Abstract
This study compared the effect of the cryopreservation protocol, the genotype and the plant growth regulators used in the recovery media on the genetic stability of mint shoot tips. Shoot tips of two micropropagated genotypes of mint were cryopreserved either using an encapsulation-dehydration or droplet-vitrification protocol. Three recovery media were tested, all of them MS based although with different plant growth regulators: (1) 0.5 mg L−1 6-benzylaminopurine (BAP); (2) 0.5 mg L−1 6-dimethylallylamino-purine (2iP) + 0.1 mg L−1 α-naphthalene acetic acid (NAA); and (3) 0.5 mg L−1 BAP + 0.1 mg L−1 NAA. DNA was extracted from three different types of samples: leaves from shoots, callus at the base of shoots and callus. RAPD markers were used to assess the genetic stability. One of the genotypes, ‘MEN 198’, showed higher percentage of stable samples than the other one, ‘MEN 186’ (97 vs. 42 %; considering all media, protocol and type of explant). The material recovered after droplet-vitrification showed higher stability compared to the encapsulation-dehydration recovered samples: 99 versus 87 % in ‘MEN 198’, and 80 versus 24 % in ‘MEN 186’. There were not large differences in the genetic stability obtained from the three types of samples in ‘MEN 196’ (85–100 %), while in ‘MEN 186’ calli were more unstable (53, 44 and 30 % stability for leaves, basal callus and callus, respectively). The effect of the recovery medium on the stability of the samples was noticeable in the more unstable types of explants but not in leaves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aromatic plants have experienced in the last years an increased attention and importance as crops due to their demand by consumers for culinary, medicinal and other applications. In this context, search of wild genotypes, breeding programmes and genotype conservation are some of the tasks involved in the development of the production of these species (Lubbe and Verpoorte 2011).
One of the most reliable methods for long-term conservation is cryopreservation (conservation of living material at temperature below −150 °C), mainly due to its capability to guarantee the genetic stability of the preserved material during the process (Martín et al. 2013). However, the stresses suffered due to the treatments applied to avoid cell damage together with the in vitro culture, required for the recovery of the plant material, may affect the quality and quantity of the response after cryopreservation. Several steps of the cryopreservation protocols should be optimized before the application of these techniques to a given species (or even to a specific genotype). The aim is not only to obtain high survival after recovery but fully viable structures, i.e. shoot apices growing to form shoots without callus formation. This makes necessary to optimize the recovery conditions after removal from liquid nitrogen (or the vapour phase; −150 °C). Besides the environmental conditions (e.g. light conditions), the composition of the growth recovery medium is also important. In some species the removal of auxins led to a reduction of callus formation (Chang and Reed 1999). Complete success in cryopreservation process implies not only a high recovery rate after liquid nitrogen immersion, but also obtaining true-to-type plants. Therefore, the study of the genetic stability of cryopreserved material should also be considered (Harding 2004; Martín and González-Benito 2005; Martín et al. 2011).
During the last years, greater attention has been given to genetic stability studies of cryopreserved plant material. In vitro cultures of shoot tips are considered more stable than other types of cultures (e.g. callus). No genetic changes were detected by RAPD or AFLP markers after cryopreservation of in vitro shoot tips of Betula pendula Roth (Ryynanen 1998), Vitis and Actinidia (Zhai et al. 2003), Arachis (Gagliardi et al. 2003), Humulus (Peredo et al. 2008) and Prunus (Helliot et al. 2002). However, examples of detection of genetic instability can also be found. One line out of eight of Rabdosia rubescens shoot tips was found to be variable after cryopreservation by encapsulation-dehydration, amounting to a variance rate of 0.01 % (Ai et al. 2012). In some cases genetic variation has been detected in control and cryopreservation-treated (without immersion in liquid nitrogen) individuals, which indicates that in vitro culture and the treatments applied (not the low temperature itself) could be responsible for such variability: this is the case of papaya shoot tips cryopreserved by vitrification (Kaity et al. 2013). On the other hand, genetic polymorphism was found in Rubus spp. shoots after a long period of in vitro culture and not in the cryopreserved shoots or in the field-grown plants derived from those shoots, indicating transitory genetic changes (Castillo et al. 2010). However, few studies have been carried out to reveal which procedures applied during cryopreservation could be related to those genetic changes. The pre-treatments applied to the plant material and the recovery medium, among other factors, could have independent or synergetic influences. When two cryopreservation protocols (encapsulation-dehydration vs vitrification) were compared with chrysanthemum in vitro-grown shoot tips, higher genetic instability was detected after the first one (Martín and González-Benito 2005). Those changes appeared from the protocol step in which tips were precultured on medium with high sucrose concentration onwards (Martín et al. 2011).
During the development of cryopreservation protocols, it is important to obtain direct regrowth and to avoid callus formation (Engelmann 2011). Therefore, it is imperative to study the factors that could influence this response. The origin of the variations is usually attributed to the application of in vitro culture conditions during the process rather that to the very low temperatures (Keller et al. 2008). The fact that cryopreservation processes involve exposure to extreme physiological conditions should be taken into account. These include not only low temperature, but also dehydration stresses, together with the use of substances potentially toxic and/or mutagenic, such as dimethyl sulfoxide, DMSO (Ashwood-Smith 1985; Ipser 1992).
