Abstract
Stabilization and utilization of poultry waste demand efficient biodegradation either by mixture of enzymes or by microbial system that can produce different types of protein-hydrolyzing enzymes. For utilization of this keratinous biomass, in the present study, genome was sequenced and annotated for a bacterium having multiple enzymatic options for hydrolysis of different soluble and insoluble protein fractions of poultry waste. Among the soluble protein substrates, optimum production of enzyme and soluble protein was observed in case of casein, whereas among the insoluble protein substrates, maximum production of enzyme was achieved when broken nails were used. Conditions for enhanced enzyme activity with concurrent degradation of keratin-rich poultry feather waste to protein-rich hydrolysate were optimized for different growth parameters. The bacterium grew well and highest protease production occurred in 144 h at mesophilic temperature (30 °C) and alkaline condition (pH 8–10) with enzyme activities of 134 and 168 U/mL, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increase in consumption of poultry products has led to a tremendous increase in poultry waste generation mainly in form of feathers with about 8.5 billion tons of chicken waste being generated from 24 billion chicken consumed globally per year [1,2,3]. This huge quantum of keratinous feather waste generated annually is a cause of concern owing to its recalcitrance which leads to disposal problems [4]. Landfilling and burning which are conventional methods for disposal of solid waste are expensive to operate and hazardous in nature, leading to environmental degradation. Treatment of feathers by physical methods such as high temperature and pressure is also not sustainable in the long run, owing to generation of non-nutritional factors resulting in animal feed product with poor digestibility [5]. Biodegradation of poultry feather waste by microorganisms has been suggested to be the best method for utilization of feather for producing animal feed and amino acids as pure chemicals [6].
In nature, materials such as bird feathers, wool, nails, hooves, horns, and hair are present in large amount but have confined uses as they are insoluble and hard to degrade by common proteolytic enzymes. The major protein present in such material is keratin, an insoluble and fibrous protein stabilized by hydrogen bonds, disulfide bonds, and hydrophobic interactions, owing to which enzymes like trypsin, papain, and pepsin are not able to degrade keratin [2, 7]. However, variety of filamentous and non-filamentous bacteria, filamentous fungi, some insects, and larvae have been reported to digest keratin by synthesizing a specific class of extracellular enzymes called keratinases which have the ability to hydrolyze keratinous waste [8]. In addition, keratinous material also contains thiol groups and reduction of these disulfide bonds is the essential pre-requisite for efficient keratin degradation [9,10,11]. Many bacterial genera have been reported for keratinolytic activity including Bacillus, Chryseobacterium, Flavobacterium, Streptomyces, and some other microorganisms from extreme environments such as Thermoanaerobacter [12]. These microorganisms can find potential applications in conversion of poultry feather waste to value-added products and in other industries where keratin processing is required such as in leather industries [13, 14]. Lateef et al. [15,16,17] have explored the potential of keratinases secreted by strains of various Bacillus species in hydrolysis of chicken feathers in addition to other areas such as synthesis of silver nanoparticles. In a recent review, Adelere and Lateef [18] have highlighted the role of keratinases as biocatalysts in diverse applications ranging from textile and leather to biomedical and detergent industries. Keratinous waste can be used as substrate for keratinase production. Among the different keratinous materials, feathers from poultries have been most commonly used for induction of keratinolytic enzymes, while human hairs which are produced in large amount from hair cutting saloons have been used only in rare cases, as demonstrated in the case of keratinase production by Stenotrophomonas sp. D-1 [19]. Efficient keratin hydrolysis by microbes is dependent on the fermentation conditions including temperature, pH, and carbon and nitrogen sources, and in the case of fungal strains, it is also dependent on the mycelial pressure or penetration [20,21,22,23]. In this study, the draft genome sequence was generated for facultative anaerobic bacterium Serratia marcescens EGD-HP20 and validation for keratin-hydrolyzing proteases was carried out. The experimental conditions were optimized for achieving high activity of extracellular keratin-hydrolyzing enzyme from S. marcescens EGD-HP20 for maximum degradation of poultry feathers.
Materials and Methods
Growth Medium and Culture Conditions
The feather keratin-hydrolyzing bacterium, S. marcescens EGD-HP20 (previously called Serratia sp. HPC 1383) was isolated from tannery wastewater treatment plant and demonstrated for its potential to hydrolyze poultry chicken feathers [14]. It was deposited in the National Centre for Microbial Resource, National Centre for Cell Science, Pune, India, and allotted the reference number MCC0036. Feathermeal consisted of feather pieces of 1–2-mm size obtained by cutting washed and dried, white chicken feathers from a local poultry which were added to basal cultivation medium (feathermeal medium (FMM)) as a substrate for enzyme production. The composition of fermentation medium and conditions used for growth and cultivation of the isolate were similar to that reported earlier [14]. All experiments were performed in triplicates as per reported methodology [14].
