Abstract
The first records in the literature compiled on the parasitic fauna of amphibians in Northeastern Brazil date back to the 1990s. Since then, several new studies have been published on parasite-host relationships, parasite communities, and descriptions of new taxa. However, only in the last decade has there been a significant increase in these studies. Given this growth, we aim to provide a complete and updated compilation of helminth records associated with amphibians from the Brazilian Northeastern region and to analyse the dynamics and network structure formed between parasites and their hosts. Therefore, 33 studies were found in the specialized literature that addressed data from eight families, 15 genera, and 34 species of anuran amphibians, distributed mainly in areas of the morphoclimatic domain of the Caatinga and Atlantic Forest remnants in the Brazilian Northeast. These data correspond to 35% of the total known species of the Caatinga, with Leptodactylidae being the most representative taxon. Regarding helminths, 51 species were recorded, belonging to 20 families and 32 genera. To evaluate the structure of the network, we used measures of connectivity, nestedness, modularity, and centrality, that were considered to identify key species. The web presented 247 interactions with a highly connected structure formed by two parasite generalist species, non-nested and non-modular. We concluded that anuran amphibians from the Brazilian Northeast possess a high parasitic diversity, being Bufonidae and Leptodactylidade taxa considered fundamental for the network structure. Herein, we provided the first analysis of the global framework of parasite communities in amphibians from Brazilian Northeast, by using antagonistic network interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Caatinga morphoclimate domain (CMD) is located in the Brazilian Northeast and holds an area of approximately 912,000 Km2 representing about 10% of the national territory. This region is characterized by a distinct seasonality with high temperatures, irregular rainfall with precipitation levels of 300–1000 mm annually and long drought periods (Ab’Saber 2003; Silvano and Segalla 2005; Queiroz et al. 2017). Despite being the only Brazilian natural region with limits restricted to the national territory, little attention has been given to the conservation of the Caatinga landscape and the diversity of its biota (Silva et al. 2004; Oliveira 2012).
The CMD presents the fourth greatest biological richness of Brazil, with a high degree of endemism. The Brazilian Caatinga presents 98 amphibian species, from which 20 are endemic and the remaining occur also in transition areas between the biomes Cerrado, Amazon Forest, and Atlantic Forest (Garda et al. 2017). The diversity of this group in the northeast region is distributed among 14 families, being Hylidae and Leptodatylidae the most representative taxa among anurans, with 39 and 28 species, respectively (Garda et al. 2017; Segalla et al. 2021).
Currently, Brazil has 1,188 amphibian species (Segalla et al. 2021), which represents 14.2% of the global diversity for this group (Frost 2024). The vast majority of the species are anurans, including 1,144 species (two exotic and invasive species) distributed in 20 families and 107 genera (Segalla et al. 2021). Considering the taxonomical diversity of amphibians, several studies have addressed aspects about their biology, ecology, taxonomy, behavior, and parasitism (Daszak et al. 2003; Cassiano-Lima et al. 2011; Roberto et al. 2013; Andrade et al. 2020; Sena et al. 2018; Madelaire et al. 2020).
Even though Brazil is well known worldwide regarding parasitological researches on anurans, the hidden diversity that these parasites represent is far from being completely unraveled. Less than 10% of the described amphibian species in Brazil have data on their helminthfauna (Campião et al. 2014). The first parasitological studies on Brazilian amphibians is from 1917 by Travassos (Vicente et al. 1991), and since then, the number of publications have been increasing (Nascimento et al. 2013; Silva et al. 2018; Oliveira et al. 2019; Silva-Neta et al. 2020).
Due to the richness of species, amphibians act as hosts to a great diversity of parasites, being infected by the main helminth groups like Nematoda, Digenea, Cestoda and Acanthocephala in larval and adult stages (Campião et al. 2014; Toledo et al. 2014). Furthermore, parasites are considered as bioindicators of environmental changes, providing information about the health and structure of ecosystems and trophic interaction networks (Campião et al. 2009; Koprivnikar et al. 2012).
Therefore, in view of the above and considering the importance and megadiversity of helminth endoparasites of amphibians, we aim 1) to provide a checklist of associations between parasites and their anuran hosts from the northeast region of Brazil based on compiled literature data from 1990 to 2022, and 2) to describe the network pattern of parasite-host interaction that exists within this set and verify which helminth and anuran species are most important in structuring the dynamics of infection in these communities.
Material and methods
Collection of data
In order to carry out this checklist, literature was surveyed using the available databases: Scientific Electronic Library Online (Scielo), Google Scholar, Science Direct, Scopus, Medline (Pubmed), Web of Science, and CAPES Portal, using the following key-words combined: helminths, parasites, endoparasites, amphibians, anuran, infection, Brazil, and Northeastern. Data published in books, book chapters, and annals related to parasites were also included in the checklist. The criteria for inclusion of scientific production was the specific approach of infection of helminth endoparasites in amphibians in the Northeast of Brazil, published from 1990 to 2022.
