 20 min. Then PVS2 solution was treated at 0 °C for 20 min. T12 was treated with PVS2 at 0 °C for 30 min without loading solution treatment. In T13-T15, a single solution of glycerol and sucrose or LS at 25 °C for 30 min and then treated with PVS2 at 0 °C for 30 min. PVS2 refers to MS (Murashige and Skoog 1962) medium containing 0.4 M sucrose with 30% glycerol, 15% DMSO and 15% ethylene glycol. Seeds were put into the 15 treatments mentioned above, and 0, 10, 20, 30 min treatments were repeated 5 times each.
Cryopreservation in liquid nitrogen
The vitrified seeds were immersed in liquid nitrogen. In order to investigate the viability of the seeds according to the storage time of liquid nitrogen, they were stored in liquid nitrogen for 1 day up to 12 weeks.
For treated seeds, the T1 and T7 treatments, which had the highest viability among the 15 treatments, were used to investigate the seed viability by preservation period. The seeds were placed in a cryovial containing T1 and T7 treatments and stored in liquid nitrogen for 1 day up to 12 weeks. Each vial was taken out from liquid nitrogen, thawed, and the viability of the seeds was investigated by the TTC (2,3,5-triphenyltetrazolium chloride) (Cottrell 1947) method.
Thawing of cryopreserved seeds
The frozen vial stored in LN was removed and quickly re-warmed in a 40° C water bath for 1 min. Again, the cryoprotectant was removed and treated with an unloading solution (unLS, MS medium with 1.2M sucrose) for 10 min. After removing the unLS, it was placed on the medium. The seeds we are then measured for viability, germination and fresh weight. Seed viability was measured using the TTC method.
Measurement of seed viability
The viability of seeds was carried out by Triphenyl tetrazolium chloride (TTC) method (Cottrell 1947). Seeds were incubated in 1% TTC solution for 1 day at 37 ± 2 ℃ in the dark. The number of zygotic embryos stained by TTC was counted, and the percentage of TTC-stained seeds represented the survival rate.
Seed viability was measured by the Triphenyl tetrazolium chloride (TTC) method (Cottrell 1947). Thirty seeds were incubated in a 1% TTC solution for 1 day at 37 ± 2 °C in the dark. The seeds were cut using a scalpel, and the number of TTC-stained zygotic embryos was counted. The viability rate of seeds represented the ratio of seeds stained with TTC among the number of seeds used for analysis. The experiment was repeated 3 times.
Germination of cryopreserved seeds
Germination of cryopreserved seeds was performed on seeds of treatments with excellent vigor (T1 and T7), treatments with poor vigor (T15), and seeds of control (C1 and C2). Seed viability was high in T3 and T4 treatments, but seed germination was not achieved afterward.
Cryopreservation solutions were removed from cryovials with a sterile disposable transfer pipette under a laminar flow hood. Seeds were rinsed with MS medium with 1.2M sucrose (pH 5.7) for 15 min, transferred to Petri dishes containing MS medium with 30 g sucrose (pH 5.7), solidified with 7.0 g/l agar and incubated under controlled environmental conditions (25 ± 2 ℃; 60 mmol m−2S−1; 16 light/8 dark hours).
Germination rates were calculated according to the Association of Official Seed Analysts Handbook (AOSA 1983). The number of germinated seeds was counted after culturing 30 ultra-low temperature preserved seeds from 4 to 12 weeks in a germination medium. The germination rate of seeds was expressed as the ratio of germinated seeds to total seeds. The experiment was repeated 3 times.
Growth of germinated plants
The germinated plants were transferred to a 100 ml Erlenmeyer flask containing 1/2MS liquid medium without growth regulators to induce growth. The fresh weight of the plants was measured after 4 weeks.
Genetic variation analysis of germinated plants
Seedlings (C1, C2, T1, T7, and T15) 3 months after germination were used to test for genetic mutations through RAPD marker analysis. For the samples used in the analysis, plants germinated in vitro were induced to shoot multiplication, and then three individuals of the same clone were randomly selected and used for genetic mutation analysis. Cultures were incubated at 25 ± 2 °C for a 16-h photoperiod under a fluorescent light of 150 μmol m−2 s−1. Explants were subcultured once every 3 weeks.