Analytical Procedures
Absorbance was measured at 600 nm in UV/VIS spectrophotometer (Shimadzu UV-1800, Japan) to indicate the change in cell density with time. Keratin-hydrolyzing enzyme activity was determined in the presence of 0.2 M Tris HCl buffer (pH 7.5) [14]. One unit of proteolytic activity was expressed as micromoles of tyrosine released per milliliter per minute (U/mL min). The soluble proteins were estimated by the method of Lowry et al. [24]
Genome Sequencing and Bioinformatics Analysis
Genomic DNA of S. marcescens EGD-HP20 was extracted using the Fast DNA SPIN Kit (MP Biomedical, USA). Whole genome shotgun sequencing was performed on Illumina MiSeq platform. The analysis of the draft genome of the strain was done by NCBI prokaryotic genome annotation pipeline (PGAP) and was provided the accession number AVSR00000000. The annotation was also validated by SEED-based genome annotation using Rapid Annotation Subsystem Technology (RAST) server [25]. A comparative visualization of draft genome against two closely related genomes using CG viewer online tool helped in genome comparison [26].
Optimization of Fermentation Parameters
Inoculum Concentration
Initial concentration of inoculum was varied by adding different cell densities (O.D. at 600 nm)—0.01, 0.1, 1, 13, and 20—to 100 mL FMM, and the effect on keratin-hydrolyzing protease activity, feather hydrolysis, and soluble protein production was studied.
Growth Temperature and pH
The growth of the isolate with subsequent enzyme activity, feather hydrolysis, and soluble protein production was evaluated under varying physical conditions of temperature and pH. The experimental flasks were incubated in shaking incubators at 15, 30, and 45 °C with incubation at 30 °C serving as control. Further, initial pH of medium was set at following values; 4, 5, 6, 7, 8, 9, and 10 with 100 mM HCl/NaOH, where pH 7 served as control though no attempt was made to control the pH during fermentation.
Fermentation Flask Volume
The level of dissolved oxygen available during fermentation was dependent on the headspace available in fermentation flasks. The following volume of media in respective capacity flasks were used during fermentation: 50 mL in a 150-mL conical flask, 100 mL in a 250-mL flask, 200 mL in a 500-mL flask, and 400 mL in a 1000-mL flask. The 250-mL flask containing 100 mL medium served as control, and enzyme production and feather hydrolysis were evaluated.
Agitation/Shaking Speed
In order to study the effect of different oxygen levels on fermentation and enzyme production, flasks were incubated under different shaking conditions. Control flasks were kept at 120 rpm while enzyme activity was compared with that produced in flasks incubated under static (0 rpm), 60 rpm, and 180 rpm.
Inorganic Nitrogen Sources
FMM containing white poultry feathers was supplemented with different inorganic nitrogen sources (0.5 g/L), such as ammonium sulfate, ammonium chloride, diammonium hydrogen phosphate, urea, and ammonium nitrate, in order to assess their effect on enzyme activity. FMM with white feathers and ammonium chloride served as control. The addition of fixed amount of different inorganic nitrogen sources resulted in variations in the concentration of nitrogen (N) in each experimental setup as follows: FMM control—0 g/L, ammonium sulfate and diammonium hydrogen phosphate—0.106 g/L, ammonium nitrate—0.175 g/L, urea—0.233 g/L, and ammonium chloride—0.4 g/L.
Organic Nitrogen Sources
Poultry feathers in FMM were replaced by soluble protein substrates (0.5 g/L), viz., casein, gelatine, beef extract, peptone, and yeast extract, and their effect on enzyme production was individually compared with control setup consisting of FMM and white poultry feathers.
Feathermeal Concentration
Based on the ability of the culture in hydrolyzing 0.1% feathermeal under above fermentation conditions, hydrolysis of higher concentration of feathermeal was explored by adding 0.1, 1, 2, 3, 4 , and 15% feathermeal in FMM. Experiment with 0.1–4% feathermeal was carried out in the 250-mL flask containing 100 mL medium; however, owing to low-bulk density and large volume occupied by 15% feathermeal, its hydrolysis was carried out in the 500-mL flask containing 200 mL medium.
Effect of Insoluble Keratinous Substrates
The ability of S. marcescens EGD-HP20 for enzyme production and subsequent hydrolysis of different insoluble keratinous substrates (1 g/L) like human hair, human nails, and green parrot feathers was compared with melanized/dark chicken feathers in addition to non-melanized/white poultry feathers which served as control.
Results and Discussion
India is one of the largest producers of poultry products generating nearly 140 million kg chicken feathers as waste product after chicken processing [14]. The poultry feather waste poses an environmental problem when it is discarded in open fields or disposed by incineration. To counter this environmental problem, proper disposal is essential and microorganisms provide a sustainable alternative for tackling this problem through enzymatic hydrolysis which can degrade chicken feathers effectively and lead to production of value-added product in the form of soluble protein hydrolysate. Thus, the feather keratin after recycling can have many industrial applications such as a raw material in animal feed preparation, biopolymer, pharmaceutical, hydrolysis of prion proteins, and biofilm production [19].
Keratin-hydrolyzing proteolytic enzymes are ubiquitous in all living beings in nature. These extracellular enzymes have many commercial applications and can be used by various industries in production of protein-rich animal feed from chicken feather waste [27, 28]. There are many microorganisms present in nature which are able to produce enzymes with similar activity as demonstrated by S. marcescens EGD-HP20. The genus Bacillus with many species, including Bacillus pumilus, B. licheniformis, B. subtilis, B. cereus, and B. megaterium, is one of the well-known bacterial genera which have been considered for commercial application in biodegradation of chicken feathers [23, 27, 29, 30]. Irrespective of the type of microorganism used, optimal production of keratin-hydrolyzing enzyme is an essential pre-requisite for effective degradation of feather waste [31].