Data analyses
To create the table of endoparasite species associated with amphibians, the following information was recorded and analyzed separately: host species, parasite, infection site and authors. The methods used for detection and identification of the hosts and parasites species in the literature cited were not taken into consideration. The data were also distributed and organized in graphics, presenting information on the anuran families.
Network analysis
We built a parasite-host interaction network from a binary matrix. The resulting matrix, with parasites as columns and hosts as rows, consisted of cells filled with 1 when interaction was recorded and 0 otherwise. To describe the structure of the web, we calculate the Connectivity, Nestedness and Modularity (Q). Connectance is provided by the ratio between the number of existing interactions and the total number of possible interactions in the network, measuring how much species are linked in the community (Jordano et al. 2006). Nestedness measures how much the set of interactions of less connected vertices is a subset of interactions of more connected vertices. Thus, a network is more nestedness the more specialist species interact with species that belong to a subset of the species with which generalists interact (Delmas et al. 2018).
Modularity assesses the extent to which species form subgroups with a higher density of internal than external interaction. Modularity analysis can provide information on the level of specialization in communities concerning the affinities of interactions (Vázquez et al. 2009). The DIRTLPAb + binary modularity maximization algorithm was used (Beckett 2016), and estimated with the computeModules() function in the Bipartite package in the R program.
As network metrics can be affected by intrinsic characteristics, such as the number of species that interact and sampling from different studies (Vizentin-Bugoni et al. 2016) the significance of the observed modularity was evaluated by comparing it with a null model. We used the Shuffles.web binary null model, similar to the one proposed by Vázquez et al. (2005), which limits the connectance and marginal totals of the matrices. The 95% confidence interval for the modularity metric (DIRTLPAb +) was estimated from 1000 simulated values, with a metric value considered significant if the confidence intervals do not overlap (0.025–0.975).
To assess the role of species in the network following centrality metrics were used: Degree and mean degree, which analyzes how much a species is interacting with another in the network (number of interactions), contributing to examining the influence of the species on other species in the network, that is, the higher the degree of a species, the more potential influence it has on other species in the network; and Closeness which measures the proximity of a species to all other species in the network, indicating how quickly/efficiently a node is likely to influence the overall network (Delmas et al. 2018).
Results
Thirty-three studies published from 1990 to 2022 were selected, from which 16 are articles, 14 scientific notes, one dissertation and two monographs. Sixteen scientific journals were identified and listed in order of the largest number of publications related to helminth fauna of anurans in Northeastern Brazil, with Herpetology Notes (n = 5) being the most representative journal, followed by Herpetological Review (n = 4), Zootaxa and Netropical Helminthology (n = 3), Acta Parasitologica, Brazilian Journal of Biology and Journal of Parasitology (n = 2) and Parasitoloy Research, Biota Neotropica, Journal of Natural History, Brazilian Journal of Veterinary Parasitology, Helminthologia, Comparative Parasitology, Cuadernos de Herpetología, Revista Brasileira de Zoologia e Biologia (n = 1), respectively. An interval of ten years was considered to observe the number of publications related to this topic. In the two first intervals, only one study was found, however, in the last decade, there was an increase of studies related to parasitology of anurans in the Brazilian Northeast, with the publication of thirty-one studies from 2010 to 2022 (Fig. 1).
These studies recorded data on parasitism in eight families, 15 genera and 35 species of amphibians, all of Anura, in terrestrial, arboreal, semi-aquatic and fossorial niches, distributed in the habitats from Caatinga and remains of Atlantic Forest in the Brazilian Northeastern region, corresponding to approximately 35% of the known species from Caatinga domain. The anuran family with the highest number of records was Leptodactylidae with 13 host species infected, followed by Hylidae with 11, Bufonidae and Odontophrynidae with three, Microhylidae with two, and Phyllomedusidae, Eleutherodactylidae, and Ranidae with only one species recorded. Regarding helminths, a total of 82 endoparasites were reported, belonging to 20 families and 32 genera. These total, 59 are nematodes, nine digeneans, six cestodes, six acanthocephalans, one monogenean, and one pentastomid. Among those, 51 were identified at species level (42 nematodes, five digeneans, one cestodes, two acanthocephalans, and one pentastomid) The remaining taxa were identified only at order, family or genus level. (Table 1, Online resource).
