Abstract
Culture conditions for high frequency plant regeneration via somatic embryogenesis from cell suspension cultures of Ranunculus kazusensis are described. Zygotic embryos formed white nodular structures and pale-yellow calluses at a frequency of 84.9% when cultured on half-strength Schenk and Hildebrandt (SH) medium supplemented with 0.1 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D). However, the frequency of white nodular structure and off-white callus formation decreased with an increasing concentration of 2,4-D up to 10 mg l−1, when the frequency reached 25%. Cell suspension cultures were established from zygotic embryo-derived pale-yellow calluses using half-strength SH medium supplemented with 0.1 mg l−1 of 2,4-D. Upon plating onto half-strength SH basal medium, over 90% of cell aggregates gave rise to numerous somatic embryos and developed into plantlets. Regenerated plantlets were successfully transplanted to potting soil and grown to maturity at a survival rate of over 90% in a growth chamber. The plant regeneration system established in this study can be applied to mass propagation and conservation of this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ranunculus kazusensis Makino is a herbaceous water plant belonging to the Ranunculaceae. This species is native to Korea and has become threatened with extinction due to habitat loss. The family Ranunculaceae consists of approximately 2,500 described species distributed among 59 genera (Tamura 1993). Crude extracts of Ranunculus spp. have fungicidal (Qasem 1996), insecticidal (Bhattacharyya et al. 1993), analgesic, and anti-inflammatory activities (Cao et al. 1992).
A few studies on plant regeneration in the genus Ranunculus have been conducted, including plant regeneration via organogenesis (Pugliesi et al. 1992) and somatic embryogenesis of R. asiaticus (Beruto and Debergh 1992, 2004), and plant regeneration in anther cultures of R. japonicus (Ko et al. 1994). However, there has been no report on plant regeneration of R. kazusensis through tissue culture.
Tissue culture and in vitro plant regeneration systems may provide an alternative means for mass proliferation and ex situ conservation of endangered plant species. Therefore, this study describes culture conditions for high frequency plant regeneration via somatic embryogenesis from zygotic embryo-derived cell suspension cultures of R. kazusensis.
Materials and methods
Plant material
Mature seeds of R. kazusensis Makino were supplied from the Seed Bank for Wild and Endangered Plant Species of the Plant Diversity Research Center of Korea. Seeds were surface-sterilized, in 70% (v/v) ethanol for 1 min, followed by a 0.4% (v/v) sodium hypochlorite solution for 20 min with occasional agitation. They were rinsed four times with sterile distilled water.
Seed germination and callus formation
The culture medium used throughout the experiments consisted of half-strength Schenk and Hildebrandt (SH) inorganic medium salts (Schenk and Hildebrandt 1972), 5 mg l−1 thiamine·HCl, 5 mg l−1 nicotinic acid, 0.5 mg l−1 pyridoxine·HCl, 1,000 mg l−1 myo-inositol, 30 g l−1 sucrose, and 4 g l−1 Gelrite. The pH of all media was adjusted to 5.8 before autoclaving. Twenty-five milliliters of medium was dispensed into plastic Petri dishes (87 × 15 mm).
Surface-sterilized seeds (approximately 2–3 mm in length) were longitudinally dissected with a forceps and scalpel. Dissected seeds were plated onto half-strength SH basal medium. Unless mentioned otherwise, all cultures were incubated at 25°C in the dark. After 2 weeks of incubation, exposed zygotic embryos were collected.
To examine the effect of 2,4-dichlorophenoxyacetic acid (2,4-D) on embryogenic callus and somatic embryo formation, zygotic embryos were placed onto half-strength SH medium containing 0, 0.1, 0.3, 1, 3, or 10 mg l−1 2,4-D. Each treatment consisted of ten explants per dish with three replicates. After 8 weeks of culture, the frequency of explants producing white nodular structures and pale-yellow calluses was scored for each treatment.
Establishment of cell suspension culture
To establish embryogenic cell suspension culture, initial pale-yellow calluses were used. For the initiation of a cell suspension culture, the calluses maintained on half-strength SH medium containing 0.1 mg l−1 of 2,4-D (approximately 1 g) were carefully disintegrated with sterile forceps and transferred to a 250-ml Erlenmeyer flask holding 20 ml of half-strength SH liquid medium containing 0.1 mg l−1 of 2,4-D. The culture was maintained on a gyratory shaker (100 rpm) at 25°C in the dark. After 2 weeks of culture, 20 ml of liquid half-strength SH medium containing 0.1 mg l−1 of 2,4-D was added. After 4 weeks of culture, 5 ml of cell suspension culture were transferred to a 250-ml Erlenmeyer flask containing 50 ml of liquid half-strength SH medium. Cell suspension cultures were subcultured at 3-week intervals.
Plant regeneration from embryogenic cell suspension culture
To regenerate whole plants, cell aggregates (1–2 mm in size) were collected from 2-week-old cell suspension cultures using a stainless steel mesh (pore size, 1 mm) and rinsed with liquid half-strength SH basal medium. Twenty-five cell aggregates were plated onto half-strength SH basal medium in each Petri dish. After 4 weeks of culture in the light (30 μmol m−2 s−1 from cool-white fluorescent lamps with a 16-h photoperiod), the somatic embryo formation frequency was determined by counting the number of green regenerated plantlets in five Petri dishes. Plantlets developed from somatic embryos were subjected to acclimation, transplanted to potting soil, and maintained in a growth chamber (25°C day/22°C night, 70 μmol m−2 s−1 from cool-white fluorescent lamps with a 16-h photoperiod).