Genome Annotation and Bioinformatics Analysis
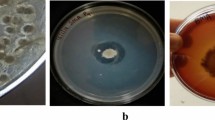
Figure 1a shows the degradation of white poultry feathers in the presence of S. marcescens EGD-HP20. Based on the ability of the isolate for hydrolyzing poultry feathers, the whole genome sequencing was carried out and draft genome was compared against two closely related genomes using CG viewer online tool [26] as shown in Fig. 1b. Comparison of draft genome of S. marcescens EGD-HP20 with reference strains, S. marcescens WW4 and S. marcescens FGI94, revealed the absence of disulfide reductase gene in the reference strains. This provided additional advantage to our strain for hydrolysis of disulfide bonds, thereby promoting keratin-hydrolyzing activity for degradation of feather keratin.
a Degradation of white poultry feathers in presence of S. marcescens EGD-HP20. b Circular representation of the genome of S. marcescens EGD-HP20. Circles from the outside show the positions of protein-coding genes (blue), tRNA genes (red) and rRNA genes (pink) on the positive (circle 1), and negative (circle 2), strands respectively. Circles 3–5 show the positions of BLAST hits detected through blast comparisons of EGD-HP20 against WW4 (circle 3) and FGI94 (circle 4) settings: query split size = 50,000 bp, query split overlap size = 0, and expect value cutoff = 0.00001. Low-complexity sequences were eliminated from the analysis. The height of the shading in the BLAST result rings is proportional to the percent identity of the hit. Overlapping hits appear as darker shading. Circles 6 and 7 show plots of GC content and GC skew plotted as the deviation from the average for the entire sequence. The origin of replication is indicated by the vertical zig-zag line. c Schematic representation of CDS distribution into subsystems in S. marcescens EGD-HP20 draft genome based on SEED database. Figure illustrates successive division of CDS for protein metabolism
The SEED-based genome annotation using RAST server analysis identified functionally categorized 574 subsystems, with 4742 CDS along with 86 RNAs. Figure 1c describes the distribution of CDS into major subsystems along with further elaboration of subsystems for protein metabolism which is further classified into its sub-components. Subsystem corresponding to protein degradation showed 48 annotation hits belonging to aminopeptidases, metallocarboxypeptidases, dipeptidases, serine endopeptidases, and omegapeptidases. Further, the different types of proteases were distributed into their homologue subtypes. Mainly, metallocarboxypeptidases can be categorized into six different subtypes like d-alanyl-d-alanine carboxypeptidase, glutamate carboxypeptidase, muramoyltetrapeptide carboxypeptidase, zinc d-Ala-d-Ala carboxypeptidase, carboxypeptidase T, and thermostable carboxypeptidase, which were found to be homologous with Serratia proteamaculans 568, Yersinia pseudotuberculosis IP 32953, etc. Whereas, serine endopeptidase exhibited four different subsystems, i.e., glutamyl endopeptidase, prolyl endopeptidase, lysyl endopeptidase, and acylamino-acid-releasing enzyme, which were found to be present in Serratia marcescens Db11, Stenotrophomonas maltophilia R551-3, Xenopus tropicalis, Xanthomonas campestris, and Vibrio fischeri. Table 1 shows the detailed classification of peptidases involved in protein degradation with reference to CDS homologues distribution.
Optimization of Fermentation Parameters
Inoculum Concentration
The keratin-hydrolyzing enzyme production was studied at different initial concentrations of inoculum; 450–500-fold increase in cell density was observed when initial concentration of inoculum was 0.01 O.D.. The rate of increase in final cell density declined as initial inoculum concentration was increased to 0.1, 1, 13, and 20 O.D., thus resulting in final cell density of 8.1, 10.3, 16, and 22.8, respectively in 144 h (Fig. 2). Highest enzyme activity and soluble protein concentration were observed to be 159 U/mL and 107 mg/L, respectively, when 20 O.D. cells were added initially. Enzyme activity was similar at lower inoculum concentration of 13 O.D. though the soluble protein dropped marginally to 83 mg/L. Both these parameters were observed to be comparatively lower at lower inoculum concentration. With reference to enzyme activity at control inoculum levels of 0.1 O.D. (considered as 100%), relative enzyme activity was 95% at 0.01 O.D. inoculum, while it was highest at 13 and 20 O.D. inoculum concentration and corresponded to 125% relative enzyme activity.
Thus, higher concentration of culture (13 and 20 O.D.) during fermentation was beneficial since higher enzyme activity and faster solubilization of feather waste to soluble protein were achieved in shorter period of 2 days while the same required 4–6 days in the case of lower inoculum concentration. There are conflicting reports on the amount of initial inoculum to be considered optimum for the highest enzyme production which was associated with the growth rates and nutrient requirements of the specific microorganisms. An inoculum of 10% was found to be optimum for high keratinase production by Bacillus subtilis KD-N2 [32], while another study by Yusuf et al. [33] found five times lower inoculum concentration (2%) to be optimum for maximum enzyme production in Alcaligenes sp. Similar findings were obtained by Shivakumar et al. [31] in B. cereus, who reported an increase in keratinase production with increase in inoculum concentration to 4% with little or no improvement in activity at higher inoculum levels. However, enzyme activity observed by Sivakumar et al. [31] was comparatively higher than that observed in the present study since the authors used supplementary carbon source along with feathers while in the present study, chicken feathers were used as sole source of carbon. The finding that inoculum above certain levels led to lower enzyme activity was also corroborated by Cai et al. [34] who showed that, at 2–5% inoculum concentration, keratinase production by Bacillus subtilis mutant strain was highest, which dropped with further increase in inoculum levels above 5%.
