Abstract
Chicken feathers are available in large quantities around the world causing environmental challenges. The feathers are composed of keratin that is a recalcitrant protein and is hard to degrade. In this work, chicken feathers were aerobically pretreated for 2–8 days at total solid concentrations of 5, 10, and 20 % by Bacillus sp. C4, a bacterium that produces both α- and β-keratinases. Then, the liquid fraction (feather hydrolysate) as well as the total broth (liquid and solid fraction of pretreated feathers) was used as substrates for biogas production using anaerobic sludge or bacteria granules as inoculum. The biological pretreatment of feather waste was productive; about 75 % of feather was converted to soluble crude protein after 8 days of degradation at initial feather concentration of 5 %. Bacteria granules performed better during anaerobic digestion of untreated feathers, resulting in approximately two times more methane yield (i.e., 199 mlCH4/gVS compared to 105 mlCH4/gVS when sludge was used). Pretreatment improved methane yield by 292 and 105 % when sludge and granules were used on the hydrolysate. Bacteria granules worked effectively on the total broth, yielded 445 mlCH4/gVS methane, which is 124 % more than that obtained with the same type of inoculum from untreated feather.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world’s average stock of chicken is about 22 billion live chickens [1], and about 5–7 % of the total weight of a normal chicken are feathers [2]. The poultry industries produce about six billion tons of chicken feathers annually. Landfilling is a major practice of disposing feather wastes in the world today, resulting in landfill gas including methane and nitrous oxides. The second largest treatment of feather waste is incineration [3]; however, a minor portion of the feathers are converted to feather meals used in animal feed preparation and in organic fertilizer through hydrothermal or chemical pretreatment methods. Nevertheless, this product has a low nutritional value due to the denaturing of the amino acid content [4, 5] and, moreover, the process is capital intensive.
The organic fraction of solid organic wastes and materials (agricultural wastes, sludge, animal by products, energy crops, and other substrates) is used for biogas production, but the use of keratin wastes such as chicken feathers, wools, hair, or nails is not yet fully explored. These wastes, if properly pretreated, can serve as a valuable feedstock for biogas production. It is important to alter the recalcitrant structure of the keratin to enhance bio-digestibility of the wastes for biogas production resulting in improved biogas yields [6]. The high degree of crosslinking of the polypeptide chain caused by extensive formation of disulfide bonds is a major challenge in processing these wastes. This strong covalent bond makes them insoluble in polar solvents [7] and resistant to proteases [8, 9].
Current processing of these wastes is based on strong acid or alkaline hydrolysis and other physicochemical methods such as hydrothermal and superheated water treatments. These treatment methods result in severe degradation and destruction of feather keratins [10]; however, the energy and/or chemicals demand related to these methods leads to economical or sustainability challenges. Enzymatic hydrolysis using keratinases is also an option, but the high cost of enzymes makes this method limited for industrial applications. Hence, the recent research focuses on biological pretreatment methods, which are sustainable, ecological, and cost effective for extracting soluble keratins. Several microorganisms (bacteria and fungi), such as Bacillus [4, 11, 12] and Aspergillus sp. [13–15] were reported to degrade keratins (chicken feathers). Hence, introducing these kinds of pretreatment can be promising and therefore there is a need to explore these effectively for their use prior to biogas production. In a previous study [16], a recombinant Bacillus megaterium strain was used for pretreatment of chicken feathers. An initial feather concentration of 4 % was pretreated prior to biogas production and then only the hydrolysate of the pretreated feather was further investigated as a biogas substrate.
In this study, the goal was to use a wild strain of bacteria for the pretreatment, and then the whole broth of the pretreated feathers including the bacteria was investigated as a substrate to produce biogas, a concept that is closer to be applied in future industrial applications. Chicken feathers at total solid concentrations of 5, 10, and 20 % (dry weight of feathers) were biologically pretreated with a naturally occurring Bacillus strain and thereafter the total broth of pretreated feathers, as well as only the feather hydrolysate for a comparison, was used for biogas production. This protein-degrading bacteria was isolated from compost and identified as Bacillus sp. C4 (2008) [4]. Moreover, this paper assesses investigations at higher initial total solid concentrations in order to minimize reactor volume and utilize larger amount of wastes. Additionally, the effects of incubation time, as well as the type of the inoculum on the biogas yield were also evaluated.