Results and discussion
Zygotic embryos cultured on half-strength SH medium containing 2,4-D began to form white globular structures and pale-yellow calluses on the radicles of germinated embryos after 2 weeks of culture (Fig. 1a). After 4 weeks of culture, white globular structure and pale-yellow compact calluses began to form on the entire surfaces of zygotic embryos (Fig. 1b). After an additional 4 weeks of culture, pale-yellow compact calluses proliferated with white globular structures were elongated. When transferred to half-strength SH basal medium, white globular structures developed into multiple somatic embryos after 4 weeks of culture (Fig. 1c), indicating that the white globular structures were an early stage of somatic embryos. In addition, pale-yellow, compact calluses subcultured on the same composition medium produced white globular structures on the surfaces, indicating that the pale-yellow compact calluses were embryogenic.
Plant regeneration of Ranunculus kazusensis Makino via somatic embryogenesis. a Zygotic embryos; b globular structure and callus formation from zygotic embryo; c plant regeneration via somatic embryogenesis; d establishment of embryogenic cell suspension cultures; e plantlets development from cell aggregates; f rooting of plantlets; g multiple plant regeneration from embryogenic cell suspension culture; h successful soil transfer of regenerated plants; i flowering of regenerated plants. Scale bars represent 2 mm (a, b, c, e, f, i), 200 μm (d), and 2 cm (g, h)
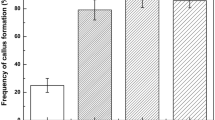
Zygotic embryos formed white nodular structures and pale-yellow calluses at a frequency of 84.9% when cultured on half-strength SH medium supplemented with 0.1 mg l−1 2,4-D (Fig. 2). The frequency of pale-yellow callus formation decreased with an increasing concentration of 2,4-D up to 10 mg l−1, where the frequency reached 25% (Fig. 2).
In preliminary studies, we examined the effect of concentrations of inorganic salts on callus induction from zygotic embryo culture of R. kazusensis using different culture media including MS (Murashige and Skoog 1962), SH, and B5 (Gamborg et al. 1968), half-strength inorganic salts of these media were more suitable than full-strength media for embryogenic callus formation (data not shown).
Zygotic embryo-derived pale-yellow calluses yielded stable cell suspension cultures after a second subculture in half-strength SH medium with 0.1 mg l−1 2,4-D. Cell suspension cultures were primarily composed of round cells containing a prominent nucleus and dense cytoplasm (Fig. 1d). After 4 weeks of plating on half-strength SH basal medium, over 90% of cell aggregates developed into somatic embryos (Fig. 1e). Somatic embryos were subsequently rooted (Fig. 1f) and developed into numerous plantlets at a frequency of approximately 90% (Fig. 1g). Plantlets were successfully transplanted to potting soil at a survival frequency of over 90% and then grown to maturity (Fig. 1h, i).
Most studies of in vitro propagation of Ranunculus spp. were achieved from land plants (Pugliesi et al. 1992; Beruto and Debergh 1992; Ko et al. 1994; Bicknell et al. 1996). In this study, we established the in vitro culture system of the aquatic plant R. kazusensis for the first time. Therefore, the plant regeneration system of R. kazusensis established in this study will be useful for mass propagation and cryopreservation of germplasm of this species.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- SH:
-
Schenk and Hildebrandt
References
Beruto M, Debergh P (1992) Somatic embryogenesis in Ranunculus asiaticus L. hybr. thalamus cultivated in vitro. Plant Cell Tissue Organ Cult 29:161–165
Beruto M, Debergh P (2004) Micropropagation of Ranunculus asiaticus: a review and perspectives. Plant Cell Tissue Organ Cult 77:221–230
Bhattacharyya PR, Nath SC, Bordoloi DN (1993) Insecticidal activity of Ranunculus sceleratus (L.) against Drosophila melanogaster and Tribolium castaneum. Indian J Exp Biol 31:85–86
Bicknell RA, Braun RH, Evans AC, Morgan ER (1996) Tissue culture of Ranunculus lyallii Hook. f. NZ J Crop Hortic Sci 24:303–306
Cao BJ, Meng QY, Ji N (1992) Analgesic and anti-inflammatory effects of Ranunculus japonicus extract. Planta Med 58:496–498
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:148–151
Ko JA, Kim YS, Kim MJ, Eun JS (1994) Immature pollen-derived plant regeneration in anther cultures of Ranunculus japonicus Thunb. Korean J Plant Biotechnol 21:293–298
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pugliesi C, Rabaglio M, Cecconi F, Baroncelli S (1992) Plant regeneration from tissue cultures of Persian buttercup (Ranunculus asiaticus L.). Plant Cell Tissue Organ Cult 28:125–128
Qasem JR (1996) Fungicidal activity of Ranunculus asiaticus and other weeds against Fusarium oxysporum f. sp. lycopersici. Ann Appl Biol 128:533–540
Schenk RU, Hildebrandt A (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50:199–204
Tamura M (1993) Ranunculaceae. In: Kubitski K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants: flowering plants dicotyledons, vol II. Springer, Berlin Heidelberg New York, pp 563–583
Acknowledgments
This work was supported by a grant from KRIBB Research Initiative Program and a grant (no. BDW0800622) from the Korea Science and Engineering Foundation. The authors are thankful to the Plant Diversity Research Center of Korea for providing plant materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Min, S.R., Liu, J.R. & Kim, S.W. Plant regeneration from zygotic embryo-derived embryogenic cell suspension cultures of Ranunculus kazusensis . Plant Biotechnol Rep 1, 57–60 (2007). https://doi.org/10.1007/s11816-006-0005-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-006-0005-0