RAPD markers are quick and easy to perform. Other advantages of these markers are that small quantities of DNA are required and previous knowledge of DNA sequences is not necessary (Kumar et al. 2009). They have been previously used in genetic stability studies (for example, Aronen et al. 1999; Gagliardi et al. 2003; Martín and González-Benito 2005; Sánchez et al. 2008). A higher number of markers is usually detected with AFLP; however, both techniques have been demonstrated to be useful for detection of variation after cryopreservation and the latter one is much more expensive and time-consuming (Martín et al. 2011).
Mint (Mentha × piperita L.) is an infertile hybrid with vegetative propagation; this makes cryopreservation a very useful conservation technique, avoiding the risks related to field collections as well as time consumption and labour costs of in vitro conservation (Martín et al. 2013). Cryopreservation conditions must be controlled to guarantee an adequate regrowth rate and the quality of the recovered material. Several cryopreservation protocols have been developed and compared for mint: controlled cooling (Towill 1988; Uchendu and Reed 2008), vitrification (Towill 1990; Volk and Walters 2006; Uchendu and Reed 2008), encapsulation-dehydration (Hirai and Sakai 1999; Sakai et al. 2000; Uchendu and Reed 2008), encapsulation-vitrification (Hirai and Sakai 1999; Sakai et al. 2000), droplet-vitrification (Senula et al. 2007), and vitrification cryo-plates (Yamamoto et al. 2012).
In order to develop appropriate cryopreservation protocols the possible influence of different factors on genetic stability should be studied. Therefore, we examined the influence of the recovery medium, cryopreservation technique and genotype on the stability of mint recovered biological material after cryopreservation. This paper presents a series of cryopreservation experiments after which DNA was extracted from different types of recovered plant material to study their RAPD profiles.
Materials and methods
Plant Material and Preconditioning Treatments
The work was carried out with two mint accessions (Mentha × piperita) received from the genebank of IPK (Leibniz Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany), namely ‘MEN 186’ and ‘MEN 198’. Shoots had been micropropagated by shoot culture for approximately 10 months in our laboratory by monthly subcultures on MS medium (Murashige and Skoog 1962), pH 5.7–5.8, with 3 % sucrose, 0.7 % agar and no growth regulators, and incubated at 25 °C, with a 16 h photoperiod and a Photosynthetic Photon Flux Density (PPFD) of 50 µmol m−2 s−1 from cool white fluorescent tubes.
Nodal segments were excised from the in vitro shoots and cultured on MS medium supplemented with 3 % sucrose, and incubated for 3 weeks at 25 °C (light)/−1 °C (dark) with a photoperiod of 16 h and 50 µmol m−2 s−1 PPFD. Subsequently, shoot tips (approx. 2 mm long, with 1–2 pairs of leaf primordia) were excised, transferred to liquid MS medium supplemented with 0.3 M sucrose, and cultured at 25 °C for one day, with a PPFD of approximately 10 µmol m−2 s−1, with 16 h photoperiod.
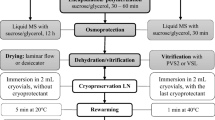
Cryopreservation by encapsulation dehydration
Pre-conditioned shoot tips were immersed in a 3 % sodium alginate solution prepared in MS liquid medium with 0.4 M sucrose. Drops of this solution, each one containing a shoot tip, were dispensed in liquid MS medium containing 100 mM CaCl2 and 0.4 M sucrose. The formed beads were maintained in that solution for approximately half an hour. Subsequently, beads were cultured in MS liquid medium containing 0.75 M sucrose, at 120 rpm, for 16–18 h (of which 8 h were in the dark and the rest under a PPFD of approximately 10 µmol m−2 s−1). After that period, beads were removed, the excess of liquid dried from the surface with sterile filter paper, and placed on open Petri dishes to be dried for 5 h under the flow of a laminar-flow bench (to 22 ± 1 % water content fresh weight basis, Teixeira et al. 2014). Beads were then included in cryovials (five beads per cryovial) and rapidly immersed in liquid nitrogen (LN), and kept there for at least 1 day. Warming took place in a water bath at 40 °C, for approximately 2 min. Beads were then cultured on the three recovery media tested. Beads containing shoot tips that had been subjected to all procedures except immersion in LN were used as the non-cryopreserved samples.
Cryopreservation by droplet-vitrification
A modified protocol of Senula et al. (2007) was used. Pre-conditioned apices were immersed in a loading solution (MS liquid medium + 2 M glycerol + 0.4 M sucrose), for 20 min at room temperature. After that, apices were maintained in the plant vitrification solution PVS2 (30 % w/v glycerol, 15 % w/v ethylene glycol and 15 % w/v DMSO and 0.4 M sucrose in liquid MS medium; Sakai et al. 1990) on ice (approx. 0 °C) for 30 min. Finally, shoot tips were placed into droplets (2 µL) of PVS2 on aluminium foil strips (five explants per strip, each one in a droplet). These were included into cryovials (one strip per cryovial); the cryovials were closed and immersed in liquid nitrogen (LN), for at least 1 day. Warming was carried by placing the cryovials in a water bath at 40 °C for 20 s. Subsequently, 1 mL of rinsing solution (1.2 M sucrose in MS) was added to each cryotube. The tubes were slightly shaken, and the content was transferred into Petri dishes with 2 mL of rinsing solution and kept at room temperature for 20 min. Shoot tips were then cultured on the three recovery media tested; those ones that had been subjected to all procedures except immersion in LN were used as the non-cryopreserved samples.