Growth Temperature and pH
Keratin-hydrolyzing protease production was studied by growing the culture at three different temperatures: 15, 30, and 45 °C out of which, the maximum enzyme production was observed at 30 °C in 144 h (134 U/mL). Relative enzyme activity was lower and was found to be 55 and 72% at 15 and 45 °C, respectively (Fig. 3a). Results showed partial hydrolysis after 72 h at 30 °C with complete degradation of feathermeal in 144 h. However, it was noted that the presence of feather shaft in the feathermeal required slightly longer time for complete solubilization. The corresponding soluble protein production was highest at 30 °C with 107 mg/L soluble protein being generated, while it was 29 and 61% lower at 45 and 15 °C, respectively. Different reports have suggested different temperatures to be optimum for keratinase production. Studies by Prakash et al. [35] indicated the highest production of enzyme and maximum growth at 35–45 °C with optimum at 37 °C whereas low growth and enzyme production were observed at 20 and 50 °C. Mesophilic temperatures between 30 and 40 °C were also shown to be optimum for the highest enzyme production and keratin hydrolysis in majority of the microbes in a recent review by Gupta and Ramnani [12]. The authors further highlighted that, in addition to microbes with temperature optima in the mesophilic range, a few microorganisms isolated from extreme environments demonstrated optimum growth and enzyme production in thermophilic (70 °C) and psychrophilic (20 °C) ranges, respectively. Park and Son [30] demonstrated that B. megaterium F7-1 degraded chicken feather completely at 30 °C in 7 days. Though, our results were in agreement with that of the above authors with 30 °C being optimum for the highest growth and enzyme production, temperature optima of Serratia marcescens EGD-HP20 for enzyme activity were earlier reported to be 60 °C, indicating that the enzyme was thermophilic in nature [14].
Effect of a growth pH and temperature on relative activity of keratin-hydrolyzing enzyme (activity at control pH 7 and control temperature of 30 °C considered as 100%) and b agitation/shaking speed and fermentation flask volume on activity of keratin-hydrolyzing enzyme (shaking speed of 120 rpm and fermentation flask volume of 250 mL considered as control)
Figure 3a also shows the relative enzyme activity at different growth pH ranging from 4 to 10, with pH 7 serving as control. Results indicated the ability of the culture to grow at both extremes of pH with increase in cell density at all pH values. Corresponding enzyme activity also followed a similar trend with increase in protease activity from 96 U/mL at pH 4.0 to 168 U/mL at pH 10. Thus, in comparison to 124 U/mL enzyme activity at control pH 7 (considered as 100% activity), the relative activity at pH range of 4–6 was found to be lower (77–89%), but the same was higher at pH range of 8–10 (117–135%). A recent report by Daroit and Brandelli [36] extensively reviewed the various aspects of keratinases which confirmed the above finding that neutral to alkaline pH was optimum for most of the keratinase-producing bacteria and fungi. The reason for alkaline pH supporting optimum keratinase production and feather degradation in most microorganisms has been attributed to the modification of cysteine residues in keratin to lathionine, thereby favoring rapid keratin degradation by making it accessible to keratinase action [37, 38]. Though experiment demonstrated the versatility of isolate for high enzyme activity at alkaline pH, soluble protein production was similar irrespective of the growth pH. Previous studies reported different pH optima for growth and enzyme production as seen in case of B. pumilis (weakly acidic environment), B. licheniformis PWD-1 (neutral conditions), Vibrio strain kr2, and Chryseobacterium strain kr6 (pH 5.0–8.0) [39,40,41]. Thus, the ability of our culture to grow at alkaline pH (pH 8–10) and optimum enzyme activity under alkaline conditions (pH 10–11) [14] indicated that the enzyme was alkaline protease which broadened the scope of this isolate for future applications in different areas including detergent and leather-processing industries.
Fermentation Flask Volume
For optimum production of keratin-hydrolyzing protease, along with agitation speed, it was also essential to provide sufficient headspace in the fermentation flask for higher oxygen levels, thereby leading to higher dissolved oxygen in the medium. Figure 3b also shows the enzyme production and feather hydrolysis during fermentation in different capacity flasks each of which provided different headspace volume, thus leading to different levels of dissolved oxygen for growth of the bacterium. Enzyme activity and soluble protein concentration were similar when fermentation was carried out in the 150- and 250-mL capacity flasks (161 U/mL and 125 mg/L). Though enzyme activity improved to 184 and 213 U/mL in the 500- and 1000-mL capacity flasks, respectively, soluble protein levels were not affected by increase in flask capacity. Thus, relative enzyme activity was found to be 132% in larger capacity flask in comparison to control setup (100 mL medium in the 250-mL flask) which was attributed to increased solubilization of oxygen from larger headspace in the bigger flasks. The beneficial effect of higher dissolved oxygen on keratinase production has been demonstrated by Cai and Zheng [32] who reported that a nominal 12% increase in headspace volume was sufficient to provide enough dissolved oxygen for 73% increase in keratinase production by Bacillus subtilis KD-N2 in the presence of hair as substrate.
