Abstract
Purpose
To investigate the possible role of physical activity (PA) on sleep disturbance in breast cancer patients.
Methods
Literature in PubMed, Embase, and the Cochrane Library was systematically searched until January 30, 2020. Randomized controlled trials that focused on the role of PA interventions on sleep disturbance were selected. The main outcome measures included the global Pittsburgh Sleep Quality Index (PSQI) score and PSQI subscales. Subgroup analysis was performed based on the study area and intervention time. The stability and authenticity of the results were measured by sensitivity analysis and publication bias analysis, respectively.
Results
Six articles were included in this meta-analysis. There were no significant differences in global PSQI scores between the PA intervention group and the usual care group (P = 0.057). As for PSQI subscales, PA intervention could improve sleep quality (weighted mean difference = 0.22; 95% confidence interval 0.04–0.40; P = 0.018). There were no significant differences in sleep duration, sleep medication, sleep latency, habitual sleep efficiency, and daytime dysfunction between the two groups (all P > 0.05).
Conclusion
PA serves as an effective intervention to improve sleep quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep is essential for health; thus, sleep problems affect physical and emotional well-being as well as immune system function. Most cancer patients and cancer survivors frequently complain about sleep disturbances, manifesting as circadian rhythm disorders, insomnia, and lethargy [1]. Compared to patients with other types of cancer, patients with breast cancer have been found to be particularly vulnerable to sleep disturbance [2]. Lowery-Allison et al. [3] reported the sleep problems in breast cancer survivors who were at 1–10 years post-treatment. They found that 38% of breast cancer survivors reported having poor sleep quality. In addition, these patients had a low quality of life (QoL), high pain severity, and severe vasomotor symptoms. Notably, sleep disturbances can aggravate the physical and mental illness syndrome of cancer survivors, including pain, fatigue, anxiety, and depression. It also increases the risk of infection and leads to a decline in overall QoL up to 10 years after diagnosis [4]. Therefore, it is necessary to propose solutions for sleep problems to improve the QoL of cancer survivors.
A previous study has indicated that physical activity (PA) is an effective approach to manage sleep problems [5]. Recent publications on PA intervention for breast cancer survivors suggested that the mortality risk for breast cancer was reduced after regular exercise [6, 7]. Further evidence indicated that both high-intensity and low- to moderate-intensity exercises could reduce general and physical fatigue in cancer survivors [8]. As for the influence of PA on sleep disturbance, no consensus conclusion has been reached. For example, Roveda et al. [9] demonstrated that sleep behaviors, such as sleep disruption, sleep efficiency, and sleep latency, in breast cancer survivors could be improved via aerobic PA. In contrast, Bernard and his colleagues [10] did not confirm a possible beneficial effect of PA on objective sleep parameters. Meanwhile, some researchers have designed meta-analyses to investigate the role of PA in sleep disturbance [11, 12]. However, there are heterogeneity in the types of exercise and sleep quality evaluation indicators included in these studies. Various types of exercise are included in a previous meta-analysis [11], such as walking, yoga, qigong, or tai chi. Moreover, self-reported sleep quality and objective sleep measurements were recorded in this systematic analysis. Thus, to accurately assess the effect of PA on sleep quality, the measurement of sleep quality should be unified, and the type of exercise should be limited.
Traditionally, the Pittsburgh Sleep Quality Index (PSQI), with an 18-item scale, is used to measure the quality of sleep and sleep disturbances; it includes seven subscales, namely, sleep quality, sleep latency, sleep duration, habitual sleep efficacy, sleep disturbances, use of sleep medication, and daytime dysfunction [13, 14]. In this study, outcome measures were assessed using the PSQI and its subscales. The aim of the current study was to explore the possible role of PA in sleep disturbance among breast cancer survivors. In brief, randomized controlled trials (RCTs) focusing on the role of PA interventions on sleep problems among patients with breast cancers were selected. The weighted mean difference (WMD) with its 95% confidence interval (CI) was calculated to assess the effect of PA on the global PSQI score and PSQI subscales. Subgroup analysis based on the study area and time of intervention was further performed. Our meta-analysis reveals that exercise can help improve the sleep quality.
Materials and methods
Selection strategy
The meta-analysis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses(PRISMA) guidelines [15]. The eligible studies were extracted after a thorough search of PubMed, Embase, and the Cochrane Library till January 30, 2020, using the following combination of search terms: “exercise,” “physical activity,” “randomized controlled trial,” “breast neoplasms.” The retrieval steps of PubMed are shown in Supplementary Table 1. The enrolled studies were selected without language limitations. To obtain more relevant literature, printouts of the studies and reference lists of included studies were manually checked.