Recovery
Beads/shoot tips from both cryopreservation protocols were cultured on MS semisolid medium supplemented with the following growth regulators: (1) 0.5 mg L−1 6-benzylaminopurine (BAP; Uchendu and Reed 2008), (2) 0.5 mg L−1 6-dimethylallylamino-purine (2iP) + 0.1 mg L−1α-naphthalene acetic acid (NAA; Senula et al. 2007); and (3) 0.5 mg L−1 BAP + 0.1 mg L−1 NAA. These media were named “Reed” (R), “Senula” (S) and “Nodes” (N), respectively. In each experiment, 2–6 replicates for non-cryopreserved samples and 4–9 replicates for cryopreserved ones were used. A replicate was a 6 cm Petri dish (10 mL medium) containing 5 beads or shoot tips. Cultures were incubated at 25 °C, the first 24 h under darkness followed by 20 days with of approximately 10 µmol m−2 s−1 PPFD and then transferred to 50 µmol m−2 s−1, always with a 16 h photoperiod. For the experiments in which DNA isolation was carried out after an 8-week culture period, explants were transferred to culture jars with MS medium without growth regulators, after the first 4-week culture on the recovery media.
RAPD analysis
Surviving explants after the two cryopreservation protocols (droplet-vitrification,”D”, and encapsulation-dehydration, “E”), from both genotypes and the three different recovery media, were collected for DNA extraction, after 4- or 8-week recovery, depending on the experiment. Non-cryopreserved, i.e. treated as the cryopreserved shoot tips except for the immersion in LN (“0”), and cryopreserved (“+”) samples were included in the study, as well as portions of leaves from in vitro shoots (in vitro control samples). From the non-cryopreserved and cryopreserved explants, samples were obtained from leaves of shoots (“L”) and the small basal callus that sometimes appeared (“cL”), and from explants that resulted only in callus growth after treatments (“C”; Fig. 1). They were stored at –80 °C until DNA isolation, which was carried out as described by Gawel and Jarret (1991) with minor modifications. DNA concentration was estimated by comparison with DNA standards in a 0.8 % (w/v) agarose gel. RAPD profiles were generated using eight arbitrary primers from Operon Technologies: OPA-11 (5′ CAATCGCCGT 3′), OPE-19 (5′ ACGGCGTATG 3′), OPF-1 (5′ ACGGATCCTG 3′), OPF-3 (5′ CCTGATCACC 3′), OPF-10 (5′ GGAAGCTTGG 3′), OPO-7 (5′ CAGCACTGAC 3′), OPO-10 (5′ TCAGAGCGCC 3′) and OPO-20 (5′ ACACACGCTG 3′). DNA amplification reactions were performed in a volume of 25 µL containing approximately 10 ng total DNA, 0.4 µM of a single decanucleotide, 0.2 mM of each dNTP, 1.5 mM of MgCl2 and 1U Taq DNA polymerase in the buffer provided by the enzyme manufacturer (Biotools). The PCR amplifications were performed in a DNA Thermal Cycler (Eppendorf) using one cycle of 1 min at 94 °C, followed by 35 cycles of 45 s at 92 °C, 1 min at 37 °C and 2 min at 72 °C, and a final cycle of 3 min at 72 °C. Aliquots of 12 µL of amplification products were loaded onto 1.5 % (w/v) agarose gels for electrophoresis (carried out at 110 V) using 1× TBE running buffer (Sambrook et al. 1989), followed by staining with ethidium bromide (0.5 µg/ml) for 7–10 min and photographed under UV light. The size of the amplified bands was related by reference to the molecular size marker (100 Base- Pair Ladder, GE Healthcare). All the amplifications were repeated at least twice, and only bands reproducible in several runs were considered for analysis.
The genetic stability of plant material obtained from both techniques (encapsulation-dehydration and droplet-vitrification) and recovered on the three different media was analyzed in an experiment carried out with ‘MEN 198’; 15–22 samples per combination of medium and technique were used, which represented between 22.4 and 68.7 % of the survived explants. In a separate experiment, samples from ‘MEN 186’ were analysed after cryopreservation following both encapsulation-dehydration and droplet-vitrification techniques (18 and 20 samples, respectively; which represented between 28.6 and 55.5 % of the survived explants) and recovered on “Reed” medium. In these two experiments, the plant material was collected after 4 weeks in culture.
In order to further study the behaviour of shoot tips after the encapsulation-dehydration protocol, the genetic stability of samples recovered in all three media was studied in both genotypes after an 8-week culture period; 12–16 samples per combination of genotype and medium were analysed (between 44.4 and 72.2 % of the survived explants).
