Abstract
Purpose
To analyze the efficacy of supervised exercise (SE) compared with control protocols on sleep parameters of women who survived breast cancer.
Methods
This systematic review with meta-analysis searched studies using the following electronic databases: PubMed, Scopus, Physiotherapy Evidence Database (PEDro), Cochrane Library, and EMBASE. The PEDro scale assessed the bias risk, and the study protocol was registered in the PROSPERO (no. CRD42023420894).
Results
Of 3,566 identified studies, 13 randomized clinical trials involving 847 women diagnosed with breast cancer were included. Interventions consisted of SE in an outpatient setting (62%) or combined protocols with supervised and home exercises. Most interventions (85%) used multicomponent protocols with aerobic and resistance exercises. Usual care and health education were the most reported controls. SE decreased the sleep disturbance score (− 31.61 [95% confidence interval = − 39.40 to − 23.83]) of the European Organisation for Research and Treatment of Cancer quality of life questionnaire and daytime dysfunction score (− 0.41 [95% confidence interval = − 0.73 to − 0.09]) of the Pittsburgh Sleep Quality Index (PSQI). Also, SE presented a tendency to improve the self-reported sleep quality score of the PSQI (p = 0.06).
Conclusion
SE increased the subjective sleep quality and immobility time and decreased sleep disturbance and daytime dysfunction symptoms in women who survived breast cancer. Most SE protocols were multicomponent, with aerobic and resistance exercises ranging from moderate to high intensity.
Implications for cancer survivors.
Supervised exercise may improve sleep quality and reduce symptoms of sleep disorders, contributing to survival outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most incident neoplasm and the main cause of death in women in high- and low-income countries [1]. Despite advances in diagnosis and treatments to cure cancer, side effects of the disease and treatments are common, such as reduced muscle strength, physical fitness, and self-esteem and increased pro-inflammatory cytokines, stress, anxiety, depressive symptoms, and sleep disorders [2, 3].
Sleep disorders affect over two-thirds of women who survived breast cancer [4, 5] and are associated with increased fatigue, reduced quality of life, cardiorespiratory performance, and functionality, and impaired memory, cognition, and motor skills [4, 6, 7]. Also, insomnia, hypersomnia, and circadian rhythm disorders are the most common sleep disorders in this population, assessed using sleep quality scales and quantitative measures, such as actigraphy [4, 5].
Exercise is an essential therapeutic intervention for patients after a cancer diagnosis, reducing cancer recurrence and breast cancer-related mortality and improving biological function and quality of life [8]. Also, studies showed beneficial effects of different exercises (e.g., aerobic, resistance, or combined) on sleep quality, improving the sleep–wake cycle and nocturnal sleep quality in women with breast cancer [9,10,11].
Previous systematic reviews assessed isolate and mind–body exercises (e.g., yoga, Tai Chi, and Qigong) and reported positive effects on women who survived breast cancer [12, 13]. However, they included different protocols of exercises, physical activities, and mind–body exercises, hindering to isolate and estimate the effects of each exercise and presence of supervision. A study suggested that supervised exercise (SE) had higher treatment adherence than unsupervised exercise, increasing continuity in exercise and quality of life and reducing side effects of breast cancer treatment [14].
Considering the exercise as a physical therapy intervention for cancer survivors and SE impact on treatment protocols, this study aimed to analyze the efficacy of SE compared with other interventions in sleep parameters of women who survived breast cancer. The research question was: “what is the efficacy of exercise compared with other interventions in sleep outcomes of women who survived breast cancer?”.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [15] and Cochrane Handbook for Systematic Reviews of Interventions [16]. The protocol was registered in the PROSPERO platform (National Institute for Health Research, UK, no. CRD42023420894).
Eligibility criteria
The inclusion criteria were randomized clinical trials (published as full articles) investigating the effects of SE (isolated or multicomponent exercises, such as aerobic, resistance, or both) compared with unsupervised exercise protocols or other interventions on sleep parameters of women who survived non-metastatic breast cancer (at any stage of clinical follow-up). Exclusion criteria were studies assessing interventions with mind–body exercises or without results related to sleep parameters (i.e., sleep quality and circadian rhythm). Language and publication date were not restrictions.