Genomic DNA from regenerants was extracted from 0.5 g leaf tissue of 2-month-old plants of each treatment with the CTAB method (Doyle and Doyle 1987). DNA content was measured from UV–Vis Spectrometer (Libra S22, Biochrome, England) at 260 and 280 nm. Purified DNA was stored at − 20 °C until further use.
For the primers used in this study, the best primers showing polymorphism in germinated plants were selected and used through previous studies. The selected primers were N-8013 (GTTTCGCTCC) and N-8017 (TGCTCTGCCC) (Bioneer, South Korea). The reaction mixture (20 µl) contained 100 ng DNA template, 2 μM of primer, AccuPower® PCR premix (Bioneer, South Korea) including 1U top DNA polymerase, 250 μM dNTPs, 1.5 mM MgCl2. The amplification was done using a thermocycler (Multigene, Labnet, USA). The cycle was comprised of an initial step at 94 °C for 3 min, 37 °C for 1 min and 72 °C for 2 min, followed by 36 cycles of 55 s at 94 °C, 55 s at 37 °C and 100 s at 72 °C, along with a final extension step of 10 min at 72 °C. Reaction products were separated on a 1.0% agarose gel using 1 × TAE buffer containing NEOgreen (Kangsan, South Korea). Sizes of the amplification products were determined using a 100 bp plus DNA ladder (Bioneer, South Korea) at 100 V for 17 min and visualized under UV light for photography and image capture.
Data analysis
A one-way ANOVA model was used to compare the seed viability, germination, regeneration rate of treated seed, and plant growth factors. Duncan's multiple range test was employed to assess the significance of differences among variants. Before all analyses, normal distribution was verified with the Shapiro–Wilk test. The data are given as means with standard errors of the mean (± SE). The values indicate statistical significance (p < 0.05). Statistical analyses were conducted in SPSS software 27.0 (SPSS Inc., Chicago, IL, USA). All experiments were repeated 5 times.
Results
Seed viability according to treatment
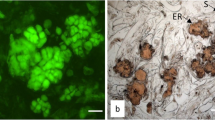
The viability of N. nucifera seeds following vitrification was investigated using the TTC method. The viability of treated seeds differed for each vitrification treatment method (Fig. 1). Among the seeds preserved at the cryopreservation temperature, viable seeds were dyed red, and seeds that were not were dyed red.
The seed viability differed depending on the type of vitrification treatment agent (Fig. 2). When seeds were treated at 25 °C for 20 min, viability was maintained regardless of whether sucrose and glycerol were treated alone or in combination`
The seed viability of N. nucifera seeds according to vitrification treatment. C1:Without cryoprotectant; without immersion in LN, C2:Without cryoprotectant, T1:G 2M, T2:S 0.4M, T3:G 2M + S 0.4M, T4:PVS2, T5:G 2M + PVS2, T6:S 0.4M + PVS2, T7:G 2M + S 0.4M + PVS2, T8:PVS2, T9:G 2M + PVS2, T10:S 0.4M + PVS2, T11:G 2M + S 0.4M + PVS2, T12:PVS2, T13:G 2M + PVS2, T14:S 0.4M + PVS2, T15:G 2M + S 0.4M + PVS2. The treatment time of each treatments is different and is shown in Table 1. Means denoted by a different letter indicate significant differences between treatments (p < 0.05). All treatments were used 50 seeds and repeated 5 times
When the PVS2 treatment was performed after the LS treatment of the seeds, the change in seed vigor was observed according to the treatment time. When treated with PVS2 for 10 min, the viability was maintained when compared to the LS-treated compartment, but when treated for more than 20 min, the viability of the seeds decreased sharply to around 50%, indicating that the treatment time should be short.
The treatments with the highest seed viability were T1 (0.2 M glycerol) and T7 (LS + PVS2 treat 10 min). On the other hand, T15 (LS + PVS2 treat 30 min) had the lowest viability of seeds, followed by T12 (PVS2 treat 30 min) and T13 (2 M glycerol + PVS2 treat 30 min).