Network analysis
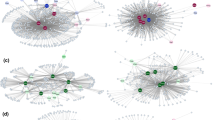
The parasite-host interaction network showed a low nestednnes pattern (NODF = 18.09), that is, a large number of species with a small number of interactions (specialists), while a small number of species with a greater number of interactions (generalists) interacted with each other (Fig. 2). The network was represented by 82 species of helminths and 35 species of anurans, resulting in a total of 247 quantitative interactions observed. The network connectance was high, presenting about 80% of the possible interactions, with the average degree of the species of 3.01 and 7.1, for helminths and anurans, respectively. The high connectance value indicates that the network forms a giant integrated component formed mainly by generalist helminth species and hosts, with many connections between them. The network was not modular (Q = 0.469), being all interactions connected except for a two pairs of species that were isolated from the other components of the network – Chiasmocleis capixaba Cruz, Caramaschi, and Izecksohn, 1997 (Microhylidae) and Lithobates palmipes (Spix, 1824) (Ranidae).
The constructed network revealed two taxa and five species with a strong interaction within the network (Fig. 2), the family Bufonidae being represented by the species Rhinella diptycha (Cope, 1862) (degree of connection: 22) and R. granulosa (Spix, 1824) (degree of connection: 17) and the family Leptodactylidae represented by three species Leptodactylus vastus Lutz, 1930 (degree of connection: 20), L. mystaceus (Spix, 1824) (degree of connection: 14) and L. fuscus (Schneider, 1799) (degree of connection: 14). Regarding the parasites, seven species stood out as the highest degree of interactions, being Raillietnema spectans Gomes, 1964 (degree of connection: 14), Physaloptera sp. (degree of connection: 13), Oswaldocruzia mazzai Travassos, 1935 (degree of connection: 12), Rhabdias sp. (degree of connection: 11) and Aplectana membranosa (Schneider, 1866), Cosmocerca parva Travassos, 1925 and the taxon Cosmocercidae each with ten interactions, respectively. Finally, the centrality evaluation, closeness degree and the betweeness degree, indicated that R. diptycha and L. vastus were the most important species in the network among the host set, follow by R. granulosa, L. fuscus and L. mystaceus, while R. spectans and O. mazzai were considered key-species for the parasite set.
Discussion
In the helminth communities analyzed, the most representative and diverse taxon was nematode, followed by Digenea, cestodes, acanthocephalans, and a single record for monogenean and pentastomid taxa. Nematoda is usually the predominant taxon in the structure and composition of the parasitic fauna in anurans (Silva et al. 2018), where most of the nematodes found have a monoxenic life cycle with their larvae being found in the soil, which makes infection possible through penetration of their host's skin. About 60% of the hosts that had their helminth fauna inventoried have terrestrial or semi-aquatic habits and life history and ecological strategies that contribute to the encounter of these with their parasites.
Digeneans tends to be the second largest group in parasite diversity (Cribb et al. 2002), often with infections in amphibians being more related to aquatic and semiaquatic frog clades (Machado et al. 2021). Diplostomidae, Glyptelmidae, Macroderoididae, and Plagiorchiidae were the digenetic taxa associated with anuran hosts in the Caatinga, divided into four genera (Glypthelmins, Lophosicyadiplostomum, Plagiorchis and Rauschiella) and three species (G. linguatula (Rudolphi, 1819), P. rangeli Artigas and Zerpa, 1961 and R. linguatula (Rudolphi, 1819)). The genus Lophosicyadiplostomum is a parasite of birds that uses anurans as intermediate hosts (Hamann and González 2009). Glypthelmins is a cosmopolitan genus of amphibian parasites, which currently has eight species formally recognized based on molecular and morphological evidence and forms a sister group with the genus Haematoloechus (Razo-Mendivil et al. 2006). Species of this genus have a heteroxenic life cycle with two hosts in their life cycle, an invertebrate as an intermediate host and a vertebrate as the definitive host (Hamann 2006). Regarding the Monogenea and Pentastomida taxa, both had only one record, which demonstrates that potentially these parasitic groups have difficulties establishing themselves or using the anuran species recorded here as hosts in their life cycles.
Of the total taxon surveyed in this study, 31 was unidentified at a specific level. Poulin (2018) reiterates the importance of identifying the species of parasites, therefore contributing to a better measurement of the existing diversity of parasites between different communities, and avoiding errors of underrated of parasite richness, imprecise estimates of diversity, or overestimation of parasites, parasitic similarities between different communities. Thus, we reinforce that the identification of parasitic species is not easy, requires time and taxonomic knowledge, and when possible, molecular techniques for species-specific identification are implemented (Müller et al. 2018; Morais et al. 2020).