Study selection
The inclusion criteria were as follows: (1) all studies were RCTs; (2) the participants in the studies were breast cancer patients; (3) the PA interventions in the experimental group included aerobic exercise, resistance training, walking, or a combination of the above, and the control group received routine care; and (4) the study outcome was sleep quality measured by the PSQI and PSQI subscales.
The exclusion criteria were as follows: (1) patients with recurrent or metastatic breast cancer and concurrent dementia; (2) the PA interventions in the experimental group were yoga, tai chi, or qigong; (3) reviews, comments, and letters; and (4) no data or incomplete data. For duplicate publications or the same data used in multiple studies, only the one with the most complete research information was included.
Data extraction and quality assessment
Two investigators independently completed literature screening according to the above inclusion and exclusion criteria and determined the studies for inclusion in this meta-analysis. In addition, the following information in each article was independently extracted by two investigators: the name of the first author, year of publication, study area, age of participants, sample size, breast cancer staging, intervention strategy, and outcome. The Cochrane Collaboration’s tool for assessing risk was used to assess the quality of included studies [16]. If disagreement occurred during data extraction and quality assessment, it would be resolved through a discussion with a third investigator.
Statistical analysis
For all studies that reported continuous data, we evaluated the overall summary using WMD with its 95% confidence interval (CI) for PSQI scores (global scores) and each item’s score (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction). Due to the obvious heterogeneity in the methodology of the included studies (such as inconsistent intensity and type of physical exercise), random effects models were used to merge effect values. Cochran’s Q and I2 tests were used to assess heterogeneity among results [7, 8]. Studies with P < 0.05 and I2 > 50% were defined as significant heterogeneity; otherwise, the heterogeneity was not significant. In addition, subgroup analysis was used to explore the possible sources of heterogeneity. We performed subgroup analysis based on the study area and intervention time. Furthermore, a sensitivity analysis was conducted by removing each included study to assess the stability of the summary results. Publication bias of the included studies was assessed by an Egger test. All statistical analyses were performed using RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen) and Stata 11.0 (StataCorp LP, College Station, TX, USA).
Results
Literature search
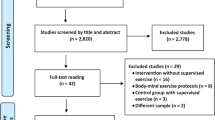
The search strategy for literature selection is shown in Fig. 1. A total of 113, 309, and 205 articles were identified in the PubMed, Embase, and the Cochrane Library databases, respectively. After removing duplicates, 435 articles remained. Of these, 419 articles were eliminated after checking the titles and abstracts. Next, 10 articles were excluded after reading the full text. In addition, manual searches failed to find studies that could be included in this analysis. Finally, six articles were included in this meta-analysis [17,18,19,20,21,22].
Characteristics of the enrolled studies
The characteristics of each eligible study are shown in Table 1. These included studies published from 2009 to 2017 that were conducted in the USA, Turkey, and Taiwan. The overall population included 485 participants (241 in the PA group and 244 in the control group), and the sample size of each study ranged from 28 to 222. There were no statistically significant differences in age and body mass index between the two groups in each included study. Except for the study by Rogers et al. in 2009 [21], the PA interventions in the rest of the studies were performed after breast cancer patients received surgery. The form of PA interventions included walking, aerobic training, or a combination of aerobic and resistance training. Moreover, the intervention times were 6, 12, or 24 weeks.
The details of the risk of bias of the included articles are displayed in Fig. 2. Random sequence generation and allocation concealment were not provided in the study by Wang et al. [22]; thus the selection bias of this study was “unclear.” Meanwhile, the allocation concealment was not described in the study by Roger et al. in 2013 [20], so the selection bias was “unclear.” In addition, none of the studies reported the blinding of participants and personnel as well as the blinding of outcome assessment. Thus, the “blinding of participants and personnel” and “blinding of outcomes assessment” had “unclear risk.” Other evaluation items were “low risk.” Overall, the methodological bias of the included literature was moderate.
Results of meta-analysis
The difference in global PSQI score between PA and traditional care is shown in Fig. 3. All six articles reported the outcome. Significant heterogeneity occurred among the included studies (I2 = 88.2%, P < 0.05). The pooled results showed that no significant difference was observed between the PA group and the control group (WMD = 2.07, 95% CI − 0.06–4.21, P = 0.057).