Experimental
Biological Treatment of Chicken Feather
White chicken feathers were collected from a slaughterhouse (Håkantorp Slakteri AB, Genomfatrsvägen, Sweden). The feathers were washed in dilute soap solution immediately after collection and rinsed with tap water and then distilled water. The washed feathers were air-dried for 3 days and then chopped into particle sizes between 1 and 10 mm, thereafter stored at room temperature before use.
The keratin-degrading bacteria used in this study was a Bacillus sp., isolated from compost and identified as Bacillus sp. C4 (2008) [4], stored at −80 °C in 50 % glycerol. The bacteria were incubated at 37 °C for 18 h on agar plates containing 1.5 % bacteriological agar in Luria-Bertani broth. It was then inoculated in 100 ml of Luria-Bertani broth (tryptone 1 % (w/v), yeast extract 0.5 % (w/v), and NaCl 0.5 % (w/v)) and incubated in a water bath at 37 °C and shaking with 160 rpm overnight (18 h).
The feathers with different total solid concentrations of 5, 10, or 20 % (dry weight of feather) were mixed with 90, 84, or 73 ml, respectively, of a modified basal medium (NH4Cl 0.5 g/l, NaCl 0.5 g/l, K2HPO4 0.5 g/l, KH2PO4 0.4 g/l, MgCl2.6H2O 0.1 g/l, yeast extract 1.5 g/l, peptone 1 g/l), and the pH was adjusted to 7.0 using K2HPO4 or KH2PO4 (1 M) in 500-ml Erlenmeyer flasks, sterilized and inoculated with 5 ml of the overnight culture, i.e., 5 % (v/v) of Bacillus sp. C4 (2008), making a total volume of 100 ml. The flasks were then placed in a water bath, and all of the cultivations were running under aerobic conditions at 37 °C, and 160 rpm for 2, 4, 6, or 8 days. All experimental setups were carried out in duplicates.
The remaining feathers in each culture was removed by vacuum filtration using 10-μm paper filters, and the percentage of feather degradation was determined according to the method presented by Park and Son [17]. After removing the un-degraded feathers, 15 ml of the liquid fraction (called hydrolysate) was used to measure the total crude protein and keratinase activity. The hydrolysate was then stored at −20 °C prior to use for biogas production.
Another setup of the experiments was carried out with identical conditions as the abovementioned one, except that the un-degraded feathers were not separated after the biological pretreatment, so that the total broth of pretreated feathers was stored at −20 °C and then used for biogas production.
Soluble Keratin Preparation and Keratinase Activity Assay
Soluble keratin was prepared from chicken feathers according to Wawrzkiewicz et al. [18] with slight modifications and used as substrate for the keratinase activity measurements. The chicken feathers (2 g) were treated at 100 °C for 2 h with 100 ml of dimethyl sulfoxide (DMSO) using a reflux condenser. Then, 300 ml of acetone cooled at −20 °C for 2 h was added to 100 ml of cooled keratin solution in DMSO and left in a refrigerator for 1 h for better precipitation. The solution was then centrifuged at 4000g for 10 min. The precipitate (cake) was washed with distilled water, and then the acetone was allowed to evaporate from the uncapped tube at room temperature for 30 min and finally the cake was suspended in 50 ml distilled water.
This prepared soluble keratin was used as substrate for the enzyme activity determination, and the assay was performed according to the method of Wawrzkiewicz et al. [18], with slight modifications. The prepared soluble keratin suspension (0.5 ml) was mixed with 3.5 ml of 0.1 M phosphate buffer pH 7.5 and 1 ml of sample solution containing the enzyme. Thereafter, the reaction mixture was incubated for 5 h at 45 °C, followed by stopping the enzymatic reaction using 3 ml of 10 % (w/v) trichloroacetic acid (TCA) and left for 30 min at 4 °C. In the control test, the reaction was stopped first with 3 ml of 10 % w/v TCA before adding 0.5 ml of prepared soluble keratin suspension. The test and control suspensions were centrifuged at 4000g for 10 min at 4 °C, and the absorbance of supernatant was measured at 280 nm using a spectrophotometer. One unit (U) keratinase activity was defined as the amount of enzyme causing 0.01 absorbance increase between the sample and the control at 280 nm under the conditions above [17].