Data analysis
Amplified fragments from the RAPD analyses of the pre-treated and cryopreserved samples as well as the in vitro plant were scored as present (1) or absent (0). Genetic similarities were calculated using the Jaccard similarity coefficient. The resultant matrix was subjected to cluster analysis by the unweighted pair-group method analysis (UPGMA) and a dendrogram was constructed from the clustering results with the TREE programme, using the package NTSYS-PC version 1.8 (Rohlf 1992).
Results
The eight primers used yielded between 70 and 73 scorable bands (depending on the experiment), ranging from 2500 to 300 bp (Fig. 2). From these results, one divergence in the presence or absence of a single amplified fragment represented a dissimilarity ranging from 1.3 to 4.6 %.
RAPD banding profiles of DNA samples from cryopreserved material using the encapsulation-dehydration protocol (E) of mint accession ‘MEN 198’ and regenerated on three different media. Amplification products were generated by primer OPO-7. M 100 Base-Pair Ladder marker. Arrows show differences in the banding profile
The analysis of the RAPD markers obtained from samples of genotype ‘MEN 198’ recovered after the two cryopreservation techniques on three different recovery media yielded 73 scorable markers for the 106 samples analysed (including the in vitro control). Fifty markers were monomorphic, being therefore 31.5 % polymorphic. However, the differences affected only to 3 out of 106 samples studied (Fig. 3). These samples (DN0cL5, EN + cL9 and ES + cL51) corresponded to calli that were formed at the base of shoots (Fig. 1a); however, the level of their dissimilarity varied. Sample DN0cL5, obtained after the droplet protocol, presented a similarity value higher than 95 % with the rest of the samples, while EN + cL9 and ES + cL51, from the encapsulation-dehydration technique, presented a considerable lower value of similarity (77 %). These results revealed the high genetic stability of genotype ‘MEN 198’, not related to the technique used nor the media, although the only two samples clearly different corresponded to the encapsulation-dehydration protocol. All of the ten callus (“C”) samples included were stable. The percentage of callus formation (“C” samples) observed in this experiment became strongly influenced by the culture medium, being “Reed” medium the one which resulted in a reduced callus formation. Higher callus formation in cryopreserved explants (“ + ”) than in non-cryopreserved (“0”) was also observed (15 vs. 1.8 %).
Dendrogram generated by the UPGMA method using Jaccard’s similarity coefficient, based on RAPD markers of samples from mint plant material recovered 4 weeks after cryopreservation of accession ‘MEN 198’, using the encapsulation-dehydration technique (E) or droplet-vitrification (D). Samples were recovered on “Senula” (S), “Reed” (R) or “Nodes” (N) medium. Samples included those immersed in liquid nitrogen (+), non-cryopreserved (0, i.e. treated similarly without immersion in liquid nitrogen) and in vitro cultures from which explants were obtained to perform the experiments (VITRO); the types of samples studied were: leaves (L), callus at the base of the shoots (cL), or callus (C). The last numbers indicate sample number. The names of the stable samples have been included at a bigger size for easiness of reading
The two cryopreservation protocols were compared in genotype ‘MEN 186’ using “Reed” as recovery medium. Seventy reliable markers were analyzed for the 39 samples studied. In this genotype, a lower percentage of polymorphic markers was obtained (15.7 %) than in the case of ‘MEN 198’. However, the number of different samples was higher than in the previous study with ‘MEN 198’: twenty showed a different pattern compared to the in vitro control, which represented 51 % of the studied samples (Fig. 4). Another important difference was that the cryopreservation method seemed to have influenced the genetic stability. All the samples from the droplet-vitrification protocol, except two, clustered together with the in vitro sample, revealing a high genetic stability. Nevertheless, the only two samples out of this cluster had a similarity coefficient close to 98 %. These samples had been obtained after recovery from LN and were callus (DR + C11) or callus obtained from the base of shoot (DR + cL20). On the other hand, all the samples recovered from the encapsulation-dehydration protocol showed some degree of variation compared to the in vitro material. Among them, all the specimens corresponding to leaves (“L”) clustered together and showed a similarity degree with the in vitro sample higher than 95 % (Fig. 4), whether they were derived from apices immersed into LN or not. However, all the samples from callus (“C”) or basal callus (“cL”) showed a similarity coefficient of 92 %.
Dendrogram generated by the UPGMA method using Jaccard’s similarity coefficient, based on RAPD markers of samples from mint plant material recovered 4 weeks after cryopreservation of accession ‘MEN 186’, using the encapsulation-dehydration technique (E) or droplet-vitrification (D). All samples were recovered on “Reed” medium (R). Samples included those immersed in liquid nitrogen (+), non-cryopreserved (0, i.e. treated similarly without immersion in liquid nitrogen) and in vitro cultures from which apices were obtained to perform the experiments (VITRO); the types of samples studied were: leaves (L), callus at the base of the shoots (cL), or callus (C). The last numbers indicate sample number
A further analysis, considering only the encapsulation-dehydration protocol, was carried out with both genotypes. This time, samples were collected after 8 weeks of recovery. As it was observed before, genotype ‘MEN 198’ was very stable (Fig. 5). Seventy two scorable markers were obtained from the 42 samples analysed (including the in vitro sample), 61 markers were monomorphic, being therefore 15.3 % polymorphic. Five different genotypes were detected; the one corresponding to the in vitro material included 71 % of the samples, and a second genotype, with a percentage of similarity higher than 98 %, included 22 % of the samples. Only two samples showed a percentage of similarity with the in vitro control lower than 95 % (near 92 %), and both corresponded to basal callus samples (“cL”) obtained after cryopreservation. No differences related to the recovery media were detected, as at the 98 % similarity 94, 92 and 100 % of the samples were stable, for “Nodes”, “Senula” and “Reed” media, respectively.