Regarding the remarkable growth of research in the last decade, we believe that it can be justified by both political and scientific issues. In the first half of the last decade, there were broad investments in public education through financial resources and research incentives in Brazil. Therefore, investing such resources in public universities boosted the development of basic and applied research in the most diverse fields of scientific knowledge (Marques 2019). Another factor that contributed to these results has been the consolidation of regionalized research groups in different universities in the Northeast that have concentrated their research and explored the diversity of different taxonomic groups of both parasites and hosts in environments of the Caatinga domain (Teles et al. 2015, 2018a, b; Silva et al. 2018, 2019; Oliveira et al. 2019; Felix-Nascimento et al. 2020) and Atlantic Forest domain (Martins-Sobrinho et al. 2017; Sena et al. 2018; Silva-Neta et al. 2020; Mascarenhas et al. 2021; Oliveira et al. 2022).
As for the parasitic diversity associated with the taxon Bufonidae, the reunion of data from Brazilian Northeast began with the study of Vicente et al. (1991), that reported the nematodes Oswaldocruzia subauricularis (Rudolphi, 1819), Rhabdias fuelleborni Travassos, 1926, and Rhabdias sphaerocephala Goodey, 1924, parasitizing Rhinella icterica (Spix, 1824) (= Bufo marinus). However, only from 2008 onwards that other helminthfauna studies for Rhinella granulosa and Rhinella diptycha (= R. jimi) were published (Anjos et al. 2008; Müller et al. 2018; Teles et al. 2018a; Amorim et al. 2019; Madelaire et al. 2020; Lima et al. 2021). Among the species of Bufonidae, R. diptycha is the only species with a record of infection by trematode (Benício et al. 2022).
Hylidae is the taxon with the greatest species occurrence in the Brazilian Northeast, being largely represented in herpetofauna surveys and species description (Roberto et al. 2017; Orrico et al. 2018), phylogeography (Menezes et al. 2016), evolution, ecology, and physiology (Jared et al. 2015; Leite-Filho et al. 2017). However, there is only one study on the helminth fauna of hylid anurans for CMD with the host Trachycephalus typhonius (Linnaeus, 1758) (Benício et al. 2022). Most of the records for hylids anurans are from humid forest vegetation and Atlantic Forest (Nascimento et al. 2013; Martins-Sobrinho et al. 2017; Machado et al. 2021). The development of more research in the semi-arid region is necessary to better understand the host-parasite dynamics between hylid species, identify the parasite species associated with these hosts, and broaden the records of the geographic distribution of their helminths in the Northeastern region. These studies contribute to the understanding of parasitic relationships and highlight the importance of the use of molecular tools for parasite identification since the findings of larvae and females hinder identification and limit discussions on parasite specificity.
Leptodactylidae was represented in the studies about endoparasites by 13 species, belonging to four genera: Leptodactylus, Physalaemus, Pseudopaludicola, and Pleurodema (Vicente et al. 1991; Campião et al. 2014; Teles et al. 2014, 2015, 2017a, b; Lins 2016; Lins et al. 2017; Müller et al. 2018; Silva et al. 2018; Oliveira et al. 2019; Madelaire et al. 2020; Silva-Neta et al. 2020; Soares et al. 2020). The literature presents a diverse parasitic fauna for hosts from Leptodactylus genus (Campião et al. 2014; Teles et al. 2017a; Müller et al. 2018; Silva-Neta et al. 2020). While studies for the Physalaemus genus are scarce (Oliveira et al. 2019; Silva-Neta et al. 2020).
Interaction networks
The network of parasite-host interactions between helminths and anurans from Brazilian Northeastern region did not show a nestedness pattern, which makes it possibly less resistant to anthropic disturbances. Dehling (2018) explains that nestedness structure of a network increases its stability and makes well-connected generalist species more resistant against extinction. Fortuna and Bascompte (2006) studying the structure of real networks and simulated networks in response to habitat loss, observed that real networks lose species faster than simulated networks. However, due to their high degree of nesting and heterogeneity, they resist for longer time to disturbance. In nestedness networks, specialist species are the first to be extinct because they have a smaller number of interactions, thus causing them to be replaced by generalist species that have a greater number of interactions and that, due to the robustness of the interaction between other high-level generalist species, degree of interaction are the last to be extinguished (Rodrigues et al. 2016).
The low nesting and modular value can make the network more susceptible to anthropic disturbances (Dehling 2018). Modularity, like nesting, increases the robustness of the network against disturbances in the system, which makes it difficult to spread negative effects to other modules. Thus, non-nestedness or low-nesting networks may be characteristic of agonistic interactions in host-parasite networks. Furthermore, we can observe a large number of peripheric species of helminths with several isolated records of specific bindings with one to three hosts (Fig. 2). This characteristic contributed the non-nestedness and not modular structuring of the network agonistic.