Agitation/Shaking Speed
The keratinolytic activity followed by soluble protein concentration was determined at different shaking speeds, i.e., 0 (static), 60, 120, and 180 rpm of which 120 rpm served as control. From Fig. 3b, it is clearly seen that enzyme activity was minimum at static condition which was found to reach 95 U/mL in 6 days. This further increased to 150 U/mL with increase in shaking speed to 120 rpm. The highest enzyme activity was obtained when the flasks were incubated at 180 rpm which was found to be 177 U/mL, indicating that the relative activity at 180 rpm was 118% in comparison to that at control shaking speed of 120 rpm which was considered as 100%. Higher shaking/agitation speed provided higher solubilization of oxygen from the headspace into the culture broth, thereby aiding in higher growth of the culture and resulting in more enzyme production and faster hydrolysis of feather waste [42]. Review by Gupta and Ramnani [12] indicated that maximum agitation/shaking speeds of up to 250 rpm have been used with 120–180 rpm being optimum for keratinolytic activity by diverse groups of microorganisms which validates our findings.
Inorganic Nitrogen Sources
The effect of different inorganic nitrogen sources on hydrolysis of poultry feathers is shown in Fig. 4a. Maximum production of enzyme was achieved in the presence of ammonium nitrate (242 U/mL) followed by ammonium sulfate (212 U/mL) whereas enzyme activity was lower in the case of urea (172 U/mL). Activity was lowest in the case of FMM control which was not supplemented with additional inorganic nitrogen source. The corresponding soluble protein generation was also found to be 1.8-fold higher in the presence of ammonium nitrate than that in control. Williams et al. [43] observed that the addition of inorganic nitrogen such as NH4Cl in fermentation media enhanced the growth and enzyme production of B. licheniformis PWD-1. Similarly, Sahoo et al. [8] also reported an improvement in enzyme production in the presence of inorganic nitrogen sources; however, in contrast to our observations, a reverse trend was seen with reference to the type of inorganic nitrogen source. The authors suggested that ammonium sulfate as inorganic nitrogen source was best suited for higher enzyme production followed by ammonium nitrate and tryptone.
Organic Nitrogen Sources
The proteolytic enzyme activity along with changes in soluble protein concentration during hydrolysis of different protein substrates, viz., casein, gelatine, beef extract, yeast extract, and peptone, were compared with FMM containing white feathers as control. From Fig. 4b, it is clearly observed that final proteolytic enzyme activity and soluble protein production were highest when culture was grown in the presence of casein (160 U/mL and 349 mg/L, respectively) followed by gelatin (141 U/mL and 312 mg/L, respectively). Corresponding enzyme production and protein generation were lowest in control medium containing white feathers (115 U/mL and 152 mg/L, respectively). Our results coincide with the findings of Wang and Hsu [44] where maximum production of enzyme by Prevotella ruminicola was achieved when the casein was added in the fermentation medium. However, Sinha and Satyanarayana [45] reported that for enrichment of bacteria, soybean meal acted as the optimum nitrogen source in production medium for maximum induction of proteolytic enzyme while studies by El-Refai et al. [27] indicated that maximum production of protease was achieved when yeast extract was used followed by casein. The presence of multi-nutrient factors in the form of high concentrations of amino acids, proteins, and vitamins was associated with the highest induction of protease by organic nitrogen sources [14, 46].
Feathermeal Concentration
The above experiments revealed the potential of S. marcescens EGD-HP20 for hydrolyzing 0.1% feathermeal under different fermentation conditions resulting in the formation of soluble protein-rich material. Based on these results, the ability of the culture to hydrolyze higher concentration of feathermeal/waste was explored.
Enzyme activity was observed to reach 302 U/mL in 144 h at control feathermeal concentration (0.1%) which increased with increase in feathermeal concentration and was found to be maximum in the presence of 15% feathermeal (593 U/mL) (Fig. 5). The enzyme activity was slightly lower (526 U/mL) when 4% feathermeal was used while the same was lowest and varied between 400 and 465 U/mL at feathermeal concentration between 1 and 3%. The key finding of this study was that enzyme activity increased gradually and reached a peak in 144 h in the presence of 0.1–4% feathermeal, while at 15% feathermeal concentration, enzyme activity increased rapidly within 2 days followed by gradual increase to reach peak value. The effect of higher enzyme activity in shorter time at 15% feathermeal was also reflected in the rapid solubilization of feather keratin to soluble protein (139 mg/L) in 48 h followed by gradual increase to 187 mg/L. Hydrolysis of 2–4% feathermeal required 120–144 h for producing similar levels of soluble protein, while at 0.1–1% feathermeal concentration, soluble protein production within the same period was 1.5–2-fold lower than that at 15% feathermeal.