Forest plots of associations between PA and traditional care based on the global PSQI score. The points represent the effect amount of a single study, and the size of the points represents the weight of the study; the horizontal line represents the confidence interval of the effect value; and the diamond represents the pooled result. PA, physical activity; PSQI, Pittsburgh Sleep Quality Index
The difference between the PA group and the control group based on the PSQI subscales is shown in Fig. 4. Specifically, three articles reported the influence of PA intervention on sleep quality, sleep duration, and sleep medication. Sleep quality was significantly improved in the PA group (WMD = 0.22, 95% CI 0.04–0.40, P = 0.018). No significant differences in sleep duration (WMD = − 0.11, 95% CI − 0.61–0.39, P = 0.660) and use of sleep medication (WMD = 0.06, 95% CI − 0.19–0.30, P = 0.641) were found between the PA group and control groups.
Forest plots of the relationship between PA and traditional care according to the score of the PSQI subscales. The points represent the effect amount of a single study, and the size of the points represents the weight of the study; the horizontal line represents the confidence interval of the effect value; and the diamond represents the pooled result. PA, physical activity; PSQI, Pittsburgh Sleep Quality Index
Four studies reported the influence of PA intervention on the improvement of sleep latency, habitual sleep efficiency, and daytime dysfunction. No significant differences were observed in sleep latency (WMD = 0.17, 95% CI − 0.20–0.54, P = 0.375), habitual sleep efficiency (WMD = 0.01, 95% CI − 0.36–0.38, P = 0.961), and daytime dysfunction (WMD = 0.10, 95% CI − 0.23–0.42, P = 0.571) between the PA group and the control group. Furthermore, two studies reported the influence of PA intervention on sleep disturbances, and the meta-analysis showed no significant difference in disturbances (WMD = 0.09, 95% CI − 0.20–0.37, P = 0.541).
Additionally, no significant heterogeneity among the enrolled studies on sleep quality, habitual sleep efficiency, and use of sleep medication was observed (P > 0.05). In contrast, obvious heterogeneity was observed on sleep duration, sleep latency, and daytime dysfunction (P < 0.05).
Subgroup analysis and sensitivity analysis
We further conducted the subgroup analysis that considered possible confounding factors, such as study area and intervention time (Fig. 5). In the case of regional impact, four studies [18,19,20,21] reported the participants from the USA, and pooled results showed that there was no significant difference between PA intervention and control groups (P = 0.146). In addition, participants from Turkey [17] and Taiwan [22] showed PA intervention could improve the global PSQI score (all P < 0.05). Furthermore, PA intervention for 12 weeks [18,19,20,21] found no significantly different effect on global PSQI score (P = 0.146), while PA intervention for 24 weeks [17] or 6 weeks [22] showed that significant between-group difference was noted for global PSQI score. However, some subgroup factors (such as PA intervention for 24 or 6 weeks; participants from Turkey or Taiwan) included only one study, and the obtained results were not representative. Thus, we did not find significant subgroup effects for all the subgroup factors. Sensitivity analysis results of global PSQI score showed that the combined result of the effect value was unstable (Fig. 6). Statistical significance was observed when we excluded the study by Rogers et al. [21] (WMD = 2.48, 95% CI 0.13–4.48, P < 0.05) or Rogers et al. [19] (WMD = 2.57, 95% CI 0.27–4.87, P < 0.05).
Forest plots for subgroup analysis stratified by study area and the length of intervention time. The points represent the effect amount of a single study, and the size of the points represents the weight of the study; the horizontal line represents the confidence interval of the effect value; and the diamond represents the pooled result
Additionally, the sensitivity analysis of PSQI subscales showed that the pooled results did not change after excluding one study each time, indicating that the conclusions were highly stable. However, the items of the PSQI subscales reported in these studies [18,19,20,21] were all from the same team (Rogers LQ and colleagues) with the same long intervention time (12 weeks). Thus, subgroup analysis based on the PSQI subscales was not performed.
Publication bias
Publication bias analysis was performed according to the global PSQI score. The result of the Egger test was P = 0.720, indicating no significant publication bias among the included studies.