Anaerobic Batch Digestion Assays
Anaerobic batch digestion assays were carried out using either feather hydrolysate or total broth of biologically pretreated chicken feathers as substrates, performed in accordance to the method described by Angelidaki et al. [19]. The assays were carried out under mesophilic conditions (37 ± 1 °C) using 118-ml serum glass bottles as reactors with active volume of 56 ml and headspace of 62 ml. The inoculum was either granulated bacteria obtained from a UASB reactor treating municipal wastewater (Hammarby Sjöstad, Stockholm, Sweden) or digested sludge from a digester treating activated sludge and operating at mesophilic conditions at a municipal wastewater treatment plant (Getteröverket, Varberg, Sweden). The inoculants were filtered through a 2-mm porosity sieve to remove large and undigested particles, and then acclimated for 5 days in an incubator at 37 °C prior to use. Substrates (feather hydrolysate or total broth) with loading of 0.25 gVS (amount of solubilized volatile solids for feather hydrolysate and volatile solids of both solubilized and undegraded feathers for total broth) was used, and then inoculum corresponding to 0.5 gVS was added, keeping a VS ratio (VSsubstrate to VSinoculum) at 1:2 in all setups. The pH in each reactor was adjusted to 7.0 using hydrochloric acid solution (2 M), and the nutrient composition was kept according to Angelidaki et al. [19]. Inoculum and water instead of substrate were used as blank to disclose any methane production by the inoculum itself. The reactors were then sealed with rubber septa and aluminum caps, and the headspace was flushed with a gas mixture of 80 % N2 and 20 % CO2 for 2 min to create anaerobic environment in each setup [19]. The reactors were then placed in an incubator at 37 ± 1 °C, and they were shaken manually once a day during the incubation period of 55 days. All experimental setups were performed in duplicates. Gas samples were taken twice a week at the beginning and once a week towards the end of the digestion period from the headspace of each reactor using a pressure-tight syringe (VICI, precious sampling Inc., USA) and then they were analyzed using a gas chromatograph (GC). Gas measurement and analysis were carried out as described previously [20]. All methane volumes are presented at standard conditions (0 °C and 1 atm).
Analytical Methods
Moisture content, pH, total nitrogen, total solids, and volatile solids were determined according to biomass analytical procedures [21]. Total nitrogen contents were measured using the Kjeldahl method [22], and then the total crude protein content was calculated by multiplying the Kjeldahl nitrogen content by 6.25. The total organic carbon was obtained by correcting the total dry weight carbon value for the ash content [23]. The fat content was determined using the Soxhlet extraction procedure [24].
The methane produced was determined by a GC (Perkin-Elmer, USA) equipped with a packed column (6′ × 1.8″ OD, 80/100, Mesh, Perkin Elmer, USA) and a thermal conductivity detector (Perkin-Elmer, USA), with an inject temperature of 150 °C. The carrier gas was nitrogen operated with a flow rate of 20 ml/min at 60 °C. A 250-μl pressure-lock gas syringe (VICI, precious sampling Inc., USA) was used for taking samples for the gas analysis, and the accumulated methane production was calculated accordingly [20].
Statistical Analysis
The experiments were randomized during biogas production analysis using statistical software, MINITAB® (version 17.1.0). Randomization was used in order to ensure that equal treatment was given to all reactors during gas sampling and analysis.
Factor effects, pretreatment impacts, confidence intervals, and standard deviations were analyzed for the anaerobic digestion experiments. The set of experimental runs was analyzed using general linear model analysis of variance (ANOVA) with accumulated methane yield as response variable, with three different factors (pretreatment time, initial percentage of feathers, and type of inoculum used with either hydrolysate or total broth of pretreated feathers) and respectively four, three, and four factor levels. The interaction effects between pretreatment time and type of inoculum used, as well as initial percentage of feathers were also evaluated. ANOVA was used to analyze data, which generate confidence intervals and the significance difference between the different factors considered in the anaerobic digestion experiments. The factors were considered significant when the probability (p value) was less than or equal to 0.05.