The present study demonstrated hydrolysis of the highest concentration of feathermeal by any bacterial isolate till date with previous report showing 60–80 g/L feathers being the maximum possible level which was completely hydrolyzed by keratinase produced by Vibrio sp. kr2 [47]. In a similar study, Reddy et al. [38] recently evaluated the enzyme production by B. pumilus strain in the presence of different feathermeal concentrations (0.5, 1.0, 1.5, 2.0, 2.5, and 3.0% w/v). The authors observed complete degradation of feathers at 0.5% of substrate concentration with enzyme production (231 ± 10 U/mL) which increased as the feathermeal concentration increased to 1% (362 ± 14 U/mL). Beyond 1% (w/v) of the substrate concentration, the enzyme production by B. pumilus GRK showed a reduction which was probably due to the insufficient aeration caused by higher concentration of substrate.
Effect of Insoluble Keratinous Substrates
Different insoluble keratinous substrates, viz., poultry chicken feathers (white and dark/brown), human hairs, human nails, and green parrot feathers, were compared for maximum enzyme production by S. marcescens EGD-HP20. Results showed that, with increase in time, the production of enzyme and protein concentration also increased and the maximum hydrolysis efficiency was achieved in 144 h in the case of human nails as indicated by 236 U/mL enzyme activity with concomitant soluble protein production of 180 mg/L (Fig. 6). There was no significant difference in enzyme activity when culture was grown in the presence of green parrot feathers and white poultry feathers (196 and 191 U/mL, respectively), a trend which was also reflected in the corresponding soluble protein generation (156 and 140 mg/L, respectively in 144 h). Hydrolysis efficiency was slightly lower in the case of dark/brown feathers (enzyme activity—179 U/mL and soluble protein—117 mg/L). Among the different substrates, human hair was degraded with difficulty owing to the lowest enzyme induction of 115 U/mL and subsequent lower soluble protein generation of 126 mg/L. Thus, the enzyme activity was observed to be 1.2–1.3-fold higher in the case of nails in comparison to white and dark/brown feathers control while the soluble protein generation was 1.3–1.5-fold higher. The melanin content in hairs and dark/brown feathers was responsible for low enzyme induction and degradability as was also confirmed by Desai et al. [48]. The authors investigated the biodegradation of different substrates including chicken feathers and other keratinous materials like bovine hair, wool, human hair, and nails by B. licheniformis K18102 out of which, comparable enzyme activity and hydrolysis were observed in all substrates except human hair. The growth of the bacterial culture in the presence of green feathers was accompanied by rapid release of the green pigment which resulted in lower recalcitrance of feathers and enhanced hydrolysis of keratin unlike the melanized keratinous substrates where melanin pigment was released with difficulty, thus making the material more recalcitrant to hydrolysis. Our observation for feather hydrolysis were in agreement with Lateef et al. [15] who also reported differential induction of keratin-hydrolyzing enzymes in the presence of diverse keratin sources, namely, hooves, horn, and feathers. Though the authors observed lower induction of protease by feather keratin, induction was considerably higher when hooves and horn were used.
Conclusion
Study identifies the bacterium that can produce multiple proteolytic enzymes which could be applied for hydrolysis of not only soluble proteins but also insoluble highly keratinized proteins such as poultry waste or broken nails. This unique ability of the bacterium has been supported by annotation of genes for enzymes assisting in keratin hydrolysis including aminopeptidases, metallocarboxypeptidases, dipeptidases, serine endopeptidases, and omegapeptidases in addition to enhanced hydrolytic potential due to the presence of gene for disulfide reductase. The isolate exhibited optimum growth and protease activity at 30 °C and alkaline pH of 10, indicating that the enzyme could provide alternate solution for replacing potentially hazardous methods currently used in industrial applications such as animal feed, detergent formulation, and leather processing.
References
Saha, S., Dhanasekaran, D., Shanmugapriya, S., & Latha, S. (2013). Nocardiopsis sp. SD5: a potent feather degrading rare actinobacterium isolated from feather waste in Tamil Nadu, India. Journal of Basic Microbiology, 53(7), 608–616.
Lo, W. H., Too, J. R., & Wu, J. Y. (2012). Production of keratinolytic enzyme by an indigenous feather–degrading strain Bacillus cereus Wu2. Journal of Bioscience and Bioengineering, 114(6), 640–647.
Gurav, R. G., & Jadhav, J. P. (2013). Biodegradation of keratinous waste by Chryseobacterium sp. RBT isolated from soil contaminated with poultry waste. Journal of Basic Microbiology, 53(2), 128–135.
Freeman, S. R., Poore, M. H., Middleton, T. F., & Ferket, P. R. (2009). Alternative methods for disposal of spent laying hens: evaluation of the efficacy of grinding, mechanical deboning, and of keratinase in the rendering process. Bioresource Technology, 100(19), 4515–4520.
Papadopoulos, M. C., El Boushy, A. R., Roodbeen, A. E., & Ketelaars, E. H. (1986). Effects of processing time and moisture content on amino acid composition and nitrogen characteristics of feather meal. Animal Feed Science and Technology, 14(3–4), 279–290.
Hussein, A. M., and Swelim, M. A. (1989). Adaptation course of Micropolyspora keratinolytica to chicken feather and consequent intensification of bioconversion of feather into soluble nitrogenous products. In: Proceedings of the Seventh Conference of Microbiology, Cairo, pp. 31–38.