Seed viability by cryopreservation period
Treated seeds showed different viability depending on the cryopreservation period (Table 2). In the case of seeds stored at room temperature without treatment, a rapid decrease in viability occurred after one week, and there was no viability after 4 weeks. C2 treated without adding cryoprotectant showed a rapid decrease in viability from 1 week after treatment, and no viable seeds were observed after 4 weeks. T1, which was treated with 0.2M glycerol, showed no difference in viability between 1 and 12 weeks. The T7 treatment added with 2M glycerol, 0.4M sucrose and PVS2 also showed no significant difference in viability between 1 and 12 weeks. Treatments with high seed vigor were C1, T1, T3, T4, and T7.
Germination rate of seeds cryopreserved in liquid nitrogen
The germination rate of cryopreserved seeds differed for each treatment (Table 3). The regeneration rate of C1 without liquid nitrogen treatment was the highest. Among the vitrification treatments, the treatment with the highest germination rate was T1, which was 86.7%. The treatment with the lowest germination rate was the T15 treatment, which was very low at 13.3%. C2 treatment without cryoprotectant treatment showed 53.3% after 1 day of treatment, 6.7% after 4 weeks, and no germination after that. The treatments in T1 and T7 showed a high germination rate for 12 weeks than C2. Statistical analysis showed no difference in germination rates at 1-day and 12 weeks storage periods. However, T3 and T4, which had good seed vigor, did not germinate.
Plant growth after cryopreserved seed germination
The growth of cryopreserved seed germinating plants differed depending on the vitrification method (Table 4; Fig. 3). The treatments with the best shoot growth were the control C1 and C2. Among the vitrification treatments, T7 was the best, followed by T15. T1 treatment had the lowest growth among the treatments. The number of stems was also the highest in the control C2 and C1, and among the treatments, T1 produced the most shoots, but there was no difference between the treatments. On the other hand, root growth also showed differences between treatments. Among the treatments, the treatment with the best root growth was the T1 treatment, and the other treatments were similar. The number of roots was the highest at T7 and T15.
The appearance of germinated plants did not show a significant difference between the control and treatments. Unlike the other treatments, C2 had yellow buds and did not develop well even with the naked eye (Fig. 3).
Genetic variation analysis of regenerated plants
Genetic variation of plants germinated from cryopreserved seeds was investigated using two RAPD primers (N-8013 and N-8017) (Fig. 4). All regenerated plants (C1, C2, T1, T7, and T15) showed RAPD polymorphisms per primer. DNA amplification products ranging from 500 to 3000 bp were observed. Band sizes using N8013 ranged from 100 to 3000, and band sizes using 8017 ranged from 100 to 2000. Plants germinated from cryopreserved seeds showed no difference in DNA polymorphism for each primer.
RAPD banding profiles of N. nucifera generated from control and cryopreserved seeds using different primers; A N8013; B N8017. Molecular weight marker (Ladder 100 bp plus). In each treatment except C1 and C2, none-cryopreservation treatment (LN−) is left, and cryopreservation treatment (LN+) is right
Discussion
After cryopreservation of lotus, an aquatic plant, seeds’ viability, germination and further growth were investigated. For cryopreservation of plant tissues, encapsulation and vitrification are most commonly used (Sakai and Engelmann 2007).
Seed viability showed a significant difference according to the cryopreservation method. Pretreatment with a loading solution such as sugar has been shown to help significantly maintain seed viability. Several previous studies have reported that sucrose pretreatment increases seed and plant viability during cryoprotection (Matsumoto et al. 1994; Kuranuki and Sakai 1995). However, in the results of this study, the glycerol pretreatments showed a high survival rate similar to that of the untreatments. Glycerol has often been used as a cryoprotectant during preculture of excised meristems and freezing of plant material (Reed and Hummer 1995). Glycerol is a polar molecule first used for mouse embryo cryopreservation and is freely miscible with water and simple alcohols (Sillanpää and Ncibi 2017). It is believed that glycerol increases the survival rate of seeds because it has a low ability to penetrate the membrane, so it is less toxic and less likely to cause osmotic shock.