Results and Discussion
Untreated Chicken Feathers and Inoculants Characterization
The feathers used in this study contained 92.05 % dry matter of which 97.59 % was organic matter (Table 1). Crude protein constituted 92.65 % of the feathers, and due to high nitrogen content in the feathers, the C/N ratio was low (3.66). The fat content was also very low (0.87 % TS). The characteristics of the two different inoculants, i.e., anaerobic sludge and granular sludge, used for the anaerobic batch digestion assays are as shown in Table 1. The anaerobic sludge used in this work contains 62.18 % of total solid as organic matter, which is slightly lower than that measured in the bacteria granules (68 %). C/N ratio was almost the same in both inoculants used, and the total solids in granules were substantially higher than that in the sludge.
Bacteria Growth and Keratinase Activity
The feather broth was sterilized prior to aerobic pretreatment with Bacillus sp. in order to deactivate all forms of biological agents. Bacillus sp. prefers yeast extract and peptone as nutrients for keratinase enzyme production [4] and therefore the medium supplemented with yeast extract and peptone. These materials will be degraded by the bacteria during the biological pretreatment. However, the excess amount of the yeast extract and peptone can be further assimilated in the subsequent digestion leading to more biogas production.
The Bacillus sp. grew well in the medium and produced keratinase resulting in degradation of feathers to soluble crude protein. The pH of the medium increased from 7.0 to 8.9, 8.6 and 8.7 for 5, 10, and 20 % concentrations, respectively, during the 8 days of degradation. This increase in pH was likely due to the production of ammonia and other alkaline compounds during the degradation of feathers as reported by Cai et al. [11]. Bacteria growth reached a maximum of 9.0 × 109, 14.3 × 109, and 1.6 × 109 CFU/ml on day 4 for 5, 10, and 20 % feather concentrations, respectively. This shows an increase in bacteria population as the feather concentration increased from 5 to 10 %. However, the population of the bacteria was reduced at higher feather concentration of 20 %, probably because at this concentration, the contact between the bacteria and its substrate was limited, since at this high initial feather concentration, larger aggregated feather particles were observed reducing the accessibility of feathers. An increase in pH from 7.5 to 8.5 was reported by Suntornsuk and Suntornsuk [25]. They found maximum bacteria growth of 8.3 × 108 CFU/ml after 3 days of incubation, and these values also increased when concentration of feathers increased using feather concentrations between 1 and 5 %.
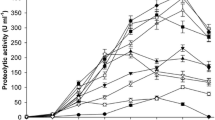
The keratinase activity of the enzyme produced by the Bacillus sp. is presented in Fig. 1. There was a mass loss of 77, 38, and 24 %, respectively for 5, 10, and 20 % of initial feather concentrations, determined after 8 days of degradation, which is an indication of the ability of the produced keratinase to degrade feathers [26]. The activity of the enzyme produced increased from day 2 to day 6 when 5 % initial concentration of feathers was applied and then decreased slightly after 6 days of incubation. However, at higher initial feather concentrations of 10 and 20 %, a sharp decrease in the enzyme activity was observed already after 4 days of degradation (Fig. 1). Ghasemi et al. [27] also reported a decrease in keratinase activity after 3 days of incubation, when 1 % of feather was used, and this was linked to feedback inhibition of the enzyme by its product.
Increase in feather concentrations from 5 to 20 % caused a suppression of keratinase activity, which resulted in a decrease in the percentage of feathers degraded (Fig. 2). At 5 % initial concentration, a maximum activity of 31.9 U was observed at day 6, corresponding to 72.3 % of substrate degradation. Furthermore, a maximum activity of 28.4 U was observed at day 4 when 10 % initial concentration of feathers was applied of which 25.0 % was degraded. Finally, a maximum enzyme activity of 26.2 U was determined for 20 % initial feather concentration at day 4 resulting in 24.9 % degradation of the substrate. Similarly, Cai et al. [11] reported reduction in keratinase activity as feather concentration increased from 1 to 20 g/l.
