Abstract
The increasing population and demand for food and feed have increased the urge to find protein sources from waste products. Due to poor management of waste valorization, it has become a pollutant to the environment. This waste can be converted into a valuable product by microbial degradation. Feather waste from poultry farms can be efficiently processed into hydrolysates, serving as an additive or in its crude form for animal feed and detergents. This approach not only reduces pollution but also boosts the economy of a country. Keratin is a hard fibrous protein, insoluble in water and organic solvents. They are accumulated in nature and are major components of feathers, nails, hairs, and wool. Microorganisms like bacteria, fungi, and actinomycetes can degrade keratin by producing the keratinase enzyme. Keratinases are thought to be promising biocatalysts for the production of animal nutrients, protein supplements, leather processing, fibre modification, detergent formulations, and pharmaceutical, cosmetic, and biomedical industries. An overview of keratin structure and composition, the mechanism of microbial hydrolysis of keratin, and their possible uses in biotechnological sectors are presented in this review.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
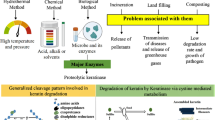
Feathers account for about 5–7% of the body weight of birds, and about 91% of chicken feathers are made up of protein (keratin), 1% lipids, and 8% water. A typical feather comprises rachis (central shaft), barbs, barbules, and shaft with keratin protein. These feather content are discarded annually in large quantities because of no proper valorization (Verma et al. 2017). Many pathogenic organisms can survive on them that release toxic pollutants, which lead to harmful effects on human health. Keratins are the structural elements of feathers, hair, nails, horns, hooves, bones, furs, claws, hides, beaks, skin, wool, scales, and bristles. About 90% of keratins are found in birds’ feathers and hairs (Gopinath et al. 2015). After chitin and cellulose, keratin is one of the most prevalent biopolymers found in nature. It is a highly insoluble, rigid fibrous material found in the tissues of mammals, amphibians, reptiles, and birds (feathers and beaks). Degradation of keratin involves two steps; the first step is keratinase adsorption to a macromolecule surface through hydrophobic and electrostatic interaction and the second step is the catalytic action. The catalysis of keratin can be of two steps; sulfitolysis or reduction of disulfide bond and the other step is proteolysis. Sulfitolysis is the major step in keratin hydrolysis before the action of keratinase, it happens in the presence of reducing compounds like disulfide reductase, sodium sulfite, dithiothreitol (DTT), and mercaptoethanol and acts synergistically with keratinase in degradation of keratin molecules (Yamamura et al. 2002). In proteolysis, keratinase can break the polypeptides into amino acids illustrated in Fig. 1. Keratin breakdown happens by multiple protease families since different enzymes favour different cleavage sites, and many enzymes are needed to convert keratin into amino acids completely. Additionally, disulfide reductase (Kasperova et al. 2013), lytic polysaccharide monooxygenases (Lange et al. 2016), and specific enzymes involved in lipoprotein signalling or fatty acid degradation have been related to biocatalytic keratin breakdown.
Conventional methods like acid hydrolysis are quite effective, but they also lose some essential amino acids, such as tryptophan, thus the residue that is left over is not very nutritious (Goda et al. 2021). Alkali hydrolysis at 80 °C with 2% NaOH for three hours produced a 25% yield which also lost some essential amino acids like cysteine, tryptophan, methionine, and tyrosine (Chilakamarry et al. 2021). Feather degradation using microorganisms is an easy and less expensive process for converting raw feathers to hydrolysates as a useful end product. Microbial degradation of keratin waste can yield high-quality protein hydrolysates (Nnolim et al. 2020a). Figure 2 represents the use of feather hydrolysates for animal feed, as a bio-fertilizer, producing coatings, films, and glues (Ozdal and Kurbanoglu 2018, 2019a, b), in cosmetics production, and pharmaceutical industries. They can also be used in peptone-substituted microbial growth media (Ozdal et al. 2017a, b; Kurbanoglu et al. 2015), plant growth hormones (Ozdal et al. 2017a, b), citric acid production (Ozdal and Kurbanoglu 2019a, b) and animal feedstocks (Nnolim and Nwodo 2021). Many studies have reported the use of single strain identification for degrading keratins, which has potential, but the use of microbial consortium showed better results which have less evidence (Kang et al. 2020). More research on microbial consortium-based degradation methods should be studied for efficient hydrolysis, and reduced loss of amino acids can be obtained. Different microorganisms have different capacities to degrade based on their biochemical factors (Callegaro et al. 2018). Some of the studies on keratin-degrading microorganisms and their applications are described in Table 1. Employment of microbial consortium, keratin can be degraded effectively and contribute to industries in an eco-friendly manner, such as pharmaceuticals, cosmetics, animal feed, and bio-fertilizers (Kang et al. 2021).