Search strategy and study selection
The search was conducted on March 2, 2023, using the PubMed, Scopus, Physiotherapy Evidence Database (PEDro), Cochrane Library, and EMBASE databases. Each database had a specific search strategy (Appendix A), and the following descriptors were combined using the Boolean operators (AND/OR): breast cancer, sleep, exercise, physical activity, physical therapy modalities, aerobic exercise, breathing exercises, and muscle training. Reference lists of included studies were also analyzed to include eligible articles.
Data screening and extraction
References were exported and analyzed using the Rayyan software (QCRI, Qatar) [17]. After excluding duplicates, two independent researchers (M.P.M.F.B. and N.T.J) screened the studies based on title and abstract. Studies meeting the inclusion criteria were selected for full-text reading, and those reporting the effect of exercise on sleep of women who survived breast cancer were included in the qualitative synthesis.
Data were extracted using an electronic spreadsheet with the following information: author, country and publication year, sample size, age, inclusion and exclusion criteria, intervention protocols, outcomes, and main results.
Study quality
Two researchers (M.P.M.F.B. and N.T.J) assessed the methodological quality of the studies using the PEDro scale, consisting of 11 criteria for clinical studies in physical therapy [18]. The score ranges from 0 to 10 points, and values ≥ 6 indicate adequate quality [19].
Outcomes
The primary outcome was sleep quality, which was assessed using validated questionnaires. Secondary outcomes were sleep parameters and circadian rhythm, assessed using validated questionnaires, patient-reported measurements (PROMIS), or actigraphy.
Meta-analysis
Meta-analysis was performed using the RevMan software version 5.3 (Cochrane Collaboration, London, UK). Odds ratio and 95% confidence interval (95% CI) were calculated for dichotomous outcomes, and mean difference and 95% CI for continuous outcomes. A fixed or random effects model was calculated based on the heterogeneity in each analysis, and statistical significance was set at p < 0.05. Data reported as median and interquartile range in the studies were converted to mean and standard deviation [20].
Results
Study selection and data extraction
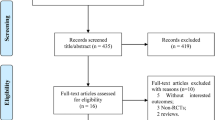
We identified 3566 studies in the electronic databases. After removing duplicates, they were screened by title and abstracts, and 42 were fully read and assessed for eligibility. Thus, 13 studies met the inclusion criteria and were included in this study (Fig. 1).
Studies characteristics
Tables 1 and 2 show the main characteristics of included studies. Studies were published between 2012 and 2023. Five studies were conducted in the USA [21,22,23,24,25], two in Iran [26, 27], two in Brazil [28, 29], and one in Turkey [30], Italy [31], South Korea [32], and North Korea [33].
A total of 847 women were included. The studies exhibited diverse sample sizes, ranging from 20 to 222 participants. On average, the total sample size was approximately 68 participants per study, with intervention and control groups each averaging around 34 participants. Generally, the studies enrolled women diagnosed with breast cancer without metastasis (Stage I to III) at least 6 months prior to the study initiation. Inclusion criteria also encompassed post-treatment or surgery periods, completion of treatments within specified time frames (ranging from three to 18 months), intervals without exercise practice, and language proficiency (English-speaking participants). Participants’ ages ranged from 30 to 70 years, with a median age of 54.4 years.
Interventions were predominantly SE in an outpatient setting (62%) or combined protocols of supervised and home exercises (38%). Of 13 studies, 85% used multicomponent protocols with aerobic and resistance exercises [21,22,23,24,25, 27, 29, 30, 33], whereas 15% used isolated aerobic exercises [26, 31]. Control interventions were mainly usual care and health education.
Nine studies used the Pittsburgh Sleep Quality Index (PSQI) to measure sleep-related outcomes. Other studies used self-reported sleep duration [23], sleep disturbance domain of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) [29, 30], or actigraphy to assess the sleep efficiency and latency [22,23,24,25, 31].
Exercise intervention
Protocols with combined exercises ranged from 8 to 12 weeks; one study of Paulo et al. extended to 36 weeks [29]. Exercise sessions were conducted between two and three times per week, lasting from 40 to 60 min.