Degradation of Feather to Soluble Crude Protein
Feather degradation during the bacterial pretreatment was observed by visualizing the disappearance of feathers into the culture medium and measuring the amount of feathers that disappeared. At different initial concentrations of 5, 10, and 20 %, 77, 38, and 24 %, respectively, (Fig. 2) of the total initial amount of feathers were dissolved after 8 days of degradation. The percentage of feather degraded increased as the duration of the degradation were prolonged; however, there was no significant difference in the amount of degraded feathers after 6- and 8-day-long degradation times, and this was observed at all the different initial feather concentrations. Park and Son [17] reported 100 % feather degradation when the initial feather concentration was 0.1 % after 10 days of treatment with B. megaterium F7–1. On the other hand, Deivasigamani and Alagappan [28] reported 85 % degradation for initial concentration of 1 % feathers and after 5 days of treatment with Bacillus sp. FK 46, while Bálint et al. [29] obtained 75 % degradation when 4 % TS feather concentration was applied after 138 h (approximately 6 days) of treatment with Bacillus licheniformis KKl. Moreover, similar results were reported by Suntornsuk and Suntornsuk [25], where 1 to 5 % of feather concentrations were investigated. These previously reported results are all in accordance with our observations, where the percentage of feathers degraded decreased as the initial feather concentration increased, which is possibly an indication of product inhibition for the enzyme. Moreover, as it was mentioned earlier at the highest initial feather concentration, i.e., at 20 %, the bacterial growth itself was also limited leading to less overall biomass concentration compared to those achieved at lower concentration feathers.
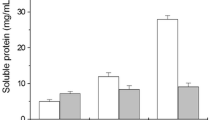
On day zero, the amount of soluble crude protein was very low (an average of 4.13 g/l), but this amount increased as days of degradation increased, which is an indication that the feathers most probably converted to soluble crude protein by the keratinase enzyme produced (Fig. 3). Beside soluble proteins, other intermediate degradation products, such as amino acids, ammonia, and sulfate can also be formed during feather degradation [26].
After 8 days of degradation, the amount of soluble crude protein increased from an average of 4.13 to 35.97 g/l, 36.97 and 45.94 g/l at initial feather concentration of 5, 10, and 20 %, respectively (Fig. 3). If we compare these values with the feather degradation shown on Fig. 2, it is clear that the main products formed during feather degradation are soluble proteins. The soluble crude protein increased slightly up to day 8, when total solid concentrations of 5 and 10 % were applied. However, this increased only up to day 4 in the case of 20 % initial concentration of feathers and thereafter it was approximately the same, as shown in Fig. 3. Our results are in line with the results obtained by Deivasigamani and Alagappan [28], where 75 % of the feather keratin was converted to soluble crude protein after 5 days of incubation when 1 % of feather concentration was used.
Anaerobic Digestion of Hydrolysate of Pretreated Feathers
Accumulated methane profiles from digesters using hydrolysates as substrate are shown in Fig. 4. Bacteria granules performed better during anaerobic digestion of untreated feathers, resulting in approximately two times more methane yield (i.e., 199 mlCH4/gVS compared to 105 mlCH4/gVS when anaerobic sludge was used). However, the highest methane yield of around 430 mlCH4/gVS was achieved from the hydrolysate of the pretreated feathers independently of which kind of inoculum was applied during the anaerobic batch digestion assays (Fig. 4). Hence, the methane yield was improved by 292 % after 55 days of anaerobic digestion when sludge was used as inoculum and, respectively, the yield was improved by 105 % when granules were used as inoculum, showing that pretreatment with Bacillus sp. C4 significantly improved the digestibility in both cases.
Effect of inoculum (sludge (a, b, c) and granules (d, e, f)) and initial feather concentrations (5, 10, or 20 %) on accumulated methane yield of hydrolysate of pretreated feathers at different days of degradation compared with the untreated. Day 2 (blue diamond suit), day 4 (red square), day 6 (green up-pointing triangle), day 8 (Х), and untreated (ж)
At 5 % initial concentration of feathers, increasing the pretreatment time did not give any significant effect on the methane production from the hydrolysate irrespective of the inoculum used. An average of 433 ± 12 mlCH4/gVS yield was observed, reaching the maximum yield after 19 days of digestion in the case of using sludge as inoculum (Fig 4a). However, when the granules were used, the maximum average methane production of 426 ± 22 mlCH4/gVS was achieved only after 45 days (Fig. 4d).