In addition, the mixed treatment of glycerol and sucrose also increased the viability of the seeds. Water-soluble sugars act as osmotic agents and nutrients and interact with the lipid layer to protect plant cells from damage caused by cold stress (Seijo 2000). In addition to these functions, it also serves as an important messenger that transmits metabolic signals. Mixed pretreatment of glycerol and sucrose is considered a significant factor in maintaining the viability of lotus seeds in cryogenic preservation of lotus seeds.
One of the keys to successful cryopreservation is optimizing the dehydration process of the cryoprotection solution to prevent damage from chemical toxicity or osmotic shock. That is, to increase the efficiency of cryopreservation, selecting an appropriate vitrification solution and dehydration time is very important (Ferrari et al. 2016). It is generally known that plant germplasm’s viability depends on the vitrification solution’s treatment time. In this study, as PVS2 treatment time increased, seed viability tended to decrease. Uchendu et al. (2014) also reported that cryopreservation of St John’s wort and tobacco showed optimal growth when exposed to PVS2 for 20 min. Additionally, PVS2 exposure times of 10–25 min at 25 °C have been reported to be optimal for several herbaceous plants (Matsumoto et al. 1994).
It was found that the order of pretreatment using the loading solution and the treatment time of the vitrification solution were complexly important for the cryopreservation of lotus flowers. In this study, the vitrification treatment time and the pretreatment time of glycerol and sucrose were significant. Even after 3 months of cryopreservation, the germination rate was maintained above 60.0%.
Vitrification is closely involved in cell dehydration (Kim et al. 2006). In particular, dehydration by vitrification is considered particularly important in the cryopreservation of lotus, an aquatic plant. Tissues must be dehydrated before cryopreservation because, below freezing, intracellular moisture forms ice crystals, ruptures cells, and causes loss of viability (Mazur 1984). Cell dehydration is performed with a strong cryoprotection solution and air drying. Most freezable cell sap is removed from the cells and becomes aqueous vitrification. Therefore, it is necessary to select an appropriate cryoprotectant.
The cryopreservation treatements showed a lower germination rate than the control. This may be due to damage to plant cells by toxic substances and excessive osmotic pressure of cryoprotectants (Niino et al. 1992). Therefore, optimal cryoprotectant and treatment time will be required to minimize cryogenic seed damage. Although many studies have investigated the cryoprotectant and treatment time, it is still necessary to investigate a treatment method with a sizeable freezing effect without significantly affecting plants.
Seedlings germinated through cryopreservation grew healthy but showed growth inhibition compared to control plants. Cryopreserved tissues report reduced growth. Berjak and Pammenter (2010) also reported that ultracold-preserved Amaryllis belladonna reduced seed germination and reduced biomass in subsequent plants. Plants germinating from cryopreserved seeds exhibited lower CO2 assimilation and stomatal density, abnormal root growth, photosynthetic apparatus damage, and less efficient leaf water potential than control seedlings. However, Villalobos et al. (2019) reported no effect on the growth of cryopreserved sorghum seeds. Growth inhibition of cryopreserved tissue is different for each plant species. However, further research on this is required.
Morphological and genetic changes were not observed in lotus plants germinated after cryopreservation. The band patterns of lotus flowers regenerated using all primers were identical to those of other in vitro seedlings.
Cryopreservation has become an important tool for long-term germplasm storage without genetic modification, using laboratory materials with unique properties and minimizing space and maintenance requirements (Sakai 1997). However, improper cryopreservation techniques can cause genetic changes in regenerated shoots (Harding and Staines 2001). Plant gene mutations can sometimes occur when plants are stressed (Engelmann 2011). Many scientists have focused on preventing genetic changes in cryopreserved and regenerated plant tissue (Skyba et al. 2010; Vasanth and Vivier 2011). In general, it is known that seeds preserved by cryopreservation are less likely to undergo genetic mutation and can be maintained in better physiological conditions (Stanwood 1980). In particular, it has been reported that the vitrification solution used to increase cryopreservation efficiency does not cause structural changes in DNA (Channuntapipat et al. 2003).