A study applied aerobic exercises using a treadmill and stationary bicycle (50 min, three times per week) with resistance exercises for legs and hips using elastic bands and a ball (40 min, twice a week) [30]. Other two studies applied aerobic (i.e., light walks) and resistance exercises for 40 min each, three times per week [27, 29]. In these studies, one used barbells (for upper limbs) and exercise machines (for lower limbs) [27], while the other used seated cable row, bench press, bridge, plank, and leg extension, curl, and press exercises [29].
Barbosa et al. [28] divided the intervention into two groups, with a 75-min session twice a week. In one group, they applied Pilates exercises, with 10 min of warm-up, 60 min of basic exercises (i.e., breath with powerhouse activation, flexion and extension of shoulders, postural education, sitting, stretching, and training of proprioception), and 5 min of global stretching [28]. The circuit group performed a 15-min warm-up, 50-min training with six circuit stations (i.e., aerobic exercise, elbow flexion, shoulder, triceps, and two exercises for lower limbs), and 10-min global stretching [28].
Jang et al. and Kim et al. used the Better Life After Cancer: Energy, Strength, and Support (BLESS) program, which combines aerobic and resistance exercises with moderate to high intensity for 12 weeks [32, 33]. It was divided into 6 weeks of SE to improve balance using movements that reduce stress and injuries in women with breast cancer and 6 weeks of home-based exercises [32, 33].
Rogers et al. (2013) gradually increased the exercise level for each woman, with two sessions per week of 75 min each on non-consecutive days [22]. Sessions included eight different exercises with 20 repetitions each for the major muscle groups, and SE transitioned to home-based exercises with personal heart rate (HR) monitors to maintain the intensity [22]. Also, two studies combined aerobic and resistance exercises for 12 weeks, with moderate-intensity treadmill walking and elastic bands (eight exercises targeting the major muscle groups). Aerobic protocol including gradually advanced from 9 to 40 min each session, four times per week, while resistance training occurred twice weekly during the same sessions of aerobic walking [23, 24]. Rogers et al. (2023) had a multicomponent intervention for 12 weeks, initially with three SE sessions per week, which transitioned to home-based exercises in the seventh week [25]. Only Dieli-Conwright et al. followed the American College of Sports Medicine (ACSM) guideline, consisting of two to three times per week of resistance exercise and 150 min of moderate aerobic exercise (e.g., walking and swimming) or more vigorous for less than 75 min [21].
Most studies controlled the intensity of aerobic exercises using the Borg scale for perceived exertion and maximum HR, being mostly moderate to high intensity (40% to 85% maximum HR). Kim et al. [32] applied low-, moderate-, and high-intensity exercises with gradual increases [32]. The resistance exercise was graduated using the one-repetition maximum (1RM), such as Monazzami et al. [27], which used 50% to 70% 1RM.
Regarding studies using exclusively aerobic exercises, Roveda et al. included two sessions per week of brisk walking for one hour, followed by 10 min of static stretching [31]. Ghavami et al. applied a 10-min light aerobic exercise (warm-up), followed by 30 min of aerobic exercise with 70 to 85% maximum HR, ending with a 10-min cool-down [26].
Risk of bias
The risk of bias in the studies was assessed using the PEDro scale (Appendix B), and the mean score was 5.7 ± 1.6 points. Seven studies (54%) scored ≥ 6 points, indicating an adequate quality. However, most studies (92%) did not meet the criteria of blinding patients and therapists. Studies by Aydin et al. [30], Jang et al. [33], Monazzami et al. [27], and Roveda et al. [31] demonstrated relatively lower PEDro scores, ranging from 3 to 4, indicating a higher risk of bias. These studies exhibited weaknesses in key domains such as concealed allocation, blinding of patients, therapists, and evaluators, as well as the adequacy of follow-up and intention-to-treat analysis. Conversely, studies by Rogers et al. [25], Rogers et al. [24], and Rogers et al. [23] stood out with higher PEDro scores of 8, 7, and 8, respectively, suggesting a lower risk of bias. These studies demonstrated robust methodological quality, with strengths in areas such as adequate follow-up and intention-to-treat analysis, contributing to increased confidence in their findings. Other studies by Barbosa et al. [28], Dieli-Conwright et al. [21], and Ghavami et al. [26] received PEDro scores of 7 or 6, indicating a moderate risk of bias, their primary vulnerabilities were related to blinding of patients, therapists, and evaluators.