Dendrogram generated by the UPGMA method using Jaccard’s similarity coefficient, based on RAPD markers of samples from mint plant material recovered 8 weeks after cryopreservation of accession ‘MEN 198’, using the encapsulation-dehydration technique (E). Samples were recovered on “Senula” (S), “Reed” medium (R) or “Nodes” (N) medium. Samples included those immersed in liquid nitrogen (+), non-cryopreserved (0, i.e. treated similarly without immersion in liquid nitrogen) and in vitro cultures from which apices were obtained to perform the experiments (VITRO); the types of samples studied were: leaves (L), callus at the base of the shoots (cL), or callus (C). The last numbers indicate sample number
In this analysis after 8-week recovery, the more unstable behaviour of genotype ‘MEN 186’ was confirmed compared to ‘MEN 198’. Only 55 % of the samples were identical to the in vitro control (Fig. 6), and 24 % showed a similarity coefficient below 95 %, being 85 % the lowest value. Regarding the recovery media used during the first 4 weeks, and considering 98 % similarity, 42 % of the samples from “Nodes” medium were stable, while samples from “Senula” and “Reed” reached 71 and 67 % stability, respectively. When the kind of tissue analysed was considered, some differences were detected. Seventy percent of leaf tissue samples (“L”) were stable, while callus at the base of the shoots (“cL”) and callus (“C”) tissues samples presented a stability percentage of 41 and 50 %, respectively. However, considering the stability of leaf samples (as the recovered plant material that would be further maintained), no effect of the media could be detected being 60 % in “Nodes”, 71 % in “Senula” and 67 % in “Reed”. On the other hand, the overall stability of “cL” and “C” samples varied with the recovery media: 29, 71 and 67 %, respectively for “Nodes”, “Senula” and “Reed”. These results could be related to an interaction between media and type of explants. In this genotype (‘MEN 186’), callus formation was again higher in those explants immersed in LN than in the non-cryopreserved. Likewise, a lower percentage of callus was observed in “Reed” medium (2 %) than in “Nodes” (10 %) and “Senula” (30 %) media. No effect of the immersion in liquid nitrogen was observed on stability, as 56 and 54 % of the non-cryopreserved and cryopreserved samples, respectively, were similar to the in vitro plant material.
Dendrogram generated by the UPGMA method using Jaccard’s similarity coefficient, based on RAPD markers of samples from mint plant material recovered 8 weeks after cryopreservation of accession 'MEN 186', using the encapsulation-dehydration technique (E). Samples were recovered on “Senula” (S), “Reed” medium (R) or “Nodes” (N) medium. Samples included those immersed in liquid nitrogen (+), non-cryopreserved (0, i.e. treated similarly without immersion in liquid nitrogen) and in vitro cultures from which apices were obtained to perform the experiments (VITRO); the types of samples studied were: leaves (L), callus at the base of the shoots (cL), or callus (C). The last numbers indicate sample number
Discussion
In the present study, the influence of recovery media and genotype have been compared in two cryopreservation protocols (encapsulation-dehydration and droplet-vitrification) in order to evaluate their effect on the genetic stability of the regenerated plants. Our results show a clear effect of the genotype and cryopreservation protocol on the genetic stability of the recovered samples. Genotype ‘MEN 198’ resulted more stable than ‘MEN 186’ and the encapsulation-dehydration protocol resulted in a higher number of unstable samples, which was more evident with the samples of ‘MEN 186’ (94 vs. 0 % stable samples for droplet-vitrification and encapsulation-dehydration, respectively).
The ‘genotype’ effect is well known in almost any tissue culture experiment. Different response of genotypes to the same treatment is a widespread result and force researchers to find the most convenient solution for each of them. Cryopreservation is not an exception, and in diverse works different results have been observed depending on the genotype (Reed 2001). For example, one out four Carica papaya genotypes of which shoot tips had been cryopreserved with a vitrification-based protocol showed a great level of variation, both in control and cryopreserved plants (Kaity et al. 2013).