Structure of keratin
Keratin, a fibrous and structural polypeptide, belongs to a heterogeneous family and is highly complex to degradation by proteolytic enzymes. Disulfide bonds and hydrogen bonds that interconnect keratin make them insoluble in water, weak acids, and organic solvents. It has been classified according to its secondary structure: 𝛼 keratins and β keratins. 𝛼-keratins are found in the hair, horns, nails, claws, wool, and hooves of mammals. It has an average molecular weight between 40 and 60 kDa, has a reduced content of sulfur, is partly crystalline, and also self-assembles into filamentous fibres (Lee et al. 2014). Feathers, claws, and beaks of birds contain β-keratins, which are insoluble sulfur compounds with disulfide bridges. The interchain hydrogen bonds that exist between the amino and carbonyl groups define the β-sheet structure. These sheets are formed by hydrophobic interactions between four β-strands and later form dimers by various types of interactions like disulfide bonds, salt bridges, and hydrophobic interactions which in turn assemble into β-keratin filaments. Based on the sulfur content that comes from cysteine residues, feathers are further divided into soft and hard keratins. Hard keratins are present in feathers, hairs, and nails, while soft keratins are found in the skin and callus (Gopinath et al. 2015).
Keratinase
Keratinase [EC 3.4.21/24/99.11] is a group of hydrolytic enzymes that can catalyze the degradation of keratins. Keratinases are serine and metalloprotease or serine-metalloprotease enzymes capable of degrading keratinous protein (Hassan et al. 2020). Though, to date, they cannot completely solubilize keratins, their nature of catalysis is still a puzzle (Gupta and Ramnani 2006). A detailed description of the diversity and classification of keratinase has been provided in the MEROPS database (Rawlings et al. 2018), based on the amino acid sequence and conserved domains. So far, at least fourteen distinct protease families represent the known keratinolytic enzymes such as S1, S8, S9, S10, S16, M3, M4, M14, M16, M28, M32, M36, M38, and M55 (Qiu et al. 2020). Based on so far recognized enzymes and the sequences filed in databases it is estimated that the molecular weight of keratinase ranges from 20 to 200 kDa. The ability to classify keratinases according to their amino acid sequence offers a distinct and understandable perspective on the mechanism and function of keratinases, indicating that figuring out the amino acid sequence of newly discovered keratinases is a crucial task (Li 2021; Gopinath et al. 2015).
Microbial degradation of keratin
Bacterial strains like Bacillus licheniformis (Lin et al. 1997), B. pumilus, B. cereus, and B. Subtilis (Nagal and Jain 2010) as well as non-sporogenic bacteria Stenotrophomonas sp. (Yamamura et al. 2002), Fervidobacterium pennavorans (Friedrich and Antranikian 1996), Lysobacter sp. (Pereira et al. 2014) and Kocuria sp. (Bernal et al. 2006) have been reported to be capable of degrading keratin. According to a study, the anaerobic bacterium Serratia marcescens EGD-HP20 is responsible for the breakdown of feathers through the production of proteolytic enzymes that hydrolyze keratin (Fuke et al. 2018). Two fungi were isolated from feathers: Fusarium oxysporum and Aspergillus sp. exhibited effective keratinase production of which Fusarium oxysporum showed high enzyme activity on the 6th day with a value of 243.25 U mL–1 and Aspergillus sp. 113.50 U mL–1 on 9th day (Preczeski et al. 2020). Recent research revealed actinomycetes strain Streptomyces werraensis KN23 showed high keratinase production reporting 51.60 U/mL and enhanced its effect by chemical mutagenesis recording its value of 106.92 U/mL (Abd El-Aziz et al. 2023). Factors like pH, temperature, carbon, nitrogen source, and agitation rate influence keratin hydrolysis (Revankar et al. 2023).
Applications
Feather lysate as animal feed
Feather meals as an additive for livestock have been practised for many years. Still, there has been concern about its nutritional value due to the inadequacy of essential amino acids in the feed. This is due to the conventional chemical-based treatment of feathers which involves higher energy and denatures some heat-labile amino acids. Chicken fed with feather lysate prepared by anaerobic fermentation of feather with Bacillus licheniformis PWD-1 showed an increase in growth response of 19.3% with that of corn-soybean meal used as control. The chickens fed with feather hydrolysate supplemented with lysine, methionine, and histidine as additives produced a similar growth curve as soybean meal (Williams et al. 1991). In a study, the Kocuria rosea strain was cultured under aerobic conditions with feathers as the substrate by submerged fermentation to obtain feather meal. Pepsin digestibility of the feather meal obtained by this method was about 88%, which is similar to the value seen in commercially available meals. Apart from that the feather meal fermented by Kocuria rosea was observed to have more amino acid contents when compared to commercial feather meal. Additionally, the bacterial biomass increased the amount of amino acids, notably lysine by 3.46%, histidine by 0.94%, and methionine by 0.69% (Bertsch and Coello 2005). The growth of broiler chicken fed with feather meal prepared by Bacillus licheniformis LMUB05 fermentation was comparable with that of the growth of broiler chicken fed standard meal (Adetunji and Adejumo 2018). Bacillus licheniformis ER-15 produced dimeric keratinases that enhanced the decaying activity of feathers at 50॰C pH 8 within 8 h. Also, 14% nitrogen, 44% carbon, and a few necessary amino acids were present in the feather meal (Tiwary and Gupta 2012). This research gives a way to replace commercial meals with feather lysate as a protein source to reduce the overhead cost of production.