At higher initial feather concentrations of 10 and 20 %, the accumulated methane yield from hydrolyzates obtained after 2 days of pretreatment was lower and it reached its maximum of 331 ± 3 mlCH4/gVS after 19 days when sludge was used (Fig. 4b, c) and a maximum yield of 324 ± 20 mlCH4/gVS after 45 days when granules were used (Figs. 4e, f) as inoculum. However, as pretreatment time increased from day 4 to day 8, the accumulated methane yield was not significantly different in cases of when either sludge or granules were used as inoculum. The reason behind this is probably the fact that at higher concentrations of 10 and 20 %, the percentage of soluble crude protein (Fig 3) in the total feather degraded (Fig. 2) at day 2 was slightly lower compared to longer pretreatment times, but at lower concentration of 5 %, the percentage of soluble crude protein in total feather degraded was almost the same independently on the length of the pretreatment time. These results are in accordance with what was found by Forgács et al. [16] who used 4 % of feather concentration in the pretreatment prior to biogas production.
Regarding the degradation rate achieved during the first 10 days of the digestion period, it was higher when anaerobic sludge was used as inoculum compared to that when granules were used (Fig. 4). The maximum methane yield at all conditions could be reached within 19 days when sludge was used while it was reached only after 45 days in the case of granules (Fig. 4). Though the free cells had a faster rate than the bacteria granules, the same overall yield was obtained in the end of the digestion time (Fig. 4).
Anaerobic Digestion of Total Broth of Pretreated Feathers
Accumulated methane profiles from digesters when the total broth of pretreated feathers was used as substrate are shown in Fig. 5. Overall, lower methane yields and slower degradation rates were obtained using anaerobic sludge as inoculum, compared to those determined in cases of granules as inoculum. For example, the accumulated methane yield of total broth of feathers pretreated for 2 days was 49 % higher when bacteria granules were used than that when sludge was used as inoculum. These results show that the granular sludge was probably able to adapt to possible inhibitors such as ammonia and sulfate [26, 30] in the total broth of pretreated feathers better than the sludge. When granules were used, the remaining un-degraded feathers were broken down better; and thereafter the intermediate products could be converted to methane on a higher rate than those obtained in the case of the free cells present in the anaerobic sludge (Fig. 5). The same trend is shown during anaerobic digestion of the untreated feathers. The methane yield from the untreated feather was 89 % higher when granules were used as inoculum compared to that when sludge was applied (Figs. 4 and 5). This suggests that granules perform better both on the untreated feather and on total broth containing a larger amount of un-degraded feathers than the sludge.
Effect of inoculum (sludge (a, b, c) and granules (d, e, f)) and initial feather concentrations (5, 10, or 20 %) on accumulated methane yield of total broth of pretreated feathers at different days of degradation compared with the untreated. Day 2 (blue diamond suit), day 4 (red square), day 6 (green up-pointing triangle), day 8 (Х), and untreated (ж)
Furthermore, in the cases of anaerobic sludge as inoculum, the methane yield was slightly lower after 2-day-long pretreatment times at all initial concentration of feathers, compared to those obtained after the longer treatments (Fig. 5a, b, c). However, there was no significant difference in the methane yields obtained (an average of 445 ± 12 mlCH4/gVS) as pretreatment time increases in cases when granules were used as inoculum (Fig. 5d, e, f).
The result from the statistical analysis (Table 2) supports our results, showing that pretreatment duration of 2 days has significant effect on the accumulated methane yield but this significant effect could be observed only at higher initial concentrations of 10 and 20 % of feathers (Fig. 6). This also shows that in the case of hydrolysate as substrate, the shortest pretreatment time of 2 days was not enough to obtain higher methane yields at higher initial concentrations irrespective of which kind of inoculum was used. The statistical analysis also shows that using total broth of pretreated feather instead of hydrolysate as substrate, gave a significant effect (p = 0.000) on the accumulated methane yields (Table 2). It is also shown on Fig. 6 that the pretreatment time had no significant effect on the accumulated methane yield when total broth was used as substrate together with bacteria granules as inoculum, but the accumulated methane yield was highly affected by the length of the pretreatment time when total broth was digested with anaerobic sludge.
The results of the statistical analysis also support our experimental results determined from total broth of pretreated feather using bacteria granules as inoculum, which show that the accumulated methane yield was basically constant after different pretreatment times and irrespective of the initial feather concentrations used.
Conclusions
-
1.