The publication bias was assessed through the funnel plot (Appendix C). Heterogeneity among the studies and the limited number of studies included in the meta-analysis for certain outcomes may have contributed to the asymmetric pattern observed in the plotted graphs.
Summary of results
The subgroup analysis based on the exercise type was not assessed due to discrepancies in outcomes measurement. In addition, meta-analysis was not feasible for some outcomes due to the limited number of studies. Most studies reported that exercise professionals performed SE; however, few did not specify who provided supervision.
Dieli-Conwright et al. [21] demonstrated that exercise reduced 54% (95% CI = 0.29 to 0.72) of the chance of women being classified as poor sleep (PSQI > 5). Although Rogers et al. [24] did not show data from both groups, they reported a 30% reduction in women with poor sleep in the multicomponent training group. Also, the exercise protocol decreased the self-reported sleep disturbance by 5.30 points (95% CI = -9.97 to -0.63) on a scale of 0 to 100 [24].
Only Roveda et al. [31] assessed circadian rhythm parameters; however, they did not observe effects in the mean estimated statistic over rhythm (mesor) (6.10 [95% CI = − 22.09 to 34.29]), acrophase (highest phase) (0.53 [95% CI = − 2.59 to 3.65]), and amplitude (difference between maximum value and mesor) (− 1.70 [95% CI = − 19.31 to 15.91]). Regarding actigraphy parameters, the immobility time significantly increased in the SE group (3.40 [95% CI = 0.39 to 6.41]), but sleep time (p = 0.36), real waking time (p = 0.23), mean activity score (p = 0.13), sleep movement, and fragmentation index showed no differences [31].
The outcomes assessed by at least two studies were included in meta-analyses (Fig. 2). Exercise decreased the sleep disturbance score (− 31.61 points [95% CI = − 39.40 to − 23.83]) of the EORTC QLQ-C30 and the daytime dysfunction score (− 0.41 points [95% CI = − 0.73 to − 0.09]) of the PSQI. Also, a tendency to improve the subjective sleep quality was observed in the PSQI (p = 0.06).
Discussion
This study assessed the efficacy of SE compared with unsupervised protocols or control groups in sleep parameters of women who survived breast cancer. SE was related to a reduced rate of women with poor sleep and scores of sleep disturbance and daytime dysfunction and increased subjective sleep quality and immobility time.
SE provided benefits for women with breast cancer since it allowed individualized attention by a healthcare professional and promoted better adherence, outcomes, and quality of life when combined with motivational tools than unsupervised exercises [34]. Also, SE is important for overcoming physical barriers and insecurities about exercising during breast cancer treatment and allows a flexible and diverse exercise program with increased and sustained engagement [35]. Specialized professional guidance for patients with cancer is essential to ensure a program that considers specific needs and medical history, preventing negative impacts on health and quality of life [35]. In addition, SE sessions allowed a high dose of exercise throughout the training program since supervision ensured the correct execution of prescribed exercises [36].
Most health professionals applying the SE included in this study were specialized in physical education or exercises. The ACSM advocates that all healthcare professionals should stimulate exercise practice. Each professional category could contribute within their specialties and seek multidisciplinary collaboration to support and encourage patients with cancer to be physically active [37]. Also, physicians involved in breast cancer treatment reported the need for health professionals to support patients during and after treatment. Moreover, the shortage of specialized professionals compared with the number of patients highlights the importance of multidisciplinary teamwork to minimize side effects of breast cancer treatment [37].
Patients with cancer often choose exercises based on psychological and physical considerations. A survey collected preferences of these patients, revealing the inclination for light exercises, such as walking outdoors and in groups [38]. Although these exercises minimize side effects of treatment and improve physical functions, guidelines for patients with cancer recommend moderate-intensity exercises, highlighting the importance of a gradual increase of intensity to reduce side effects and improve physical fitness [38].