Feather lysate as biofertilizer
Feather hydrolysate as a biofertilizer is currently needed as it is an environment-friendly, cost-effective product for agricultural production, as chemical fertilizers are harmful to the soil. Feather lysate with amino acids and peptides can be used as a plant growth promoter and slow-release nitrogenous fertilizer (Bhari et al. 2021). A feather degrading bacteria Bacillus tropics LS 27 isolated from a poultry dumping site degraded chicken feathers and the feather hydrolysate showed antioxidant properties and obtained 1.5 mg/mL by DPPH scavenging assay. It also showed that plants supplemented with 20% feather hydrolysate showed increased plant growth-promoting properties when utilized as a liquid biofertilizer (Stanly and Umesh 2023). By composting pig bristles in a mixture containing sawdust and lignite dust, the keratinolytic Bacillus cereus PCM 2849 strain shows improvement in the decomposition of organic waste. The carbon and nitrogen ratio, carbon solubility, oxidation index of mineral forms of nitrogen, and humification ratio were all favourably impacted by the bacterial inoculum, which also improved the transformation of mineral compounds (Choinska-Pulit et al. 2019). Amycolatopsis sp. MBRL 40 was found in another study to have antifungal efficacy against four significant fungal pathogens Pyricularia oryzae, Rhizoctonia solani, Fusarium oxysporum, Curvularia oryzae, and plant growth regulators like indole-3-acetic acid (IAA) production and phosphate solubilization. Rice seeds treated with Amycolatopsis sp. MBRL 40 showed increased germination, vigor indices, and seedling growth. Protein hydrolysate obtained from Trichoderma asperellum showed improved activity on crop health (Calin et al. 2019). The growth rate of plants was observed higher when the feather hydrolysate pellet was supplemented with bioinoculant compared to plants provided only with feather hydrolysate pellet (Tamreihao et al. 2017). When chicken feathers are used as foliar feeding to banana plants, Chryseobacterium sp. RBT degraded them and banana fruit’s protein content rose from 15 mg/g to 16 mg/g and its amino acid content from 2 mg/g to 2.96 mg/g. Furthermore, the fertilizing action of banana fruit significantly increased its anti-oxidant capacity through the presence of flavonoids and phenolics. Two native Arbuscular Mycorrhizal Fungi (AMF), Glomus caesaris and Acaulospora bireticulata obtained from sandy soil and Bacillus licheniformis ASU from chicken feathers. Hydrolysate from the feathers and AMF or a combination of both was inoculated in pots where faba bean seeds were grown. When compared to non-inoculated plants, the total dry biomass of plants co-inoculated with AMF and feather hydrolysate (FH) increased substantially. Therefore, the combination of FH and AMF may develop into a useful biofertilizer in the future (Nafady et al. 2018).
Partially hydrolyzed keratin for producing films, coatings, and glues
A study used microcrystalline cellulose (0.2%) and glycerol (3.5%) in NaOH to create the bioplastic film and keratin hydrolysate were obtained after chemical treatment of feathers for 48 h at 60 °C where the thickness of the bioplastic film was found to be 1.12 × 10− 4 mm (Sharma et al. 2018). Using non-soluble keratin for biodegradable and eco-friendly composites has developed a new process to combat sustainable economic needs. Keratin powder was added to polylactic acid pellets to obtain composites using hot-melt extrusion technology. The samples exhibited the same thermal stability compared to Poly lactic acid alone with decreased toughness (Pulidori et al. 2022). A study developed an efficient biomaterial that converted keratin waste to bio-composite film in combination with ginger starch (Oluba et al. 2021). Feather protein-based resins were created by utilizing three pH values, two formaldehyde-phenol (F-P) ratios, and two hydrolysis techniques. Feather hydrolysate was prepared with an F-P ratio of 2.0 at pH 10.5 and 30% phenol substitution with feather protein. It was made from one part feather meal hydrolyzed in an alkaline solution with two parts phenol, and it performed better than a commercial phenol-formaldehyde resin. These results suggest that feather hydrolysate functions as an efficient co-polymer in these types of resin formulations and could represent a reasonably priced additional raw material for the preparation of phenol-formaldehyde-type wood adhesive resins (Jiang et al. 2008).
Biogas production from keratin waste
The conversion of keratin waste to biogas is an eco-friendly and cost-effective method. A two-process system was developed that combines keratin degradation and biogas production. Chicken feather degradation by recombinant Bacillus megaterium strain showed a yield of 0.51 mg/mL soluble proteins after 8 days of cultivation. During the anaerobic batch digestion process methane gas produced about 0.35 Nm3/kg of dry feathers (Forgacs et al. 2011). A similar study was carried out where keratin was first degraded by Bacillus licheniformis KK1 and then the hydrolysate obtained was optimized and metabolized by Thermococcus litoralis generating hydrogen gas as a fermentation byproduct (Balint et al. 2005). These methods can be effective and environment friendly where waste is converted into a byproduct along with biogas production that can reduce the manpower and cost for their production.