Most interactions between helminths and their anuran hosts that occurred comprise very specifically and unique links, such as the sympatric occurrence of the species Aplectana crucifer, A. lopesi, A. meridionalis, A. travossosi, and Cosmocerca travassossi in L. mystaceus, P. silvoi in D. muelleri and, in particular, several taxa of digenetic trematodes and cestodes occurring singly in different anuran host species (Table 1, Online resource).
Another fundamental issue is how the data were used in this work. In this study, unequal factors such as collection methodologies, sampled location, the type of landscape (the Caatinga domain or Atlantic Forest domain), the collection effort employed, and especially the temporality in which these works passed and have been developed may have influenced the descriptive parameters of the network (Pilosof et al. 2013; Runghen et al. 2021). Therefore, future studies with a well-designed sample developed only in a morphoclimatic domain may come to corroborate or refute the network pattern presented in this work.
Network analyses enable to record interactions amongst the components of those system, the way that communities are organized, and the complexity of these interactions (Bascompte and Jordano 2007; Metz et al. 2007; Schreiber 2008; Bellay et al. 2013; Mello et al. 2015). Recently, studies on ecological networks have been encompassing mutualistic plant-pollinator and plant-seed-disperser and antagonist relationships (Lewinsohn et al. 2006; Bascompte and Jordano 2007; Mello 2010; Francisco et al. 2019). Specifically, antagonistic interactions benefit only one of the involved species, such as herbivory and parasitism (Schowalter 2011; Souza et al. 2015; Lopes et al. 2020).
The network analysis identified the anuran and parasite core species that have higher interaction frequency aiming at verifying what are the most important species that structure and balance the host-parasite interaction network in this study. Raillietnema spectans (Cosmocercidae) and O. mazzai (Molineidae) are core parasite species and could be considered generalists, given the number of host species parasitized. Besides, they configure as the most important parasite species for the structure of network. However, other species also had a relatively high frequency of interactions in the network, such as species from the taxa Cosmocercidae, Physalopteridae, and Rhabdiasidae.
Aplectana membranosa and C. parva are common, generalist species that occur widely in amphibians in the northeast as well as in other geographic locations (Gonçalves et al. 2002; Luque et al. 2005; Silva et al. 2018; Oliveira et al. 2019; Silva-Neta et al. 2020; González et al. 2021; Mascarenhas et al. 2021). Both are monoxenic nematodes that infect their hosts through ingestion of larvae or penetration through the skin (Anderson 2000) and that can also be considered important in structuring the parasite community of their hosts. Physaloptera is a diverse genus that infects all classes of terrestrial vertebrates, and rarely fishes (Teles et al. 2018a). Although, among amphibians, there are only two species described Physaloptera amphibia Linstow, 1889 and Physaloptera tigrinae Ali and Farooqui, 1969, using these as definitive hosts in the Palearctic and Oriental region, respectively (Pereira et al. 2012). For the Neotropical region, the taxon Physaloptera sp. is usually found in the larval stage in different anuran taxa, which makes it possible to infer that this parasite uses anuran hosts as paratenic hosts to reach its definitive hosts (Mascarenhas et al. 2021).
The genus Rhabdias occurs throughout the world and are lung parasites of amphibians and reptiles (Tkach et al. 2014). Recent studies of the phylogeny of this genus with Neotropical species revealed the monophyly of Rhabdiasidae as well as the existence of an infection pattern of Rhabdias species associated with the phylogeny of their hosts (see Müller et al. 2018). In our study, Rhabdias sp. treated here as a single taxon in our analysis may represent about five possible new species of the genus considering their occurrence records by different hosts of the taxa Bufonidae, Hylidae, Leptodactylidae, Odontophrynidae, and Phyllomedusidae. The hidden diversity of Rhabdias in the Neotropics is evident and the presence of cryptic species occurring in different host taxa requires studies that use molecular techniques to identify these parasites as well as for a better understanding of these systematic and host-parasite relationships (Müller et al. 2018).
The host species Rhinella diptycha and R. granulosa (Bufonidae) and Anuran leptodactylids like L. fuscus, L. mystaceus and L. vastus presented the highest degree of interaction, from which some are common to the host species of each genus. The data corroborate previous studies, reinforcing that phylogenetically close hosts tend to present similar parasitic faunas when compared with non-related hosts (Bellay et al. 2011; Bellay et al. 2013; Krasnov et al. 2012; Lima et al. 2012). We assume that these host species present more interactions due to the phylogenetic proximity, their sympatric occurrence, and their similar generalist habits, ingesting a great variety of prey. Thus, because they harbor greater helminth diversity shared, we can considered the species cited above key-species relative importance in structure network.