However, many studies have reported genetic variations. The genetic stability of regenerated plants was assessed using morphological, biochemical and molecular assays (Matsumoto 2017). The RAPD assay in this study has the advantage of being relatively simple and fast and allowing random screening of large portions of the genome. However, it is sometimes questioned due to its lack of reproducibility, but it is very widely used for genetic stability analysis of tissue culture-derived plants. (Martín et al. 2015; Mandal et al. 2008; Kulus et al. 2019). Genetic diversity was tested using the RAPD method in this study, and there was no difference from the control plants. However, molecular-level studies such as more precise markers and sequencing will be needed to identify more specific genetic variants.
Optimal conditions for cryopreservation of N. nucifera using the vitrification method were summarized (Fig. 5). Lotus seeds were immersed in the loading solution for 20 min before antifreeze treatment and treated for 20 min in PVS2. The seeds were then placed in nitrogen liquid (− 196 °C) for various storage times. Up to 12 weeks, the germination rate was maintained at more than 90%. After thawing at 36–39 °C, the seeds were treated in unLS for 10 min and cultured in MS medium.
Conclusions
The lotus is an ethnic-aquatic plant species with unique biological and nutritional properties. Despite the importance of resources, research on the cryopreservation of aquatic plant seeds is still insufficient. This study established optimal conditions for seed viability, germination, and growth and genetic mutation tests at cryogenic temperatures. This study is judged to have obtained results that prove all of the research hypotheses mentioned above. First, it was found that the cryopreservation of lotus seeds, which are aquatic plants, was not different from that of terrestrial plants. Even cryopreservation of aquatic plants can be pretreated with loading solution followed by appropriate treatment with PVS2 solution followed by cryopreservation. Second, the cryopreservation of terrestrial plants was effective when cryopreserved by the vitrification method using a PVS2 solution. Third, germinated plants were obtained from cryopreserved seeds. Fourth, plants germinated from cryopreserved seeds did not have morphological and genetic variations, so cryopreservation effectively preserved the germplasm of aquatic plants, including lotus for a long period. However, additional research is needed to determine whether the cause of the rapid decrease in the germination rate of lotus seeds following cryogenic storage is due to physiological changes such as accumulation of germination inhibitors or secondary dormancy. In addition, because the freeze-preservation period is too short, long-term preservation studies and studies on the relationship between seed vigor and germination rate and genetic variation by long-term monitoring are needed. However, this study is expected to be widely used for cryopreserved aquatic plants and other wild genetic resources.
Abbreviations
- DMSO:
-
Dimethylsulphoxide
- LN:
-
Liquid nitrogen
- LS:
-
Loading solution
- MS:
-
Murashige and Skoog (1962) medium
- PVS2:
-
Plant vitrification solution 2
- RAPD:
-
Random amplified polymorphic DNA
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
- unLS:
-
Unloading solution
References
Ahn YH, Sakai A (1994) Survival by vitrification of Dendranthema graniflorum L. shoot tips for cryopreservtion. Hortic Environ Biotechnol 35:499–506
Association of Official Seed (AOSA) (1983) Analysts Seed Vigor Testing Handbook. 32:88
Barrientos-Priego AF, Borys MW, Escamilla-Prado E, Ben-Ya'acov A, Cruz-Torres, Lopez-Lopez L (1992) A study of the avocado germplasm resources, 1988–1990. IV. Findings in the Mexican Gulf region. In: Lovatt CJ (ed) Proceedings of the Second World Avocado Congress, California, April 21–26 1991, pp 551–558
Benson EE (2008) Cryopreservation theory. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, New York, pp 15–32