Feather hydrolysate in cosmetics
Hydrolyzed keratin peptides obtained from wool were applied to the skin, improving hydration and elasticity. It can also be applied with wool internal lipids, enhancing absorption and desorption profile (Barba et al. 2008). Keratin hydrolysates from chicken feather waste showed potential antioxidant and anti-tyrosinase activity (Kshetri et al. 2020). Keratin peptides obtained from enzymatic hydrolysis of chicken feathers by Bacillus subtilis AMR tend to penetrate hair or nail cuticles and hydrate the hair follicles. The keratin peptides made the hair fibres more hydrated, and the sealed cuticles in the fibres treated with the hydrolysates also showed a significant improvement in brightness and softness by Scanning Electron Microscope (SEM) analysis (Villa et al. 2013). Hydrolysates of keratin by alkaline-enzymatic hydrolysis strengthen the skin barrier by reducing transepidermal water loss and hydrating the skin. Moisturizing properties were examined in both men and women at different time intervals and concentrations. According to the results of the hydration measurement, adding 2% keratin hydrolysate to the ointment base during the monitored measurement interval (1–48 h) results in an increase in stratum corneum hydration of 11–19% for male volunteers and 12–22% for female volunteers and it also showed a reduction in transepidermal water loss (Mokrejs et al. 2017a).
Pharmaceutical applications
2% of keratin hydrolysate combined with an ointment base can act as a good humectant when tested over 48 h and has increased skin hydration. The results interpreted a 14-23% increase in hydration of stratum corneum and for trans-epidermal water loss about 4% of keratin hydrolysate was preferred for the reduced trans-epidermal water loss of about 26-46%. Such formulations are stable in their structure and do not cause phase separation (Mokrejs et al. 2017b). Acrylic acid and acrylamide monomers (AAm) were added with keratin hydrolysate obtained from bovine hair using the alkali technique to create superabsorbent hydrogels through free radical graft copolymerization. After 48 h, the highest swelling ratio of 1791% was observed at pH 9. With a swelling capacity of 1430.7%, the ideal formulation of the synthesized hydrogel contains 3 g of keratin hydrolysate and 4 g of AAm copolymer. This suggested technique effectively transformed low-biodegradable keratin waste into superabsorbent hydrogel that can be used in cosmetics and biomedical applications (Arican et al. 2021).
Conclusion
Poultry processing industries and farms produce enormous amounts of feather-keratin waste which creates a problem of solid waste in the environment. Microbial conversion of feather waste is an eco-friendly and sustainable approach. Chicken feather waste can be processed into a useful by-product for various industries mainly in the cosmetics and bio-fertilizer domain. Feather hydrolysates contain rich amino acids which could be potent for poultry feed development. In the future, feathers can be converted into enriched products that can support industries at a large scale without looking for any other source of raw material that can cause damage to the environment. This review highlights the versatile uses of keratin hydrolysates in various biotechnology sectors which paved a new way to combat environmental pollution by transforming community waste into a valuable product.
Data availability
All the data used for the study has been cited and included in the references.
Abbreviations
- DTT:
-
Dithiothreitol
- IAA:
-
Indole-3-Acetic Acid
- AMF:
-
Arbuscular Mycorrhizal Fungi
- FH:
-
Feather Hydrolysate
- SEM:
-
Scanning Electron Microscope
- Aam:
-
Acrylic acid and Acrylamide monomers
References
Abd El-Aziz NM, Khalil BE, Ibrahim HF (2023) Enhancement of feather degrading keratinase of Streptomyces swerraensis KN23, applying mutagenesis and statistical optimization to improve keratinase activity. BMC Microbiol 23:158. https://doi.org/10.1186/s12866-023-02867-0
Adelere IA, Lateef A (2019) Degradation of keratin biomass by different microorganisms. Keratin Protein Biopolym. Extr Waste Biomass Appl 123–162. https://doi.org/10.1007/978-3-030-02901-2_5
Adetunji CO, Adejumo IO (2018) Efficacy of crude and immobilized enzymes from Bacillus licheniformis for production of biodegraded feather meal and their assessment on chickens. Environ Technol Innov 11:116–124. https://doi.org/10.1016/j.eti.2018.05.002