Chiasmocleis capixaba and Lithobates palmipes interacted with only one parasite each, which did not interact with any other amphibian. Parasite species with high specificity to stablish their interactions are selective regarding their host choice, which is reflected in the high modularity of the analyzed network (Lopes et al. 2020). Svensson-Coelho et al. (2014) stated that highly specialized associations are indicative of the existence of species groups that interact more among themselves, possessing functional traces that are compatible among the species. Thus, the antagonistic networks and high phylogenetic proximity among parasite lineages are highlighted, as well as their host specialization.
About half of the helminth species (n = 42, 51.2%) listed in this study maintain a single interaction with one host or with up to three anuran hosts (see Fig. 2 and supplementary material), which makes it possible for parasite species that have higher specialization are more prone to be affected by the removal of a host species from the network if there are no records of other hosts. Such fact stresses the importance of host species that are considered generalists for the establishment of the network (Burgos et al. 2007; Lopes et al. 2020). Moreover, according to Lopes et al. (2020), the establishment of the networks are key mechanisms for the maintenance of interactions and might be considered important in an evolutionary context of host-parasite relationships.
Studies analyzing the network structure of biological communities allow us to better understand the processes and complexity of ecosystems by being able to extract the properties of an ecological system according to the number and distribution of links between interacting organisms (Runghen et al. 2021). We concluded that anuran amphibians from the Brazilian Northeastern region present a high parasitic diversity. Some hosts of generalist habits are infected by several helminths, being considered important for the network structure, as observed for some Bufonidae and Leptodactylidade. Even, more specialized species retain a smaller number of interactions, which is valid for both parasites and hosts, in a way that extremely specific interactions do not interact with the network itself and are presented as a weak point considering its specificity. On the other hand, although we have evidenced the existence of a great diversity of macroendoparasite helminths associated with these hosts, within this diversity there is a high percentage of taxa yet to be described and identified at a specific level, making it necessary to implement greater efforts in the field knowledge of this yet unidentified diversity.
The present study provided the first analysis of the global structure of parasite communities in amphibians from the Brazilian Northeastern region, by using antagonistic network interactions. Despite the increase in the number of studies in the last decade, the growing number of descriptions of new anuran species and the high diversity of endoparasitic helminths for this vertebrate host group, the development of more research is imperative. Such studies must aim taxonomical and ecological approaches of parasites in amphibians, mostly nematodes that are the most common, as well as assess host-parasites interactions and life-cycle of these parasites. It is thus expected that with the increase of sampling efforts, new records of interactions will emerge and unravel the hidden diversity of amphibian parasites.
Data availability
Data supporting the results of this study are available for free consultation and use as 'supplementary files' on the website of the journal Biologia.
References
Ab’Saber AN (2003) Os domínios de natureza no Brasil: Potencialidades paisagísticas, 1st edn. Atêlie Editorial, São Paulo
Alcantara EP, Ferreira-Silva C, Silva LAF, Lins AGS, Ávila RW, Morais DH, da Silva RJ (2018) Helminths of Dermatonotus muelleri (Anura: Microhylidae) from Northeastern Brazil. J Parasitol 104(5):550–556. https://doi.org/10.1645/16-160
Almeida WO, Freire EMX, Lopes SG (2008) A new species of Pentastomida infecting Tropidurus hispidus (Squamata: Tropiduridae) from caatinga in northeastern Brazil. Braz J Biol 68:199–203
Amorim DM, Olivera RH, Dyna C, Sousa DM, Santos MEP, Lima LS, Pinto LC, Ávila RW (2019) Nematodes parasites of Rhinella jimi Stevaux, 2002 (Anura: Bufonidae) in areas of Caatinga, northeastern Brazil. Neotrop Helminthol 13(2):265–271