The ACSM guideline designed for patients with cancer recommends aerobic exercises with moderate to high intensity (e.g., swimming, walking, dancing, cycling, and treadmill) for three or more days per week, with 30-min sessions [39]. For resistance exercises, it recommends home-based activities involving strength, elastic bands, dumbbells, or exercises using body weight, especially for lower and upper limbs and lumbar and trunk muscle groups [39]. Also, the frequency should be two or three days per week with two to four sets of 10 to 15 (light weights) or 8 to 12 repetitions (moderate or high weights), resting one day between sessions [39]. Thus, the studies included in this review followed these recommendations.
Combined resistance and aerobic exercises may provide physical, psychological, and social benefits for women who survived breast cancer [29]. Thus, regular exercise is a non-pharmacological treatment for poor sleep quality of these women [40]. Also, poor sleep may be associated with increased cardiovascular and metabolic risks, highlighting the importance of physical and psychological well-being to prevent depression [41].
The findings of this study corroborated evidence supporting exercise as an intervention to improve quality of life and perceptions of physical function and reduce anxiety and depressive symptoms, sleep disorders, and fatigue [39, 42]. Also, exercise does not offer risks for exacerbating upper limb lymphedema and is considered safe during and after cancer treatment, reducing side effects [39, 42].
The study had some limitations, such as the variability of outcome measures for sleep, limiting the number of meta-analyses and preventing comparison by types of exercises. Also, the methodological quality of some studies was compromised due to the high risk of bias, which may affect the reliability of reported effect sizes. Furthermore, the inclusion of patients across diverse phases of oncological treatment precluded the execution of subgroup analyses, thereby limiting the estimation of the influence of each treatment on observed effects. Lastly, clinical trials did not provide information on the effects of exercise on vasomotor symptoms associated with disrupted sleep. Future studies should investigate this aspect to enhance the comprehensiveness of the existing literature.
This study highlighted the need for multidisciplinary teamwork to provide exercise practice and reduce negative effects of cancer treatment on sleep of women with breast cancer. It also provided updated information for healthcare professionals regarding SE benefits on sleep outcomes and may support and guide physical therapists by presenting evidence to improve the sleep of these women.
Conclusion
SE protocols for women who survived breast cancer increased sleep quality and immobility time and reduced sleep disturbance and daytime dysfunction symptoms. Most protocols were multicomponent (i.e., aerobic and resistance exercises) with moderate to high intensity. However, the high heterogeneity of some outcomes and the risk of bias may influence the magnitude of the effect.
Data availability
This manuscript use data previously published by other authors and all data are presented into results section.
References
Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Global Burden of Disease 2019 Cancer Collaboration, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420.
Xu S, Thompson W, Ancoli-Israel S, Liu L, Palmer B, Natarajan L. Cognition, quality-of-life, and symptom clusters in breast cancer: using Bayesian networks to elucidate complex relationships. Psychooncology. 2018;27:802–9.
Imanian M, Imanian M, Karimyar M. Sleep quality and fatigue among breast cancer patients undergoing chemotherapy. IJHOSCR [Internet]. 2019 [cited 2023 Apr 27]; Available from: https://publish.kne-publishing.com/index.php/IJHOSCR/article/view/1894
Lourenço A, Dantas AAG, Souza JC, Araujo CM, Araujo DN, Lima INDF, et al. Sleep quality is associated with disability and quality of life in breast cancer survivors: a cross‐sectional pilot study. Eur J Cancer Care [Internet]. 2021 [cited 2023 Mar 15];30. Available from: https://onlinelibrary.wiley.com/doi/https://doi.org/10.1111/ecc.13339
Momayyezi M, Fallahzadeh H, Farzaneh F, Momayyezi M. Sleep quality and cancer-related fatigue in patients with cancer. J Caring Sci. 2021;10:145–52.
Otte JL, Davis L, Carpenter JS, Krier C, Skaar TC, Rand KL, et al. Sleep disorders in breast cancer survivors. Support Care Cancer. 2016;24:4197–205.