Berjak P, Pammenter NW (2010) Effects of cryopreservation of recalcitrant Amaryllis belladonna zygotic embryos on vigor of recovered seedlings: a case of stress ‘hangover’? Physiol Plant 139:205–219
Chambers PA, Lacoul P, Murphy KJ, Thomaz SM (2008) Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 595:9–26
Channuntapipat C, Sedgley M, Collins G (2003) Changes in methylation and structure of DNA from almond tissues during in vitro culture and cryopreservation. J Am Soc Hortic Sci 128:890–897
Choi YC, Ryu KS, Bang HS (2000) Cryopreservation of Mulberry (Morus) seeds in liquid nitrogen (LN2). Korean J Seric Sci 42:1–5
Cottrell HJ (1947) Tetrazolium salt as a seed germination indicator. Nature 159:748
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Engelmann F (2004) Plant cryopreservation: progress and prospects. In Vitro Cell Dev Biol Plant 40:427–433
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev Biol Plant 47:5–16
Engelmann F (2012) Germplasm collection, storage and preservation. In: Altman A, Hazegawa PM (eds) Plant biotechnology and agriculture. Academic Press, Oxford, pp 255–267
Ferrari EAP, Columbo RC, Faria RT, Takane RJ (2016) Cryopreservation of seeds of Encholirium spectabile Martius ex Schultes f. by the vitrification method. Rev Cienc Agron 47:172–177
Galdiano RF, Lemos EGM, Faria RT, Vendrame WA (2012) Cryopreservation of Dendrobium hybrid seeds and protocorms as affected by phloroglucinol and Supercool X1000. Sci Hortic 148:154–160
Gonzalez-Arnao MT, Engelmann F (2006) Cryopreservation of plant germplasm using the encapsulation-dehydration technique: review and case study on sugarcane. CryoLett 27:155–168
Gowthami R, Sharma N, Pandey R, Agrawal A (2021) A model for integrated approach to germplasm conservation of Asian lotus (Nelumbo nucifera Gaertn.). Genet Resour Crop Evol 68:1269–1282
Harding K, Staines H (2001) Biometric analysis of phenotypic characters of tomato shoot tips recovered from tissue culture. dimethyle sulphoxide treatment and cryopreservation. CryoLett 22:255–262
Kaviani B (2011) Conservation of plant genetic resources by cryopreservation. Aust J Crop Sci 5:778–800
Kim SW, Oh MJ (2009) Establishment of plant regeneration and cryopreservation system from zygotic embryo-derived embryogenic cell suspension cultures of Ranunculus kazusensis. Methods Mol Biol 547:107–115
Kim HH, Cho EG, Park SU (2006) Analysis of factors affecting the cryopreservation of garlic shoot tips. J Biomed Nanotechnol 2:129–132
Kulus D, Rewers M, Serocka M, Mikula A (2019) Cryopreservation by encapsulation-dehydration affects the vegetative growth of chrysanthemum but does not disturb its chimeric structure. Plant Cell Tissue Organ Cult 138:153–166
Kuranuki Y, Sakai A (1995) Cryopreservation of in vitro grown shoot tips of tea (Camelia sinensis) by vitrification. CryoLett 16:345–352
Mandal BB, Ahuja-Ghosh S, Srivastava PS (2008) Cryopreservation of Dioscorea rotundata poir.: a comparative study with two cryogenic procedures and assessment of true-to-type of regenerants by RAPD analysis. CryoLett 29:399–408
Martín C, Kremer C, González I, González-Benito ME (2015) Influence of the cryopreservation technique, recovery medium and genotype on genetic stability of mint cryopreserved shoot tips. Plant Cell Tissue Org Cult 122:185–195
Matsumoto T (2017) Cryopreservation of plant genetic resources: conventional and new methods. Rev Agric Sci 5:13–20
Matsumoto T, Sakai A, Yamada K (1994) Cryopreservation of in vitro-grown apical meristems of Wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13:442–446
Mazur P (1984) Freezing of living cells: mechanisms and implications. Am J Physiol 247:C125–C142
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Niino TA, Yakuwa SSH, Nojiro K (1992) Cryopreservation of in vitro grown shoot tips of apple and pear by vitrification. Plant Cell Tissue Organ Cult 28:261–266
Panis B (2008) Cryopreservation of monocots. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, New York, pp 241–280