Anderson RC (2000) Nematode parasites of vertebrates, their development and transmission, 2nd edn. CABI, Wallingford, UK
Andrade FS, Haga IA, Ferreira JS, Recco-Pimentel S, Toledo LF, Bruschi DP (2020) A new crypticspecies of Pithecopus (Anura, Phyllomedusidae) in north-eastern Brazil. Eur J Taxon 723:108–134. https://doi.org/10.5852/ejt.2020.723.1147
Anjos LA, Silva LEM, Almeida WO, Costas JGM, Vasconcelos A (2008) Chaunus jimi (NCN) endoparasites. Herpetol Rev 39(3):337
Araujo-Filho JA, Brito SV, Almeida WO, Morais DH, Avila RW (2015) A new species of Parapharyngodon (Nematoda: Pharyngodonidae) infecting Dermatonotus muelleri (Anura: Microhylidae) from Caatinga, Northeastern Brazil. Zootaxa 2(3):386–390. https://doi.org/10.11646/ZOOTAXA.4012.2.10
Ávila RW, Almeida WO, Ferreira FS, Gaiotti MG, Lima SMQ, Morais DH, Neves GP, Pinheiro AP, Silva JAF, Vasconcellos A et al (2017) Fauna da estação ecológica de Aiuaba: integração de informações para subsídio de planos de conservação e o uso sustentável. In: Mantovani W, Monteiro RF, Anjos L, Cariello MO (eds) Pesquisa em unidades de conservação no domínio da Caatinga. Edn UFC, Fortaleza, pp 405–438
Bascompte J, Jordano P (2007) Plant-animal mutualistic networks: The architecture of biodiversity. Annu Rev Ecol Evol Syst 38:567–593. https://doi.org/10.1146/annurev.ecolsys.38.091206.095818
Bastos RP, Pombal JP Jr (1996) A new species of Hyla (Anura: Hylidae) from eastern Brazil. Amphibreptil 17:325–331
Beckett SJ (2016) Improved community detection in weighted bipartite networks. Roy Soc Open Sci 3:140536. https://doi.org/10.1098/rsos.140536
Bellay S, Oliveira EF, Almeida-Neto M, Lima Junior DP, Takemoto RM, Luque JL (2013) Developmental stage of parasites influences the structure of fish-parasite networks. PLoS One 8(10):e75710. https://doi.org/10.1371/journal.pone.0075710
Bellay S, Lima DP, Takemoto RM, Luque JL (2011) A host-endoparasite network of Neotropical marine fish: are there organizational patterns? Parasitology 138:1945–1952. https://doi.org/10.1017/S0031182011001314
Benício RA, Santos RS, Freire SM, Ávila RW, Silva RJ, Fonseca MG (2022) Diversity of helminth parasites in amphibians from northeastern Brazil. Biologia 77:2571–2579. https://doi.org/10.1007/s11756-022-01132-5
Bezerra CH, Braga RR, Borges-Nojosa DM, Silva GA (2012) Occurrence of spargana infection in Dermatonotus muelleri Boettger, 1885 (Anura, Microhylidae) from a coastal complex in Northeastern Brazil. Herpetol Notes 5:69–71
Burgos E, Ceva H, Perazzo RP, Devoto M, Medan D, Zimmermann M, María Delbue A (2007) Why nestedness in mutualistic networks? J Theor Biol 249(2):307–313. https://doi.org/10.1016/j.jtbi.2007.07.030
Campião KM, Silva RJ, Ferreira VL (2009) Helminth parasites of Leptodactylus podicipinus (Anura: Leptodactylidae) from south-eastern Pantanal, State of Mato Grosso do Sul, Brazil. J Helminthol 83(4):345–349. https://doi.org/10.1017/S0022149X09289358
Campião KM, Morais DH, Dias OT, Aguiar A, Toledo GM, Tavares LER, Silva JR (2014) Checklist of helminth parasites of amphibians from South America. Zootaxa 3846(1):1–93. https://doi.org/10.11646/zootaxa.3843.1.1
Cassiano-Lima D, Borges-Nojosa DM, Cascon P, Cechin SZ (2011) The reproductive mode of Adelophryne maranguapensis Hoogmoed, Borges & Cascon, 1994 (Anura, Eleutherodactylidae) an endemic and threatened species from the remnants in northern Brazil. North-West J Zool 7(1):92–97
Cribb TH, Chisholm LA, Bray RA (2002) Diversity in the Monogenea and Digenea: does lifestyle matter? Int J Parasitol 32(3):321–328. https://doi.org/10.1016/s0020-7519(01)00333-2
Cruz CAG, Caramaschi U, Izecksohn E (1997) The genus Chiasmocleis Méhely, 1904 (Anura, Microhylidae) in the Atlantic Rainforest of Brazil with description of three new species. Alytes 15(2):49–71
Daszak P, Cunningham AA, Hyatt AD (2003) Infectious disease and amphibian population declines. Divers Distrib 9:141–150. https://doi.org/10.1046/j.1472-4642.2003.00016.x
Dehling DM (2018) The structure of ecological networks. In: Dáttilo W, Gray VR (eds) Ecological networks in the tropics: an integrative overview of species interactions from some of the most species-rich habitats on earth. Nature. https://doi.org/10.1007/978-3-319-68228-0