De Nys L, Anderson K, Ofosu EF, Ryde GC, Connelly J, Whittaker AC. The effects of physical activity on cortisol and sleep: a systematic review and meta-analysis. Psychoneuroendocrinology. 2022;143:105843.
Hayes SC, Steele ML, Spence RR, Gordon L, Battistutta D, Bashford J, et al. Exercise following breast cancer: exploratory survival analyses of two randomized, controlled trials. Breast Cancer Res Treat. 2018;167:505–14.
Courneya KS, Segal RJ, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, et al. Effects of exercise dose and type on sleep quality in breast cancer patients receiving chemotherapy: a multicenter randomized trial. Breast Cancer Res Treat. 2014;144:361–9.
Kim C-J, Kang D-H, Park J-W. A meta-analysis of aerobic exercise interventions for women with breast cancer. West J Nurs Res. 2009;31:437–61.
Waltman NL, Twiss JJ, Ott CD, Gross GJ, Lindsey AM, Moore TE, et al. The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: a 24-month randomized controlled trial. Osteoporos Int. 2010;21:1361–9.
Yang H, Yang Z, Pan H, Zhou Q. Effects of physical activity on sleep problems in breast cancer survivors: a meta-analysis. Support Care Cancer. 2021;29:4023–32.
Matthews EE, Janssen DW, Djalilova DM, Berger AM. Effects of exercise on sleep in women with breast cancer. Sleep Med Clin. 2018;13:395–417.
Westphal T, Rinnerthaler G, Gampenrieder SP, Niebauer J, Thaler J, Pfob M, et al. Supervised versus autonomous exercise training in breast cancer patients: a multicenter randomized clinical trial. Cancer Med. 2018;7:5962–72.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583.
Lasserson TJ, Thomas J, Higgins JP. Starting a review. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions [Internet]. 1st ed. Wiley; 2019 [cited 2023 Apr 24]. p. 1–12. Available from: https://onlinelibrary.wiley.com/doi/https://doi.org/10.1002/9781119536604.ch1
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
Shiwa SR, Costa LOP, Moser LAD de L, Aguiar de IC, Oliveira LVF de. PEDro: a base de dados de evidências em fisioterapia. Fisioter Mov. 2011; 24, 523–33.
Armijo-Olivo S, da Costa BR, Cummings GG, Ha C, Fuentes J, Saltaji H, et al. PEDro or Cochrane to assess the quality of clinical trials? A meta-epidemiological study. Bayer A, editor. PLoS ONE. 2015;10:e0132634.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Norris MK, Fox FS, et al. Aerobic and resistance exercise improve patient-reported sleep quality and is associated with cardiometabolic biomarkers in Hispanic and non-Hispanic breast cancer survivors who are overweight or obese: results from a secondary analysis. Sleep. 2021;44:zsab111.
Rogers LQ, Fogleman A, Trammell R, Hopkins-Price P, Vicari S, Rao K, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12:323–35.
Rogers LQ, Vicari S, Trammell R, Hopkins-Price P, Fogleman A, Spenner A, et al. Biobehavioral factors mediate exercise effects on fatigue in breast cancer survivors. Med Sci Sports Exerc. 2014;46:1077–88.
Rogers LQ, Fogleman A, Trammell R, Hopkins-Price P, Spenner A, Vicari S, et al. Inflammation and psychosocial factors mediate exercise effects on sleep quality in breast cancer survivors: pilot randomized controlled trial: Mediators of exercise effects on sleep in breast cancer survivors. Psychooncology. 2015;24:302-310.
Rogers LQ, Courneya KS, Oster RA, Anton PM, Phillips S, Ehlers DK, et al. Physical activity intervention benefits persist months post-intervention: randomized trial in breast cancer survivors. J Cancer Surviv [Internet]. 2023 [cited 2023 Mar 14]; Available from: https://springerlink.bibliotecabuap.elogim.com/https://doi.org/10.1007/s11764-022-01329-2
Ghavami H, Akyolcu N. The impact of lifestyle interventions in breast cancer women after completion of primary therapy: a randomized study. J Breasth Health. 2017;13:94–9.