Increase in feather concentration from 5 to 20 % did not result in significant increase in the amount of feathers degraded. Pretreatment of feather wastes with Bacillus sp. C4 (2008) was successful, as an average of 75.5 % of the feather keratin was converted to soluble crude protein by the enzyme produced after 8 days of degradation.
-
2.
Chicken feather wastes can effectively be used for biogas production. Anaerobic digestion of these wastes is possible even without any pretreatment; the granules used as inoculum resulted in almost two times more methane production from the untreated feather (an average yield of 199 mlCH4/gVS) than when anaerobic sludge was used as inoculum (an average yield of 105 mlCH4/gVS). However, the final methane yield observed from samples treated biologically by using naturally occurring bacteria that can produce both α- and β-keratinases reached approximately the same level. Accordingly, the methane yield could be increased by 292 % when sludge was used as inoculum on the hydrolysate of the pretreated feather (initial concentration of 5 %) after 55 days of anaerobic digestion and respectively by 105 % when granules were used as inoculum on the hydrolysate compared to those obtained from untreated feathers. The same trend was observed in the case of anaerobic digestion of total broth of treated feathers, an increase by 237 % was obtained when sludge was used as inoculum and, respectively, an increase by 124 % was achieved when bacteria granules were applied as inoculum.
-
3.
Sludge used as inoculum performed better on the feather hydrolysate, while granules worked better on total broth of the pretreated feathers. A pretreatment duration of 2 days is recommended when 5 % initial feather concentration is applied while 4 days are recommended for higher, i.e., 10 % and 20 %, initial concentrations, when hydrolysate of pretreated feathers is used for methane production. Total broth of pretreated feathers can be used effectively with granules for methane production at shorter pretreatment duration of 2 days irrespective of the initial feather concentrations in between the investigated range of 5–20 %.
References
FAOSTAT. (2013) Live animals. Food and Agriculture Organization of the United Nations. FAO Statistics Division 2016. http://faostat.fao.org/site/573/DesktopDefault.aspx?PageID=573#ancor. Accessed 18 Feb 2016.
Matikevičienė, V., Masiliūnienė, D. and Grigiškis, S. (2015) Degradation of keratin containing wastes by bacteria with keratinolytic activity. Environment. Technology. Resources. Proceedings of the International Scientific and Practical Conference, pp. 284–289.
Wang, L., Xin, J., Li, X., & Wang, Y. (2015). The variability of biomass burning and its influence on regional aerosol properties during the wheat harvest season in North China. Atmospheric Research, 157, 153–163.
Fellahi, S., Zaghloul, T. I., Feuk-Lagerstedt, E., & Taherzadeh, M. J. (2014). A bacillus strain able to hydrolyze alpha- and beta-keratin. Journal of Bioprocessing and Biotechniques, 4, 7.
Johnson, D. K. and Elander, R. T. (2009), In Biomass Recalcitrance, Blackwell Publishing Ltd., pp. 436–453.
Taherzadeh, M. J., & Karimi, K. (2008). Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. International Journal of Molecular Sciences, 9, 1621–1651.
Zhao, W., Yang, R., Zhang, Y., & Wu, L. (2012). Sustainable and practical utilization of feather keratin by an innovative physicochemical pretreatment: high density steam flash-explosion. Green Chemistry, 14, 3352–3360.
Korniłłowicz-Kowalska, T., & Bohacz, J. (2011). Biodegradation of keratin waste: theory and practical aspects. Waste Management, 31, 1689–1701.
Onifade, A. A., Al-Sane, N. A., Al-Musallam, A. A., & Al-Zarban, S. (1998). A review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresource Technology, 66, 1–11.
Barone, J. R., Schmidt, W. F., & Gregoire, N. T. (2006). Extrusion of feather keratin. Journal of Applied Polymer Science, 100, 1432–1442.
Cai, C.-G., Lou, B.-G., & Zheng, X.-D. (2008). Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. Journal of Zhejiang University. Science. B, 9, 60–67.
Zaghloul, T. I., Embaby, A. M., & Elmahdy, A. R. (2011). Biodegradation of chicken feathers waste directed by Bacillus subtilis recombinant cells: scaling up in a laboratory scale fermentor. Bioresource Technology, 102, 2387–2393.