Arican F, Uzuner-Demir A, Sancakli A, Ismar E (2021) Synthesis and characterization of superabsorbent hydrogels from waste bovine hair via keratin hydrolysate graft with acrylic acid (AA) and acrylamide (AAm). Chem Pap 75(12):6601–6610. https://doi.org/10.1007/s11696-021-01828-z
Balint B, Bagi Z, Toth A, Rakhely G, Perei K, Kovacs KL (2005) Utilization of keratin-containing biowaste to produce biohydrogen. Appl Microbiol Biotechnol 69(4):404–410. https://doi.org/10.1007/s00253-005-1993-3
Barba C, Mendez S, Roddick-Lanzilotta A, Kelly R, Parra JL, Coderch L (2008) Cosmetic effectiveness of topically applied hydrolysed keratin peptides and lipids derived from wool. Skin Res Technol 14(2):243–248. https://doi.org/10.1111/j.1600-0846.2007.00280.x
Bernal C, Cairo J, Coello N (2006) Purification and characterization of a novel exocellular keratinase from Kocuria rosea. Enzyme Microb Technol 38(1):49–54. https://doi.org/10.1016/j.enzmictec.2005.02.021
Bertsch A, Coello N (2005) A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Bioresour Technol 96(15):1703–1708. https://doi.org/10.1016/j.biortech.2004.12.026
Bhari R, Kaur M, Singh RS (2019) Thermostable and halotolerant keratinase from Bacillus aerius NSMk2 with remarkable dehairing and laundary applications. J Basic Microbiol 59(6):555–568. https://doi.org/10.1002/jobm.201900001
Bhari R, Kaur M, Sarup Singh R (2021) Chicken Feather Waste Hydrolysate as a Superior Biofertilizer in Agroindustry. Curr Microbiol 78(6):2212–2230. https://doi.org/10.1007/s00284-021-02491-z
Cai C, Lou B, Zheng X (2008) Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. J Zhejiang Univ Sci B 9(1):60–67. https://doi.org/10.1631/jzus.B061620
Calin M, Raut I, Arsene ML, Capra L, Gurban AM, Doni M et al (2019) Applications of fungal strains with keratin-degrading and Plant Growth promoting characteristics. Agronomy. Multidisciplinary Digit Publishing Inst 9(9):543. https://doi.org/10.3390/agronomy9090543
Callegaro K, Welter N, Daroit DJ (2018) Feathers as bioresource: microbial conversion into bioactive protein hydrolysates. Process Biochem 75:1–9. https://doi.org/10.1016/j.procbio.2018.09.002
Chilakamarry CR, Mahmood S, Saffe SNBM, Arifin MAB, Gupta A, Sikkandar MY et al (2021) Extraction and application of keratin from natural resources: a review. 3 Biotech 11(5):220. https://doi.org/10.1007/s13205-021-02734-7
Choinska-Pulit A, Laba W, Rodziewicz A (2019) Enhancement of pig bristles waste bioconversion by inoculum of keratinolytic bacteria during composting. Waste Manag 84:269–276. https://doi.org/10.1016/j.wasman.2018.11.052
Duffeck CE, de Menezes CLA, Boscolo M, da Silva R, Gomes E, da Silva RR (2020a) Keratinases from Coriolopsis byrsina as an alternative for feather degradation: applications for cloth cleaning based on commercial detergent compatibility and for the production of collagen hydrolysate. Biotechnol Lett 42(11):2403–2412. https://doi.org/10.1007/s10529-020-02963-5
Duffeck CE, de Menezes CLA, Boscolo M, da Silva R, Gomes E, da Silva RR (2020b) Citrobacter diversus-derived keratinases and their potential application as detergent-compatible cloth-cleaning agents. Braz J Microbiol 51(3):969–977. https://doi.org/10.1007/s42770-020-00268-3
Forgacs G, Alinezhad S, Mirabdollah A, Feuk-Lagerstedt E, Horvath IS (2011) Biological treatment of chicken feather waste for improved biogas production. J Environ Sci 23(10):1747–1753. https://doi.org/10.1016/s1001-0742(10)60648-1
Friedrich AB, Antranikian G (1996) Keratin degradation by Fervidobacterium pennavorans, a Novel Thermophilic Anaerobic species of the Order Thermotogales. Appl Environ Microbiol 8:2875–2882. https://doi.org/10.1128/aem.62.8.2875-2882.1996
Fuke P, Gujar VV, Khardenavis AA (2018) Genome annotation and validation of keratin-hydrolyzing proteolytic enzymes from Serratia marcescens EGD-HP20. Appl Biochem Biotechnol 184(3):970–986. https://doi.org/10.1007/s12010-017-2595-0
Goda DA, Bassiouny AR, Abdel Monem NM, Soliman NA, Abdel-Fattah YR (2021) Feather protein lysate optimization and feather meal formation using YNDH protease with keratinolytic activity afterward enzyme partial purification and characterization. Sci Rep Nat Publishing Group 11(1):14543. https://doi.org/10.1038/s41598-021-93279-5
Gong J-S, Wang Y, Zhang D-D, Zhang R-X, Su C, Li H et al (2015) Biochemical characterization of an extreme alkaline and surfactant-stable keratinase derived from a newly isolated actinomycete Streptomyces aureofaciens K13. RSC Adv Royal Soc Chem 5(31):24691–24699. https://doi.org/10.1039/C4RA16423G