Monazzami A, Momenpour R, Alipoor E, Yari K, Payandeh M. The effects of concurrent training on the body composition, quality of life, and sleep quality of postmenopausal women with breast cancer. J Kermanshah Univ Med Sci. 2020;24(3):e101186.
de Barbosa KP, da Silva LGT, Garcia PA, de Freitas CA, da Silva ECF, Pereira TV, et al. Effectiveness of Pilates and circuit-based exercise in reducing arthralgia in women during hormone therapy for breast cancer: a randomized, controlled trial. Support Care Cancer. 2021;29:6051–9.
Paulo TRS, Rossi FE, Viezel J, Tosello GT, Seidinger SC, Simões RR, et al. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial. Health Qual Life Outcomes. 2019;17:17.
Aydin M, Kose E, Odabas I, Meric Bingul B, Demirci D, Aydin Z. The effect of exercise on life quality and depression levels of breast cancer patients. Asian Pac J Cancer Prev. 2021;22:725–32.
Roveda E, Vitale JA, Bruno E, Montaruli A, Pasanisi P, Villarini A, et al. Protective effect of aerobic physical activity on sleep behavior in breast cancer survivors. Integr Cancer Ther. 2017;16:21–31.
Kim S, Ko YH, Song Y, Kang MJ, Lee H, Kim SH, et al. Pre-post analysis of a social capital-based exercise adherence intervention for breast cancer survivors with moderate fatigue: a randomized controlled trial. Support Care Cancer. 2020;28:5281–9.
Jang MK, Han J, Kim SH, Ko YH, Kim SY, Kim S. Comparison of fatigue and fatigability correlates in Korean breast cancer survivors and differences in associations with anxiety, depression, sleep disturbance, and endocrine symptoms: a randomized controlled trial. BMC Cancer. 2021;21:855.
Reverte-Pagola G, Sánchez-Trigo H, Saxton J, Sañudo B. Supervised and non-supervised exercise programs for the management of cancer-related fatigue in women with breast cancer: a systematic review and meta-analysis. Cancers. 2022;14:3428.
Depenbusch J, Sweegers MG, Aaronson NK, Wengström Y, Backman M, Arraras JI, et al. PERSPECTIVEs on supervised exercise programs in people with metastatic breast cancer- a qualitative study in four European countries. Support Care Cancer. 2023;31:281.
Stout NL, Baima J, Swisher AK, Winters-Stone KM, Welsh J. A systematic review of exercise systematic reviews in the cancer literature (2005–2017). PM R. 2017;9(9S2):S347–84.
Schmitz KH, Campbell AM, Stuiver MM, Pinto BM, Schwartz AL, Morris GS, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA A Cancer J Clin. 2019;69:468–84.
Avancini A, Pala V, Trestini I, Tregnago D, Mariani L, Sieri S, et al. Exercise levels and preferences in cancer patients: a cross-sectional study. IJERPH. 2020;17:5351.
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51:2375–90.
Tseng T-H, Chen H-C, Wang L-Y, Chien M-Y. Effects of exercise training on sleep quality and heart rate variability in middle-aged and older adults with poor sleep quality: a randomized controlled trial. J Clin Sleep Med. 2020;16:1483–92.
Shorofi SA, Nozari-Mirarkolaei F, Arbon P, Bagheri-Nesamie M. Depression and sleep quality among iranian women with breast cancer. Asian Pac J Cancer Prev. 2021;22:3433–40.
Peterson LL, Ligibel JA. Physical activity and breast cancer: an opportunity to improve outcomes. Curr Oncol Rep. 2018;20:50.
Acknowledgements
The authors thank Universidade Federal de Pernambuco and CAPES for the financial support provided for translating the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Moura Ferraz Barbosa, M.P., de Jesus, N.T., Bergmann, A. et al. Efficacy of supervised exercise on sleep of women who survived breast cancer: a systematic review with meta-analysis. J Cancer Surviv (2024). https://doi.org/10.1007/s11764-024-01532-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11764-024-01532-3