Kanchana, R., & Mesta, D. (2013). Native feather degradation by a keratinophilic fungus. International Journal of Chemtech Research, 5, 2947–2954.
Kim, J.-D. (2003). Preliminary characterization of keratinolytic enzyme of aspergillus flavus K-03 and its potential in biodegradation of keratin wastes. Mycobiology, 31, 209–213.
Mazotto, A. M., Couri, S., Damaso, M. C. T., & Vermelho, A. B. (2013). Degradation of feather waste by Aspergillus niger keratinases: comparison of submerged and solid-state fermentation. International Biodeterioration & Biodegradation, 85, 189–195.
Forgács, G., Alinezhad, S., Mirabdollah, A., Feuk-Lagerstedt, E., & Horváth, I. S. (2011). Biological treatment of chicken feather waste for improved biogas production. Journal of Environmental Sciences, 23, 1747–1753.
Park, G.-T., & Son, H.-J. (2009). Keratinolytic activity of Bacillus megaterium F7-1, a feather-degrading mesophilic bacterium. Microbiological Research, 164, 478–485.
Wawrzkiewicz, K., Łobarzewski, J., & Wolski, T. (1987). Intracellular keratinase of Trichophyton gallinae. Journal of Medical and Veterinary Mycology, 25, 261–268.
Angelidaki, I., Alves, M., Bolzonella, D., Borzacconi, L., Campos, J. L., Guwy, A. J., Kalyuzhnyi, S., Jenicek, P., & Van Lier, J. B. (2009). Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Science and Technology, 59, 927–934.
Teghammar, A., Yngvesson, J., Lundin, M., Taherzadeh, M. J., & Horváth, I. S. (2010). Pretreatment of paper tube residuals for improved biogas production. Bioresource Technology, 101, 1206–1212.
APHA-AWWA-WEF. (2005) Standard methods for the examination of water and wastewater 21st Edition. 21st Edition ed., American Public Health Association, 800 I Street, NW, Washington, DC 20001–3710.
LABCONCO. (2015) A guide to Kjeldahl nitrogen determination. Methods and Apparatus. LABCONCO, An Industry Service Publication. Accessed 8th of April. http://www.expotechusa.com/catalogs/labconco/pdf/KJELDAHLguide.PDF. ed.
Haug, R. T. (1993), In The Practical Handbook of Compost Engineering., Taylor & Francis, pp. 247–257
Carpenter, D. C. (2010), In Food Analysis Laboratory Manual: Food Science Texts Series, (Nielsen, S. S., ed.), Springer US, pp. pp 29–37.
Suntornsuk, W., & Suntornsuk, L. (2003). Feather degradation by Bacillus sp. FK 46 in submerged cultivation. Bioresource Technology, 86, 239–243.
Korniłłowicz-Kowalska, T. (1997). Studies on the decomposition of keratin wastes by saprotrophic microfungi. I. Criteria for evaluating keratinolytic activity. Acta Mycologica, 32, 51–79.
Ghasemi, Y., Shahbazi, M., Rasoul-Amini, S., Kargar, M., Safari, A., Kazemi, A., & Montazeri-Najafabady, N. (2012). Identification and characterization of feather-degrading bacteria from keratin-rich wastes. Annals of Microbiology, 62, 737–744.
Deivasigamani, B., & Alagappan, K. M. (2008). Industrial application of keratinase and soluble proteins from feather keratins. Journal of Environmental Biology, 29, 933–936.
Bálint, B., Bagi, Z., Tóth, A., Rákhely, G., Perei, K., & Kovács, K. (2005). Utilization of keratin-containing biowaste to produce biohydrogen. Applied Microbiology and Biotechnology, 69, 404–410.
Deublein, D. and Steinhauser, A. (2011) Biogas from waste and renewable resources. ed. Wiley-VCH.
Acknowledgments
The authors greatly acknowledge Håkantorp Slakteri AB, Genomfatrsvägen, Sweden for supplying the chicken feathers and Alex Osagie Osadolor for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patinvoh, R.J., Feuk-Lagerstedt, E., Lundin, M. et al. Biological Pretreatment of Chicken Feather and Biogas Production from Total Broth. Appl Biochem Biotechnol 180, 1401–1415 (2016). https://doi.org/10.1007/s12010-016-2175-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2175-8