This is not the first report on the negative effect of the encapsulation-dehydration protocol on the genetic stability of the cryopreserved material. Genetic instability in chrysanthemum regenerated plantlets was observed when encapsulation-dehydration was the shoot cryopreservation method used, but no variants were found after the vitrification protocol (Martín and González-Benito 2005). Similarly, Ai et al. (2012) used sequence-related amplified polymorphism (SRAP) markers to detect the DNA-level variation on shoots of Rabdosia rubescens recovered after an encapsulation-dehydration protocol and observed a variance rate of 0.01 %. Furthermore, different cryopreservation protocols can render, not only dissimilarities in the genetic stability of recovered specimens, but also differences in the recovery capability of the cryopreserved material. Matsumoto (2001) compared the effect of different cryopreservation protocols on wasabi (Eutrema japonicum Miq.) shoot-tips and detected a significant lower recovery with encapsulation-dehydration than with encapsulation-vitrification or vitrification. The histological study of the regenerated material showed that nearly all of the cells remained viable in the vitrification protocols (combined or not with encapsulation), while massive structural damage was observed in the encapsulated-dehydrated meristems, except for the dome area. Similar results have been found comparing survival and recovery (shoot development) rates in cryopreservation of mint genotypes ‘MEN 186’ and ‘MEN198’ using the two types of protocols considered in this work (Kremer et al. in press). Shoot regrowth after encapsulation-dehydration was lower than after droplet-vitrification in pretreated non-cryopreserved (no LN) explants of both genotypes when recovery took place on “Nodes” or “Senula” media; however, on “Reed” medium recovery percentages were similar after both protocols, and higher than with the other two media. In cryopreserved apices, recovery was low (<50 %) in both methods, except when recovery was carried out on “Reed” medium; the effect of encapsulation-dehydration process seems to affect not only the genetic stability, but also the recovery. Matsumoto et al. (2013), working again with wasabi plants regenerated from long-term cryopreserved shoot tips, did not observed differences in the RAPD profiles of the cryopreserved material using a vitrification protocol; however, in this study, analyses of encapsulation-dehydration protocol were not included.
The negative effect of the encapsulation-dehydration protocol on stability is related to the treatments, as it can be observed both in pretreated and in cryopreserved (immersed in LN) samples. For example, in the encapsulation-dehydration experiments of genotype ‘MEN 198’, the genetic stability of non-cryopreserved and cryopreserved samples was 77 and 68 %, respectively. Similarly, the values observed in the more unstable genotype ‘MEN 186’ were 56 % for the non-cryopreserved samples and 55 % for cryopreserved ones. The main effect of the pre-treatment, rather than the ultra low temperature per se, could also be observed in a vitrification protocol in papaya since variants were found among vitrification-treated (not immersed in LN) plants (Kaity et al. 2013). The origin of the genetic instability in cryopreserved cultures, when it occurs, has been discussed (Harding 2004; Kulus and Zalewska 2014), and the effect of extreme low temperatures has been considered not the main source of variation. Our results in mint confirm this theory: the genetic instability appears during the pre-treatment in those protocols prone to instability, as encapsulation-dehydration seems to be (Martín et al. 2011).
In this work, not only the effect of the cryopreservation technique employed, but also the recovery media composition (i.e. plant growth regulators) has been taken into account to analyze the genetic stability of the mint samples. Previous studies by our group (Kremer et al. in press) have demonstrated that the use of growth medium without auxins (i.e. medium “Reed”) produced higher recovery (shoot development) after cryopreservation than when auxins were included (i.e. “Nodes” and “Senula” media). Similarly, reduction of callus appearance and increased regrowth was observed after cryopreservation of quince nodal section by removing auxin from the recovery medium (Lynch et al. 2014). In the present work, in the more unstable genotype (‘MEN 186’) better stability results were observed in samples regenerated on “Senula” medium after cryopreservation ("Senula" 71.4 % vs "Nodes" 57.1 % and "Reed" 37.5 %). Considering both genotypes, and for the encapsulation-dehydration protocol, the samples that accumulated a higher quantity of variation corresponded to “Nodes” medium, mainly when the type of sample considered was callus (alone or at the base of the shoot). The highest callus formation was also found in this medium, and the increment of callus response was mainly observed in the explants immersed in LN (Kremer et al. in press). The effect of cryopreservation on callus formation was reported by He et al. (1998), who found that cryopreservation improved the rate of callus induction in Oryza meyeriana (Zoll. & Moritzi) Baill. In fact, most of the variable samples detected in this work corresponded to callus at the base of the shoots, though also some leaf samples showed instability. Although media composition is usually no taken into account, compared with other major factors such as the technique used, recovery medium is often important whether meristems or cell cultures are involved (Reed 1993).
The capability of the explants to recover into a well differentiated structure, without callus formation, has a very important role in the genetic stability of the resulted plant, and is directly affected by the medium composition. The dedifferentiation process in callus, followed by redifferentiation events when indirect shoots are developed, leads to frequent genetic modifications (Munthali et al. 1996; Al-Zahim et al. 1999; Salvi et al. 2001). Similarly, plants derived from calli of Oryza rufipogon cryopreserved by slow cooling showed 4.78–7.25 % change from control plants (seed-derived), when analysing molecular data generated using simple sequence repeat markers (SSR), suggesting that both callus induction and cryopreservation induced genetic variations (Zeliang et al. 2010). Although the correlation between callus formation and higher genetic stability is usually considered, the capability to regenerate genetically stable shoots from unstable calluses has been reported in chrysanthemum (Miñano et al. 2014). Analyzing different tissues from organogenic callus in chrysanthemum ‘Red Reagan’ the authors found that even when the original callus showed genetic variations, the shoots originated from it were stable, pointing to some kind of selective regeneration from stable callus cells.