Gopinath SCB, Anbu P, Lakshmipriya T, Tang T-H, Chen Y, Hashim U et al (2015) Biotechnological Aspects and Perspective of Microbial Keratinase Production. BioMed Res. Int 2015:140726. https://doi.org/10.1155/2015/140726
Gupta R, Ramnani P (2006) Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol 70(1):21–33. https://doi.org/10.1007/s00253-005-0239-8
Hassan MA, Taha TH, Hamad GM, Hashem M, Alamri S, Mostafa YS (2020) Biochemical characterisation and application of keratinase from Bacillus thuringiensis MT1 to enable valorisation of hair wastes through biosynthesis of vitamin B-complex. Int J Biol Macromol 153:561–572. https://doi.org/10.1016/j.ijbiomac.2020.03.032
Jeong J-H, Lee O-M, Jeon Y-D, Kim J-D, Lee N-R, Lee C-Y, Son H-J (2010) Production of keratinolytic enzyme by a newly isolated feather-degrading Stenotrophomonas maltophilia that produces plant growth-promoting activity. Process Biochem 45(10):1738–1745. https://doi.org/10.1016/j.procbio.2010.07.020
Jiang Z, Qin D, Hse C-Y, Kuo M, Luo Z, Wang G et al (2008) Preliminary study on Chicken feather protein–based Wood adhesives. J Wood Chem Technol Taylor Francis 28(3):240–246. https://doi.org/10.1080/02773810802347073
Kang D, Jacquiod S, Herschend J, Wei S, Nesme J, Sørensen SJ (2020) Construction of simplified microbial consortia to degrade recalcitrant materials based on Enrichment and Dilution-to-extinction cultures. Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.03010
Kang D, Huang Y, Nesme J, Herschend J, Jacquiod S, Kot W et al (2021) Metagenomic analysis of a keratin-degrading bacterial consortium provides insight into the keratinolytic mechanisms. Sci Total Environ 761:143281. https://doi.org/10.1016/j.scitotenv.2020.143281
Kasperova A, Kunert J, Raska M (2013) The possible role of dermatophyte cysteine dioxygenase in keratin degradation. Med Mycol 51(5):449–454. https://doi.org/10.3109/13693786.2013.794310
Kshetri P, Ningthoujam DS (2016) Keratinolytic activities of alkaliphilic Bacillus sp. MBRL 575 from a novel habitat, limestone deposit site in Manipur, India. SpringerPlus 5(1):595. https://doi.org/10.1186/s40064-016-2239-9
Kshetri P, Roy SS, Chanu SB, Singh TS, Tamreihao K, Sharma SK et al (2020) Valorization of chicken feather waste into bioactive keratin hydrolysate by a newly purified keratinase from Bacillus sp. RCM-SSR-102. J Environ Manage 273:111195. https://doi.org/10.1016/j.jenvman.2020.111195
Kurbanoglu EB, Ozdal M, Ozdal OG, Algur OF (2015) Enhanced production of prodigiosin by Serratia marcescens MO-1 using ram horn peptone. Braz J Microbiol 46:631–637. https://doi.org/10.1590/S1517-838246246220131143
Lange L, Huang Y, Busk PK (2016) Microbial decomposition of keratin in nature-a new hypothesis of industrial relevance. Appl Microbiol Biotechnol 100(5):2083–2096. https://doi.org/10.1007/s00253-015-7262-1
Langeveld JPM, Wang J-J, Van de Wiel DFM, Shih GC, Garssen GJ, Bossers A et al (2003) Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. J Infect Dis 188(11):1782–1789. https://doi.org/10.1086/379664
Lateef A, Adelere IA, Gueguim-Kana EB (2015) Bacillus safensis LAU 13: a new source of keratinase and its multi-functional biocatalytic applications. Biotechnol Biotechnol Equip 29(1):54–63. https://doi.org/10.1080/13102818.2014.986360
Lee H, Noh K, Lee SC, Kwon IK, Han DW, Lee IS et al (2014) Human hair keratin and its-based biomaterials for biomedical applications. Tissue Eng Regenerative Med. https://doi.org/10.1007/s13770-014-0029-4
Li Q (2021) Structure, application, and Biochemistry of Microbial Keratinases. Front Microbiol 12:674345. https://doi.org/10.3389/fmicb.2021.674345
Liberato V, Benevenuti C, Coelho F, Botelho A, Amaral P, Pereira N et al (2019) Clostridium sp. as Bio-catalyst for fuels and chemicals production in a Biorefinery Context. Catalysts 9(11):962. https://doi.org/10.3390/catal9110962
Lin X, Wong SL, Miller ES, Shih JC (1997) Expression of the Bacillus licheniformis PWD-1 keratinase gene in B. subtilis. J Ind Microbiol Biotechnol 19(2):134–138. https://doi.org/10.1038/sj.jim.2900440
Manivasagan P, Sivakumar K, Gnanam S, Venkatesan J, Kim S-K, Production (2014) Biochemical characterization and detergents application of keratinase from the marine actinobacterium Actinoalloteichus sp. ma-32. j Surfac Deterg 17(4). https://doi.org/10.1007/s11743-013-1519-4