Riffel, A., Daroit, D. J., & Brandelli, A. (2011). Nutritional regulation of protease production by the feather-degrading bacterium Chryseobacterium sp. kr6. New Biotechnology, 28(2), 153–157.
Sahoo, D. K., Das, A., Thatoi, H., Mondal, K. C., & Mohapatra, P. K. D. (2012). Keratinase production and biodegradation of whole chicken feather keratin by a newly isolated bacterium under submerged fermentation. Applied Biochemistry and Biotechnology, 167(5), 1040–1051.
Cao, L., Tan, H., Liu, Y., Xue, X., & Zhou, S. (2008). Characterization of a new keratinolytic Trichoderma atroviride strain F6 that completely degrades native chicken feather. Letters in Applied Microbiology, 46(3), 389–394.
Daroit, D. J., Corrêa, A. P. F., & Brandelli, A. (2009). Keratinolytic potential of a novel Bacillus sp. P45 isolated from the Amazon basin fish Piaractus mesopotamicus. International Biodeterioration & Biodegradation, 63(3), 358–363.
Mabrouk, M. E. (2008). Feather degradation by a new keratinolytic Streptomyces sp. MS-2. World Journal of Microbiology and Biotechnology, 24(10), 2331–2338.
Gupta, R., & Ramnani, P. (2006). Microbial keratinases and their prospective applications: an overview. Applied Microbiology and Biotechnology, 70(1), 21.
Laxman, R. S., Sonawane, A. P., More, S. V., Rao, B. S., Rele, M. V., Jogdand, V. V., & Rao, M. B. (2005). Optimization and scale up of production of alkaline protease from Conidiobolus coronatus. Process Biochemistry, 40(9), 3152–3158.
Khardenavis, A. A., Kapley, A., & Purohit, H. J. (2009). Processing of poultry feathers by alkaline keratin hydrolyzing enzyme from Serratia sp. HPC 1383. Waste Management, 29(4), 1409–1415.
Lateef, A., Oloke, J. K., Gueguim Kana, E. B., Sobowale, B. O., Ajao, S. O., & Bello, B. Y. (2010). Keratinolytic activities of a new feather-degrading isolate of Bacillus cereus LAU 08 isolated from Nigerian soil. International Biodeterioration and Biodegradation, 64(2), 162–165.
Lateef, A., Adelere, I. A., Gueguim-Kana, E. B., Asafa, T. B., & Beukes, L. S. (2015). Green synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13. International Nano Letters, 5, 29–35.
Lateef, A., Adelere, I. A., & Gueguim-Kana, E. B. (2015). Bacillus safensis LAU 13: a new source of keratinase and its multi-functional biocatalytic applications. Biotechnology and Biotechnological Equipment, 29(1), 54–63.
Adelere, I. A., & Lateef, A. (2016). Keratinases: emerging trends in production and applications as novel multifunctional biocatalysts. Kuwait Journal of Science, 43(3), 118–127.
Riffel, A., & Brandelli, A. (2002). Isolation and characterization of a feather-degrading bacterium from the poultry processing industry. Journal of Industrial Microbiology & Biotechnology, 29(5), 255–258.
Moreira, F. G., de Souza, C. G., Costa, M. A., Reis, S., & Peralta, R. M. (2007). Degradation of keratinous materials by the plant pathogenic fungus Myrothecium verrucaria. Mycopathologia, 163(3), 153–160.
Thys, R. C. S., Lucas, F. S., Riffel, A., Heeb, P., & Brandelli, A. (2004). Characterization of a protease of a feather degrading Microbacterium species. Letters in Applied Microbiology, 39(2), 181–186.
Wawrzkiewicz, K., Wolski, T., & Łobarzewski, J. (1991). Screening the keratinolytic activity of dermatophytes in vitro. Mycopathologia, 114(1), 1–8.
Zaghloul, T. I., Al-Bahra, M., & Al-Azmeh, H. (1998). Isolation, identification, and keratinolytic activity of several feather-degrading bacterial isolates. Applied Biochemistry and Biotechnology, 70(1), 207–213.
Lowry, O. H., Rosenbrourgh, N. J., Farr, N. J., & Randall, J. R. (1951). Protein measurement with the folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Aziz, R. K., Bartels, D., Best, A. A., DeJongh, M., Disz, T., Edwards, R. A., & Meyer, F. (2008). The RAST Server: rapid annotations using subsystems technology. BMC Genomics, 9(1), 75.
Grant, J. R., & Stothard, P. (2008). The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Research, 36(suppl 2), W181–W184.
El-Refai, H. A., AbdelNaby, M. A., Gaballa, A., El-Araby, M. H., & Fattah, A. A. (2005). Improvement of the newly isolated Bacillus pumilus FH9 keratinolytic activity. Process Biochemistry, 40(7), 2325–2332.
Romero, F. J., García, L. A., Salas, J. A., Díaz, M., & Quirós, L. M. (2001). Production, purification and partial characterization of two extracellular proteases from Serratia marcescens grown in whey. Process Biochemistry, 36(6), 507–515.