In conclusion, the success of a cryopreservation protocol depends of many factors which can affect differently to each genotype. Reducing as much as possible the effect of each of these factors in order to establish an universal protocol for each species would be desirable. However, different critical points can be found in each technique or genotype, although some common factors can be considered to offer a more suitable protocol. In our case, the use of droplet-vitrification protocol instead of encapsulation-dehydration seems to facilitate a more stable pathway for mint cryopreservation. Likewise, our results pointed to the pre-treatment factors as genetic variation source and not the LN immersion per se. Further studies on the effect of these pre-treatments would be necessary to understand the genetic instability process linked to encapsulation-dehydration protocols and to offer safer procedures. Likewise, phenotypical analyses of ex vitro plants obtained after cryopreservation using these protocols should be considered in the future to relate phenotype to the polymorphism revealed by the molecular markers.
References
Ai P-F, Lu L-P, Song J-J (2012) Cryopreservation of in vitro-grown shoot-tips of Rabdosia rubescens by encapsulation-dehydration and evaluation of their genetic stability. Plant Cell Tiss Organ Cult 108:381–387
Al-Zahim MA, Ford-Lloyd BV, Newbury HJ (1999) Detection of somaclonal variation in garlic (Allium sativum L.) using RAPD and cytological analysis. Plant Cell Rep 18:473–477
Aronen TS, Krajnakova J, Häggman HM, Ryynänen LA (1999) Genetic fidelity of cryopreserved embryogenic cultures of open-pollinated Abies cephalonica. Plant Sci 142:163–172
Ashwood-Smith MJ (1985) Genetic damage is not produced by normal cryopreservation procedures involving either glycerol or dimethyl sulfoxide: a cautionary note, however, on possible effects of dimethyl sulfoxide. Cryobiology 22:427–433
Castillo NRF, Bassil NV, Wada S, Reed BM (2010) Genetic stability of cryopreserved shoot tips of Rubus germplasm. In Vitro Cell Dev Biol-Plant 46:246–256
Chang YJ, Reed BM (1999) Extended cold acclimation and recovery medium alteration improve regrowth of Rubus shoot tips following cryopreservation. CryoLetters 20:371–376
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev Biol-Plant 47:5–16
Gagliardi R, Pacheco G, Carneiro L, Valls J, Vieira M, Mansur E (2003) Cryopreservation of Arachis species by vitrification of in vitro grown shoot apices and genetic stability of recovered plants. CryoLetters 24:103–110
Gawel NJ, Jarret RL (1991) A modified CTAB DNA extraction procedure of Musa and Ipomoea. Plant Mol Biol Rep 9:262–266
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. CryoLetters 25:3–22
He G, Shu L, Liao L, Yin X, Sheng L, Wang X (1998) Somatic cell preservation and protoplast regeneration of important disease resistant wild rice Oryza meyeriana Baill. Sci China 41:393–399
Helliot B, Madur D, Dirlewanger E, De Boucaud MT (2002) Evaluation of genetic stability in cryopreserved Prunus. In Vitro Cell Dev Biol-Plant 38:493–500
Hirai D, Sakai A (1999) Cryopreservation of in vitro-grown axillary shoot-tip meristems of mint (Mentha spicata L.) by encapsulation-vitrification. Plant Cell Rep 19:150–155
Ipser J (1992) Effect of dimethylsulfoxide on methylmethanesulfonate induced chromosomal aberrations in Crepis capillaris cultivated in vitro. Biol Plant 35:137–139
Kaity A, Drew RA, Ashmore SE (2013) Genetic and epigenetic integrity assessment of acclimatized papaya plants regenerated directly from shoot-tips following short- and long-term cryopreservation. Plant Cell Tiss Organ Cult 112:75–86
Keller ERJ, Senula A, Kacznarczyk A (2008) Cryopreservation of herbaceous dicots. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, NewYork, pp 281–332
Kremer C, Martín C, González I, González-Benito ME (in press) Regeneration in mint (Mentha × piperita) cryopreserved apices: can the cryopreservation technique, regeneration medium and genotype affect the final results? Acta Horticulturae
Kulus D, Zalewska M (2014) Cryopreservation as a tool used in long-term storage of ornamental species—a review. Sci Hortic 168:88–107
Kumar P, Gupta VK, Misra AK, Modi DR, Pandey BK (2009) Potential of molecular markers in plant biotechnology. Plant Omics 2:141–162
Lubbe A, Verpoorte R (2011) Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind Crop Prod 34:785–801
Lynch PT, Siddika A, Mehra A, Benelli C, Lambardi M (2014) Cryopreservation of quince (Cydonia oblonga Mill.). CryoLetters 35:188–196
Martín C, González-Benito ME (2005) Survival and genetic stability of Dendranthema grandiflora Tzvelev shoot apices after cryopreservation by vitrification and encapsulation-dehydration. Cryobiology 51:281–289
Martín C, Cervera MT, González-Benito ME (2011) Genetic stability analysis of chrysanthemum (Chrysanthemum × morifolium Ramat) after different stages of an encapsulation-dehydration cryopreservation protocol. J Plant Physiol 168:158–166