Mokrejs P, Hutta M, Pavlacková J, Egner P (2017a) Preparation of keratin hydrolysate from Chicken Feathers and its application in cosmetics. J Vis Exp JoVE 12956254. https://doi.org/10.3791/56254
Mokrejs P, Hutta M, Pavlackova J, Egner P, Benicek L (2017b) The cosmetic and dermatological potential of keratin hydrolysate. J Cosmet Dermatol 16(4):e21–e27. https://doi.org/10.1111/jocd.12319
Nafady NA, Hassan EA, Abd-Alla MH, Bagy MMK (2018) Effectiveness of eco-friendly arbuscular mycorrhizal fungi biofertilizer and bacterial feather hydrolysate in promoting growth of Vicia faba in sandy soil. Biocatal Agric Biotechnol 16:140–147. https://doi.org/10.1016/j.bcab.2018.07.024
Nagal S, Jain PC (2010) Feather degradation by strains of Bacillus isolated from decomposing feathers. Braz J Microbiol 41(1):196–200. https://doi.org/10.1590/S1517-838220100001000028
Nnolim NE, Nwodo UU (2021) Microbial keratinase and the bio-economy: a three-decade meta-analysis of research exploit. AMB Express 11(1):12. https://doi.org/10.1186/s13568-020-01155-8
Nnolim NE, Ntozonke N, Okoh AI, Nwodo UU (2020a) Exoproduction and characterization of a detergent-stable alkaline keratinase from Arthrobacter sp. KFS-1. Biochimie 177:53–62. https://doi.org/10.1016/j.biochi.2020.08.005
Nnolim NE, Udenigwe CC, Okoh AI, Nwodo UU (2020b) Microbial Keratinase: Next Generation Green Catalyst and prospective applications. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.580164
Okoroma EA, Purchase D, Garelick H, Morris R, Neale MH, Windl O, Abiola OO (2013) Enzymatic formulation capable of degrading scrapie prion under mild digestion conditions. PLoS ONE 8(7):e68099. https://doi.org/10.1371/journal.pone.0068099
Oluba OM, Obi CF, Akpor OB, Ojeaburu SI, Ogunrotimi FD, Adediran AA et al (2021) Fabrication and characterization of keratin starch biocomposite film from chicken feather waste and ginger starch. Sci Rep 11(1):8768. https://doi.org/10.1038/s41598-021-88002-3
Ozdal M, Kurbanoglu EB (2018) Valorisation of chicken feathers for xanthan gum production using Xanthomonas campestris MO-03. J Genet Eng Biotechnol 16(2):259–263. https://doi.org/10.1016/j.jgeb.2018.07.005
Ozdal M, Kurbanoglu EB (2019a) Use of chicken feather peptone and Sugar Beet Molasses as low cost substrates for Xanthan production by Xanthomonas campestris MO-03. Fermentation. Multidisciplinary Digit Publishing Inst 5(1):9. https://doi.org/10.3390/fermentation5010009
Ozdal M, Kurbanoglu EB (2019b) Citric acid production by Aspergillus Niger from Agro-industrial By-Products: molasses and chicken feather peptone. Waste Biomass Valorization 10(3):631–640. https://doi.org/10.1007/s12649-018-0240-y
Ozdal M, Gurkok S, Ozdal OG (2017) Optimization of rhamnolipid production by Pseudomonas aeruginosa OG1 using waste frying oil and chicken feather peptone. 3 Biotech 7(2):117. https://doi.org/10.1007/s13205-017-0774-x
Ozdal M, Gukok S, Ozdal OG, Kurbanoglu EB (2017b) Rhamnolipid production by Pseudomonas aeruginosa OG1 using waste frying oil and ram horn peptone. AIP Conf Proc 1833(1):020102. https://doi.org/10.1063/1.4981750
Patinvoh RJ, Feuk-Lagerstedt E, Lundin M, Sarvari Horvath I, Taherzadeh MJ (2016) Biological pretreatment of chicken feather and Biogas production from total broth. Appl Biochem Biotechnol 180(7):1401–1415. https://doi.org/10.1007/s12010-016-2175-8
Paul T, Jana A, Mandal AK, Mandal A, Das Mohpatra PK, Mondal KC (2016) Bacterial keratinolytic protease, imminent starter for NextGen leather and detergent industries. Sustain Chem Pharm 3:8–22. https://doi.org/10.1016/j.scp.2016.01.001
Pereira JQ, Lopes FC, Petry MV, Medina da C LF, A Brandelli (2014) Isolation of three novel Antarctic psychrotolerant feather-degrading bacteria and partial purification of keratinolytic enzyme from Lysobacter sp. A03. Int Biodeterior Biodegrad 88:1–7. https://doi.org/10.1016/j.ibiod.2013.11.012
Preczeski KP, Dalastra C, Czapela FF, Kubeneck S, Scapini T, Camargo AF et al (2020) Fusarium oxysporum and aspergillus sp. as keratinase producers using swine hair from agroindustrial residues. Front Bioeng Biotechnol 8:71. https://doi.org/10.3389/fbioe.2020.00071
Pulidori E, Micalizzi S, Bramanti E, Bernazzani L, De Maria C, Pelosi C et al (2022) Valorization of not soluble byproducts deriving from green keratin extraction from poultry feathers as filler for biocomposites. J Therm Anal Calorim 147(9):5377–5390. https://doi.org/10.1007/s10973-021-11166-7
Qiu J, Wilkens C, Barrett K, Meyer AS (2020) Microbial enzymes catalyzing keratin degradation: classification, structure, function. Biotechnol Adv 44:107607. https://doi.org/10.1016/j.biotechadv.2020.107607