Lin, X., Lee, C. G., Casale, E. S., & Shih, J. C. (1992). Purification and characterization of a keratinase from a feather-degrading Bacillus licheniformis strain. Applied and Environmental Microbiology, 58(10), 3271–3275.
Park, G. T., & Son, H. J. (2009). Keratinolytic activity of Bacillus megaterium F7-1, a feather-degrading mesophilic bacterium. Microbiological Research, 164(4), 478–485.
Sivakumar, T., Shankar, T., Thangapand, V., & Ramasubram, V. (2013). Optimization of cultural condition for keratinase production using Bacillus cereus TS1. Microbiology Insights, 3(1), 1–8.
Cai, C., & Zheng, X. (2009). Medium optimization for keratinase production in hair substrate by a new Bacillus subtilis KD-N2 using response surface methodology. Journal of Industrial Microbiology & Biotechnology, 36(7), 875–883.
Yusuf, I., Ahmad, S. A., Phang, L. Y., Syed, M. A., Shamaan, N. A., Khalil, K. A., & Shukor, M. Y. (2016). Keratinase production and biodegradation of polluted secondary chicken feather wastes by a newly isolated multi heavy metal tolerant bacterium-Alcaligenes sp. AQ05-001. Journal of Environmental Management, 183, 182–195.
Cai, C. G., Lou, B. G., & Zheng, X. D. (2008). Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. Journal of Zhejiang University-Science B, 9(1), 60–67.
Prakash, P., Jayalakshmi, S. K., & Sreeramulu, K. (2010). Purification and characterization of extreme alkaline, thermostable keratinase, and keratin disulfide reductase produced by Bacillus halodurans PPKS-2. Applied Microbiology and Biotechnology, 87(2), 625–633.
Daroit, D. J., & Brandelli, A. (2014). A current assessment on the production of bacterial keratinases. Critical Reviews in Biotechnology, 34(4), 372–384.
Friedrich, A. B., & Antranikian, G. (1996). Keratin degradation by Fervidobacterium pennavorans, a novel thermophilic anaerobic species of the order thermotogales. Applied and Environmental Microbiology, 62(8), 2875–2882.
Reddy, M. R., Reddy, K. S., Chouhan, Y. R., Bee, H., and Reddy, G. (2017). Effective feather degradation and keratinase production by Bacillus pumilus GRK for its application as bio-detergent additive. Bioresources Technology, 243, 254–263.
Kim, J. M., Lim, W. J., & Suh, H. J. (2001). Feather-degrading Bacillus species from poultry waste. Process Biochemistry, 37(3), 287–291.
Sangali, S., & Brandelli, A. (2000). Feather keratin hydrolysis by a Vibrio sp. strain kr2. Journal of Applied Microbiology, 89(5), 735–743.
Riffel, A., Lucas, F., Heeb, P., & Brandelli, A. (2003). Characterization of a new keratinolytic bacterium that completely degrades native feather keratin. Archives of Microbiology, 179(4), 258–265.
Nadeem, M., Qazi, J. I., & Baig, S. (2009). Effect of aeration and agitation rates on alkaline protease production by Bacillus licheniformis UV-9 mutant. Turkish Journal of Biochemistry, 34(2), 89–96.
Williams, C. M., Richter, C. S., Mackenzie, J. M., & Shih, J. C. (1990). Isolation, identification, and characterization of a feather-degrading bacterium. Applied and Environmental Microbiology, 56(6), 1509–1515.
Wang, H. T., & Hsu, J. T. (2005). Optimal protease production condition for Prevotella ruminicola 23 and characterization of its extracellular crude protease. Anaerobe, 11(3), 155–162.
Sinha, N., & Satyanarayana, T. (1999). Alkaline protease production by thermopile Bacillus licheniformis. Enzyme and Microbial Technology, 8, 370–372.
Esakkiraj, P., Sankaralingam, S., Usha, R., Palavesam, A., & Immanuel, G. (2011). Solid-state protease production using anchovy waste meal by moderate halophile Serratia proteamaculans AP-CMST isolated from fish intestine. Annales de Microbiologie, 61(4), 749–755.
Grazziotin, A., Pimentel, F. A., Sangali, S., de Jong, E. V., & Brandelli, A. (2007). Production of feather protein hydrolysate by keratinolytic bacterium Vibrio sp. kr2. Bioresource Technology, 98(16), 3172–3175.
Desai, S. S., Hegde, S., Inamdar, P., Sake, N., & Aravind, M. S. (2010). Isolation of keratinase from bacterial isolates of poultry soil for waste degradation. Engineering in Life Sciences, 10(4), 361–367.
Acknowledgements
The authors are thankful to the Director, CSIR-National Environmental Engineering Research Institute for providing necessary facilities for this work [KRC Manuscript No. CSIR-NEERI/KRC/2017/JULY/EBGD/10]. Funds from Department of Biotechnology (DBT), New Delhi, for project -BT/PR4799/PID/6/642/2012 are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fuke, P., Gujar, V.V. & Khardenavis, A.A. Genome Annotation and Validation of Keratin-Hydrolyzing Proteolytic Enzymes from Serratia marcescens EGD-HP20. Appl Biochem Biotechnol 184, 970–986 (2018). https://doi.org/10.1007/s12010-017-2595-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2595-0