Martín C, Senula A, González I, Acosta A, Keller ERJ, González-Benito ME (2013) Genetic identity of three mint accessions stored by different conservation procedures: field collection, in vitro and cryopreservation. Genet Resour Crop Evol 60:243–249
Matsumoto T (2001) Cryopreservation of in vitro-cultured meristems of wasabi. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm—current research progress and applications. JIRCAS/IPGRI, Tsukuba (Japan)/Rome (Italy), pp 212–216
Matsumoto T, Akihiro T, Maki S, Mochida K, Kitagawa M, Tanaka D, Yamamoto S-I, Niino T (2013) Genetic stability assessment of wasabi plants regenerated from long-term cryopreserved shoot tips using morphological, biochemical and molecular analysis. CryoLetters 34:128–136
Miñano HS, Ibáñez MA, González-Benito ME, Martín C (2014) Sequential study of the genetic stability of callus and regenerated shoots in chrysanthemum. Propag Ornam Plants 14:57–67
Munthali MT, Newbury HJ, Ford-Lloyd BV (1996) The detection of somaclonal variants of beet using RAPD. Plant Cell Rep 15:474–478
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Peredo EL, Arroyo-García R, Reed BM, Revilla MA (2008) Genetic and epigenetic stability of cryopreserved and cold-stored hops (Humulus lupulus L.). Cryobiology 57:234–241
Reed BM (1993) Responses to ABA and cold acclimation are genotype dependent for cryopreserved blackberry and raspberry meristems. Cryobiology 30:179–184
Reed BM (2001) Genotype considerations in temperate fruit crop cryopreservation. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm—current research progress and applications. JIRCAS/IPGRI, Tsukuba (Japan)/Rome (Italy), pp 200–205
Rohlf FJ (1992) NTSYS-PC: numerical taxonomy and multivariate analysis system. Exeter Software, New York
Ryynanen L (1998) Effect of abscisic acid, cold hardening, and photoperiod on recovery of cryopreserved in vitro shoot tips of silver birch. Cryobiology 36:32–39
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrussinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sakai A, Matsumoto T, Hirai D, Niino T (2000) Newly development encapsulation-dehydration protocol for plant cryopreservation. CryoLetters 21:53–62
Salvi ND, George L, Eapen S (2001) Plant regeneration from leaf base callus of turmeric and random amplified polymorphic DNA analysis of regenerated plants. Plant Cell Tiss Organ Cult 66:113–119
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York
Sánchez C, Martinez MT, Vidal N, San-Jose MC, Valladares S, Vieitez AM (2008) Preservation of Quercus robur germplasm by cryostorage of embryogenic cultures derived from mature trees and RAPD analysis of genetic stability. CryoLetters 29:493–504
Senula A, Keller J, Sanduijav T, Yohannes T (2007) Cryopreservation of cold-acclimated mint (Mentha spp.) shoot tips using a simple vitrification protocol. CryoLetters 28:1–12
Teixeira AS, González-Benito ME, Molina-García AD (2014) Determination of glassy state by cryo-SEM and DSC in cryopreservation of mint shoot tips by encapsulation-dehydration. Plant Cell Tiss Organ Cult 119:269–280
Towill LE (1988) Survival of shoot tips from mint species after short-term exposure to cryogenic conditions. Hort Science 23:839–841
Towill LE (1990) Cryopreservation of isolated mint shoot tips by vitrification. Plant Cell Rep 9:178–180
Uchendu EE, Reed B (2008) A comparative study of three cryopreservation protocols for effective storage of in vitro grown mint (Mentha spp.). CryoLetters 29:181–188
Volk GM, Walters C (2006) Plant vitrification solution 2 lowers water content and alters freezing behavior in shoot tips during cryoprotection. Cryobiology 52:48–61
Yamamoto S, Rafique T, Fukui K, Sekizawa K, Niino T (2012) V-cryo-plate procedure as an effective protocol forcryobanks: case study of mint cryopreservation. CryoLetters 33:12–23
Zeliang PK, Pattanayak A, Iangrai B, Khongwir EA, Sarma BK (2010) Fertile plant regeneration from cryopreserved calli of Oryza rufipogon Griff. and assessment of variation in the progeny of regenerated plants. Plant Cell Rep 29:1423–1433
Zhai Z, Wu Y, Engelmann F, Chen R, Zhao Y (2003) Genetic stability assessments of plantlets regenerated from cryopreserved in vitro cultured grape and kiwi shoot tips using RAPD. CryoLetters 24:315–322
Acknowledgments
This research was supported by the Spanish Government project AGL2010-21989-C02-01. C. K. is supported by a Grant from Universidad Politécnica de Madrid. Technical support from C. Sansegundo, M. Huertas and C. Ruiz is appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martín, C., Kremer, C., González, I. et al. Influence of the cryopreservation technique, recovery medium and genotype on genetic stability of mint cryopreserved shoot tips. Plant Cell Tiss Organ Cult 122, 185–195 (2015). https://doi.org/10.1007/s11240-015-0760-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0760-0