Rawlings ND, Barrett AJ, Thomas PD, Huang X, Bateman A, Finn RD (2018) The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res 46(D1):D624–D632. https://doi.org/10.1093/nar/gkx1134
Revankar AG, Bagewadi ZK, Bochageri NP, Yunus Khan TM, Mohamed Shamsudeen S (2023) Response surface methodology based optimization of keratinase from Bacillus velezensis strain ZBE1 and nanoparticle synthesis, biological and molecular characterization. Saudi J Biol Sci 30(10):103787. https://doi.org/10.1016/j.sjbs.2023.103787
Riffel A, Brandelli A (2002) Isolation and characterization of a feather-degrading bacterium from the poultry processing industry. J Ind Microbiol Biotechnol 29(5):255–258. https://doi.org/10.1038/sj.jim.7000307
Schommer VA, Wenzel BM, Daroit DJ (2020) Anaerobic co-digestion of swine manure and chicken feathers: effects of manure maturation and microbial pretreatment of feathers on methane production. Renew Energy 152:1284–1291. https://doi.org/10.1016/j.renene.2020.01.154
Schrooyen PM, Dijkstra PJ, Oberthür RC, Bantjes A, Feijen J (2001) Partially carboxymethylated feather keratins. 2. Thermal and mechanical properties of films. J Agric Food Chem 49(1):221–230. https://doi.org/10.1021/jf0004154
Sharma S, Gupta A, Kumar A, Kee CG, Kamyab H, Saufi SM (2018) An efficient conversion of waste feather keratin into ecofriendly bioplastic film. Clean Technol Environ Policy 20(10):2157–2167. https://doi.org/10.1007/s10098-018-1498-2
Stanly L, Umesh M (2023) Bioconversion of chicken feather waste into feather hydrolysate by multifaceted keratinolytic Bacillus tropicus LS27 and new insights into its antioxidant and plant growth-promoting properties. Biomass Convers Biorefinery 1–11. https://doi.org/10.1007/s13399-023-04664-1
Tamreihao K, Devi LJ, Khunjamayum R, Mukherjee S, Ashem RS, Ningthoujam DS (2017) Biofertilizing potential of feather hydrolysate produced by indigenous keratinolytic Amycolatopsis sp. MBRL 40 for rice cultivation under field conditions. Biocatal Agric Biotechnol 10:317–320. https://doi.org/10.1016/j.bcab.2017.04.010
Tiwary E, Gupta R (2012) Rapid Conversion of Chicken Feather to feather meal using Dimeric Keratinase from Bacillus licheniformis ER15. J Bioprocess Biotech 2:123. https://doi.org/10.4172/2155-9821.1000123
Verma A, Singh H, Anwar S, Chattopadhyay A, Tiwari KK, Kaur S et al (2017) Microbial keratinases: industrial enzymes with waste management potential. Crit Rev Biotechnol 37(4):476–491. https://doi.org/10.1080/07388551.2016.1185388
Villa ALV, Aragao MRS, dos Santos EP, Mazotto AM, Zingali RB, de Souza EP et al (2013) Feather keratin hydrolysates obtained from microbial keratinases: effect on hair fiber. BMC Biotechnol 13:15. https://doi.org/10.1186/1472-6750-13-15
Williams C, Lee C, Garlich J, SHIH J (1991) Evaluation of a bacterial feather fermentation product, Feather-Lysate, as a feed protein. Poult Sci 70:85–94. https://doi.org/10.3382/ps.0700085
Yamamura S, Morita Y, Hasan Q, Yokoyama K, Tamiya E (2002) Keratin degradation: a cooperative action of two enzymes from Stenotrophomonas Sp. Biochem Biophys Res Commun 294(5):1138–1143. https://doi.org/10.1016/S0006-291X(02)00580-6
Zhou L, Xie X, Wu T, Chen M, Yao Q, Zhu H, Zou W (2022) Compound enzymatic hydrolysis of feather waste to improve the nutritional value. Biomass Convers Biorefinery 12(2):287–298. https://doi.org/10.1007/s13399-020-00643-y
Acknowledgements
The authors thank the SRM Institute of Science and Technology for providing support to pursue the research. I sincerely thank my supervisor for the constant support.
Author information
Authors and Affiliations
Contributions
KM - Study conception and design, literature search, data collection, analysis and interpretation of results, and writing the original draft. PK– Review and Editing. LMS– Conceptualization, Supervision, Review, and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest for the submission of this manuscript.
Declaration statement
The work reported in this manuscript has not been published elsewhere and is not currently under any other considerations.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukesh, K., Kannan, P. & Saleena, L.M. Keratin hydrolysates: a sustainable product in biotechnology sectors by microbial conversion. Biologia 79, 2535–2543 (2024). https://doi.org/10.1007/s11756-024-01725-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-024-01725-2