Discussion
Sleep problems have a negative impact on the health of cancer survivors. In addition, poor sleep quality contributes to poorer functional well-being and stronger fatigue intensity among breast cancer survivors [23]. It has been reported that PA intervention plays a role in improving QoL, and regular exercise is associated with improvement of overall sleep health [24]. This meta-analysis provided a comprehensive analysis of the benefits of PA interventions in breast cancer survivors. The results demonstrated that the benefits of PA interventions on sleep might manifest as significant improvements in sleep quality in breast cancer survivors (WMD = 0.22, 95% CI 0.04–0.40, P = 0.018). However, no significant differences were found in the global PSQI score and other PSQI subscales (all P > 0.05) between the PA intervention and control groups. The PSQI is used to measure overall sleep quality during the past month, which is a useful tool for the assessment of subjective sleep quality in nonclinical and clinical settings [25]. It measures sleep quality from seven dimensions, and the sum of these seven components produces an overall score to distinguish people with good sleep quality from those with poor sleep quality [13]. Additionally, this scale has good reliability and validity, and it has been widely used in general medical and psychiatric studies. Notably, a higher PSQI score is associated with poorer sleep quality [26]. Armbruster et al. [27] examined the impact of PA intervention on the sleep quality of endometrial cancer survivors using the PSQI score and found that the mean global PSQI score did not statistically change from baseline to PA intervention for 6 months. In the current study, despite the PSQI score changed after the PA intervention compared with that of the baseline score, no statistically significant difference in global PSQI score was observed between the PA and control groups, which were in line with the results of Armbruster et al.’s study [27].
Regarding PSQI subscales, our meta-analysis results showed that PA interventions could significantly improve sleep quality, while PA interventions had no effect on sleep latency, sleep duration, habitual sleep efficacy, sleep disturbances, use of sleep medication, and daytime dysfunction. A systematic analysis by Mercier et al. [12] reported that 48% of qualitative reviews showed a beneficial effect of exercise on poor sleep quality. In addition, Rogers et al. [18] revealed that PA intervention significantly improved sleep quality and reduced daytime dysfunction as well as sleep disturbances in breast cancer survivors at 3 months; only some of the results were consistent with our study. We suspected that these differences might be caused by different periods of intervention. In this analysis, the intervention period of patients ranged from 6 to 24 weeks. Moreover, sleep latency represents the time spent falling asleep, sleep duration represents total time of sleep, and sleep efficiency refers to sleep time divided by time spent in bed [28]. Kreutz et al. [11] found no significant difference in the effect of physical exercise on these indicators among patients with breast cancer, which was consistent with our findings.
According to the data from the study by Humpel et al. [29], sufficient exercise as per the national guidelines was only reported by 19% of breast cancer survivors, suggesting that the effect of PA on sleep dysfunction might be limited by the duration or intensity of PA. The duration of PA in the included studies varied from each other, such as 6, 12, or 24 weeks. Although we concluded in the three included studies that PA intervention had a significant impact on sleep quality of breast cancer survivors, they were limited to only one among several PSQI subscales and did not correlate with the global PSQI scores, and the association should be further studied after including more patients and more detailed information.
To date, the mechanism by which exercise affects sleep quality is largely unclear. Some common signs and symptoms of cancer are related to pro-inflammatory cytokines. Therefore, one crucial mechanism through which physical exercise exerts effects on sleep is by reducing chronic low-grade inflammation [30]. It is also known that sleep disorders are associated with increases in markers of systemic inflammation, such as C-reactive protein, interleukin-6, and tumor necrosis factor-α [31]. Thus, it is possible that the effects of PA on sleep quality in breast cancer patients can be attributed to changes in inflammation. Another hypothesis may be that exercise is involved in a dual-process model of circadian rhythm and homeostatic regulation [32, 33], and it stimulates the recovery function during sleep, triggering an increase in body temperature. Then, the decrease in body temperature after exercise can promote the latency to fall asleep and slow-wave sleep [34]. However, the evidence is still limited. Meanwhile, our present analysis did not conduct an in-depth study on the mechanisms through which exercise affect sleep, which would be the focus of our future research.
In this meta-analysis, significant heterogeneity existed, which might be attributed to inconsistent intensity and type of physical exercise in each enrolled study. All statistics of PA in enrolled studies were self-reported, and thus, the data might be under- or over-reported. Moreover, subgroup analysis based on the study area and follow-up time was performed to explore heterogeneity in the meta-analysis, but the results showed that no significant subgroup effects were found for any subgroup factors.