Pawlowska B, Szewczyk-Taranek B (2014) Droplet vitrification cryopreservation of Rosa canina and Rosa rubiginosa using shoot tips from in situ plants. Sci Hortic 168:151–156
Phillips G, Willby N, Moss B (2016) Submerged macrophyte decline in shallow lakes: what have we learnt in the last forty years? Aquat Bot 135:37–45
Pritchard HW, Poyner ALC, Seaton PT (1999) Interspecific variation in orchid seed longevity in relation to ultra-dry storage and cryopreservation. Lindleyana 14:92–101
Rajametov S, Lee YY, Kim YC, Lee S, Yi JY, Jeon YA, Sung JS, Lee GA, Gwak JG (2014) Response of pre and post treatments for cryopreservation of Korean ginseng seeds on recovering viability. Korean J Breed Sci 46:408–416
Reed BM, Hummer KE (1995) Conservation of germplasm of strawberry (Fragaria species). In: Bajaj YPS (ed) Cryopreservation of plant germplasm I, vol 32. Springer Verlag, Berlin, pp 354–370
Sakai A (1997) Conservation of plant genetic resources in vitro. In: Razdan MK, Cocking EC (eds) Geneneral aspects, vol I. Science Publishers, New Hampshire, pp 53–66
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLett 28:151–172
Sakai A, Kobayashi S (1990) A simple and efficient procedure for cryopreservation of nucellar cells of navel orange by vitrification. Cryobiology 27:657
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Seaton PT, Hailes NSJ (1989) Effect of temperature and moisture content on the viability of Cattleya aurantiaca seed. In: Pritchard HW (ed) Modern methods in orchid conservation: the role of physiology, ecology and management. Cambridge University Press, Cambridge, pp 17–30
Seijo G (2000) Effects of preculture with sucrose and ABA on cell suspensions water status and its relation with vitrification resistance. Rev Bras Fisiol Veg 12:166–180
Sillanpää M, Ncibi CA (2017) Sustainable bioeconomy: biochemical. Springer, Berlin Heidelberg
Skyba M, Urbanová M, Kapchina-Toteva V, Košuth J, Harding K, Čellárová E (2010) Physiological, biochemical and molecular characteristics of cryopreserved Hypericum Perforatum L. shoot tips. CryoLett 31:249–260
Stanwood PC (1980) Tolerance of crop seeds to cooling and storage in liquid nitrogen (-196 C). J Seed Tech 5:26–31
Stanwood PC, Bass LN (1981) Seed germplasm preservation using liquid nitrogen. Seed Sci Technol 9:423–437
Uchendu EE, Shukla MR, Reed BM, Saxena PK (2014) An efficient method for cryopreservation of St. John’s wort and tobacco: role of melatonin. Acta Hortic 1039:233–241
Vasanth K, Vivier MA (2011) Improved cryopreservation procedure for long term storage of synchronised culture of grapevine. Biol Plant 55:365–369
Villalobos A, Arguedas M, Escalante D, Martínez J, Zevallos BE, Cejas I, Yabor L, Martínez-Montero ME, Sershen S, Feijoo JCL (2019) Cryopreservation of sorghum seeds modifies germination and seedling growth but not field performance of adult plants. J App Bot Food Qual 92:94–99
Zamecnik J, Faltus M, Bilavcik A (2021) Vitrification solutions for plant cryopreservation: modification and properties. Plants 10:2623
Acknowledgements
The authors are grateful to the Korea National Arboretum researcher who distributed lotus seeds and advised on the direction of the study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SHY, MSC and MJJ: conceived and conceptualized the manuscript. SHY: wrote and prepared the original draft. SHY, KBP, YWS, DHK: conducted the experiments. MJJ: provided the plant material and contributed to the experiments. SHY, KBP: analysed data. SHY, KBP, MSC: contributed to review and critically revise the manuscript. SHY and MSC: visualized and carefully supervised the work and the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or fnancial relationships that could be construed as a potential confict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yong, S.H., Park, K.B., Seol, Y.W. et al. Optimization conditions for cryopreservation of Nelumbo nucifera Gaertn., aquatic plant. Plant Biotechnol Rep 17, 625–635 (2023). https://doi.org/10.1007/s11816-023-00860-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-023-00860-7