The strengths of our analysis are listed as follows. A unified sleep quality assessment tool, the PSQI, was used in our study, which could reduce heterogeneity in outcome evaluation to some extent. The low risk of publication bias in the included studies suggested highly credible results. Additionally, sensitivity analysis demonstrated that our results were stable. Unfortunately, some limitations of this study should also be acknowledged. First, the studies included in this meta-analysis were limited, and most of them were from the same authors (Rogers et al.). Although no significant effect of PA on the PSQI global score was found, RCTs with a larger sample size were needed to verify this conclusion. Second, various types of PA interventions were included in the study, which might have resulted in heterogeneity. Finally, there was no follow-up to track the continuous effect post-intervention due to the limitations of the original study.
Till date, nontraditional care such as PA has been recommended for patients with cancer. The current study showed that sleep quality in breast cancer survivors could benefit from PA intervention. Although our data suggested that physical exercise could improve the PSQI sleep quality score, and no significant differences between two groups was noted for sleep duration, sleep medication, sleep latency, habitual sleep efficiency, and daytime dysfunction between the two groups. Thus, further RCTs with large samples and of high quality are needed to verify this hypothesis.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Voiss P, Höxtermann MD, Dobos G, Cramer H (2019) Cancer, sleep problems, and mind-body medicine use: results of the 2017 National Health Interview Survey. Cancer 125(24):4490–4497
Colagiuri B, Christensen S, Jensen AB, Price MA, Butow PN, Zachariae R (2011) Prevalence and predictors of sleep difficulty in a national cohort of women with primary breast cancer three to four months postsurgery. J Pain Symptom Manag 42(5):710–720
Lowery-Allison AE, Passik SD, Cribbet MR, Reinsel RA, O'Sullivan B, Norton L, Kirsh KL, Kavey NB (2018) Sleep problems in breast cancer survivors 1-10 years posttreatment. Palliat Support Care 16(3):325–334
Garland SN, Mahon K, Irwin MR (2019) Integrative approaches for sleep health in cancer survivors. Cancer J 25(5):337–342
Palesh O, Aldridge-Gerry A, Ulusakarya A, Ortiz-Tudela E, Capuron L, Innominato PF (2013) Sleep disruption in breast cancer patients and survivors. J Natl Compr Cancer Netw 11(12):1523–1530
Sternfeld B, Weltzien E, Quesenberry CP Jr, Castillo AL, Kwan M, Slattery ML, Caan BJ (2009) Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomark Prev 18(1):87–95
Palesh O, Kamen C, Sharp S, Golden A, Neri E, Spiegel D, Koopman C (2018) Physical activity and survival in women with advanced breast cancer. Cancer Nurs 41(4):E31–E38
Kampshoff CS, Chinapaw MJ, Brug J, Twisk JW, Schep G, Nijziel MR, van Mechelen W, Buffart LM (2015) Randomized controlled trial of the effects of high intensity and low-to-moderate intensity exercise on physical fitness and fatigue in cancer survivors: results of the Resistance and Endurance exercise After ChemoTherapy (REACT) study. BMC Med 13:275
Roveda E, Vitale JA, Bruno E, Montaruli A, Pasanisi P, Villarini A, Gargano G, Galasso L, Berrino F, Caumo A, Carandente F (2017) Protective effect of aerobic physical activity on sleep behavior in breast cancer survivors. Integr Cancer Ther 16(1):21–31
Bernard P, Ivers H, Savard MH, Savard J (2016) Temporal relationships between sleep and physical activity among breast cancer patients with insomnia. Health Psychol 35(12):1307–1315
Kreutz C, Schmidt ME, Steindorf K (2019) Effects of physical and mind-body exercise on sleep problems during and after breast cancer treatment: a systematic review and meta-analysis. Breast Cancer Res Treat 176(1):1–15
Mercier J, Savard J, Bernard P (2017) Exercise interventions to improve sleep in cancer patients: a systematic review and meta-analysis. Sleep Med Rev 36:43–56
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213
de la Vega R, Tomé-Pires C, Solé E, Racine M, Castarlenas E, Jensen MP, Miró J (2015) The Pittsburgh Sleep Quality Index: validity and factor structure in young people. Psychol Assess 27(4):e22–e27
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Br Med J 339:b2535
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10:ED000142
Ghavami H, Akyolcu N (2017) The impact of lifestyle interventions in breast cancer women after completion of primary therapy: a randomized study. J Breast Health 13(2):94–99
Rogers LQ, Courneya KS, Oster RA, Anton PM, Robbs RS, Forero A, McAuley E (2017) Physical activity and sleep quality in breast cancer survivors: a randomized trial. Med Sci Sports Exerc 49(10):2009–2015
Rogers LQ, Fogleman A, Trammell R, Hopkins-Price P, Spenner A, Vicari S, Rao K, Courneya KS, Hoelzer K, Robbs R, Verhulst S (2015) Inflammation and psychosocial factors mediate exercise effects on sleep quality in breast cancer survivors: pilot randomized controlled trial. Psychooncology 24(3):302–310
Rogers LQ, Fogleman A, Trammell R, Hopkins-Price P, Vicari S, Rao K, Edson B, Verhulst S, Courneya KS, Hoelzer K (2013) Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther 12(4):323–335
Rogers LQ, Hopkins-Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, Verhulst S, Hoelzer K, Naritoku C, Jones L, Dunnington G, Lanzotti V, Wynstra J, Shah L, Edson B, Graff A, Lowy M (2009) A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc 41(4):935–946
Wang YJ, Boehmke M, Wu YW, Dickerson SS, Fisher N (2011) Effects of a 6-week walking program on Taiwanese women newly diagnosed with early-stage breast cancer. Cancer Nurs 34(2):E1–E13
Vargas S, Wohlgemuth WK, Antoni MH, Lechner SC, Holley HA, Carver CS (2010) Sleep dysfunction and psychosocial adaptation among women undergoing treatment for non-metastatic breast cancer. Psychooncology 19(6):669–673
Reynolds GO, Otto MW, Ellis TD, Cronin-Golomb A (2016) The therapeutic potential of exercise to improve mood, cognition, and sleep in Parkinson’s disease. Mov Disord 31(1):23–38
Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A (2016) The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev 25:52–73
Grutsch JF, Wood PA, Du-Quiton J, Reynolds JL, Lis CG, Levin RD, Ann Daehler M, Gupta D, Quiton DF, Hrushesky WJ (2011) Validation of actigraphy to assess circadian organization and sleep quality in patients with advanced lung cancer. J Circadian Rhythms 9:4
Armbruster SD, Song J, Gatus L, Lu KH, Basen-Engquist KM (2018) Endometrial cancer survivors’ sleep patterns before and after a physical activity intervention: a retrospective cohort analysis. Gynecol Oncol 149(1):133–139
Henneghan AM, Carter P, Stuifbergan A, Parmelee B, Kesler S (2018) Relationships between self-reported sleep quality components and cognitive functioning in breast cancer survivors up to 10 years following chemotherapy. Psychooncology 27(8):1937–1943
Humpel N, Iverson DC (2010) Sleep quality, fatigue and physical activity following a cancer diagnosis. Eur J Cancer Care 19(6):761–768
Meneses-Echávez JF, Correa-Bautista JE, González-Jiménez E, Schmidt Río-Valle J, Elkins MR, Lobelo F, Ramírez-Vélez R (2016) The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Cancer Epidemiol Biomark Prev 25(7):1009–1017
Irwin MR, Olmstead R, Carroll JE (2016) Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 80(1):40–52
Aoyama S, Shibata S (2017) The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front Neurosci 11:63
Yamanaka Y, Hashimoto S, Takasu NN, Tanahashi Y, Nishide SY, Honma S, Honma K (2015) Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am J Physiol Regul Integr Comp Physiol 309(9):R1112–R1121
Aritake-Okada S, Tanabe K, Mochizuki Y, Ochiai R, Hibi M, Kozuma K, Katsuragi Y, Ganeko M, Takeda N, Uchida S (2019) Diurnal repeated exercise promotes slow-wave activity and fast-sigma power during sleep with increase in body temperature: a human crossover trial. J Appl Physiol (1985) 127(1):168–177
Funding
This study was supported by the Fundamental Research Funds for the Central Universities (no. B210202175), the Hohai University Disciplinary Planning Program (no. 1013–418246) and Social Science Fund of Jiangsu Province (no. 19TYD001).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: QZ and HY; acquisition of data: ZY and HP; analysis and interpretation of data: ZY and HP; statistical analysis: ZY and HP; obtained funding: QZ; drafting the manuscript: HY; and revision of the manuscript for important intellectual content: QZ. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Yang, H., Yang, Z., Pan, H. et al. Effects of physical activity on sleep problems in breast cancer survivors: a meta-analysis. Support Care Cancer 29, 4023–4032 (2021). https://doi.org/10.1007/s00520-020-05914-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05914-y