Delmas E, Benson M, Brice MH, Burkle LA, Dalla Riva GV, Fortin MJ, Newman EA, Olesen JM, Pires MM, Yeakel JD, Poisot T (2018) Analysing ecological networks of species interactions. Biol Rev 94:16–36. https://doi.org/10.1111/brv.12433
Felix-Nascimento G, Vieira FM, Muniz-Pereira LC, Moura GJB, Ribeiro LB, Oliveira JB (2020) Two new species of Cosmocercidae (Nematoda: Cosmocercoidea) of Leptodactylus macrosternum Miranda-Ribeiro (Anura: Leptodactylidae) from Caatinga Biome, Brazil. Zootaxa 4877(2):274–290. https://doi.org/10.11646/zootaxa.4877.2.3
Francisco TM, Couto DR, Garbin ML, Muaylaert RL, Ruiz-Miranda CR (2019) Low modularity and specialization in a commensalistic epiphyte–phorophyte network in a tropical cloud forest. Biotrop 51(4): 1–10. https://doi.org/10.1111/btp.12670
Frost DR (2024) Amphibian species of the world: An online reference. Version 6.2. Electronic database accessible at https://amphibiansoftheworld.amnh.org/index.php. American Museum of Natural History, New York, USA. https://doi.org/10.5531/db.vz.0001. Accessed 12 Mar 2024
Garda AA, Stein MG, Machado RB, Lion MB, Juncá FA, Napoli MF (2017) Ecology, biogeography, and conservation of amphibians of the caatinga. In: Silva JMC, Leal IR, Tabarelli M (eds) Caatinga. Springer, Cham, pp 133–149
Gonçalves AQ, Vicente JJ, Pinto RM (2002) Nematodes of Amazonian vertebrates deposited in the Helminthological Collection of the Oswaldo Cruz Institute with new records. Rev Bras Zool 19(2):453–465. https://doi.org/10.1590/S0101-81752002000200011
González CE, Hamann MI, Duré MI (2021) Nematodes of amphibians from the South American Chaco: Distribution, host specificity and ecological aspects. Diversity 13(7):321. https://doi.org/10.3390/d13070321
Hamann MI, González CE (2009) Larval digenetic trematodes in tadpoles of six amphibian species from Northeastern Argentina. J Parasitol 95(3):623–628. https://doi.org/10.1645/GE-1738.1
Hamann MI (2006) Seasonal maturation of Glypthelmins vitellinophilum (Trematoda: Digenea) in Lysapsus limellus (Anura: Pseudidae) from an Argentinian subtropical permanent pond. Braz J Biol 66(1):85–93. https://doi.org/10.1590/S1519-69842006000100011
Hoogmoed MS, Borges DM, Cascon P (1994) Three new species of the genus Adelophryne (Amphibia: Anura:Leptodactylidae) from northeastern Brazil, with remarks on the other species of the genus. Zoologische Mededelingen 68(24):271–300
Jared C, Mailho-Fontana PL, Antoniazzi MM, Mendes VA, Barbaro KC, Rodrigues MT, Brodie ED (2015) Venomous frogs use heads as weapons. Cur Biol 25(16):2166–2170. https://doi.org/10.1016/j.cub.2015.06.061
Jordano P, Bascompte J, Olesen JM (2006) The ecological consequences of complex topology and nested structure in pollination webs. In: Waser NM, Ollerton J (eds) Plant-pollinator interactions: from specialization to generalization. The University of Chicago Press, Chicago, pp 173–199
Koprivnikar J, Marcogliese D, Rohr J, Orlofske S, Raffel T, Johnson P (2012) Macroparasite infections of amphibians: What can they tell us? Eco Health 13(22):3566. https://doi.org/10.1007/s10393-012-0785-3
Krasnov BR, Fortuna MA, Mouillot D, Khokhlova IS, Shenbrot GI, Poulin R (2012) Phylogenetic signal in module composition and species connectivity in compartmentalized host-parasite networks. Am Nat 179(4):501–511. https://doi.org/10.1086/664612
Kuzmin Y, Tkach VV, Brooks DR (2007) Two new species of Rhabdias (Nematoda: Rhabdiasidae) from the marine toad, Bufo marinus (L.) (Lissamphibia: Anura: Bufonidae) in Central America. J Parasitol 93:159–165
Kuzmin Y, Melo FTV, Silva-Filho HF, Santos JN (2016) Two new species of Rhabdias Stiles et Hassall, 1905 (Nematoda: Rhabdiasidae) from anuran amphibians in Pará, Brazil. Folia Parasitol 63:15. https://doi.org/10.14411/fp.2016.015
Leite-Filho E, Oliveira FA, Eloi FJ, Liberal CN, Lopes AO, Mesquita DO (2017) Evolutionary and ecological factors influencing an anuran community structure in an Atlantic rainforest urban fragment. Copeia 105(1):64–74. https://doi.org/10.1643/CH-15-298
Lewinsohn TM, Prado PI, Jordano P, Bascompte J, Olesen JM (2006) Structure in plant-animal interaction assemblages. Oikos 113(1):174–184
Lima DP Jr, Giacomini HC, Takemoto RM, Agostinho AA, Bini LM (2012) Patterns of interactions of a large fish–parasite network in a tropical floodplain. J Anim Ecol 81(4):905–913. https://doi.org/10.1111/j.1365-2656.2012.01967.x
Lima AES, Duarte RG, Lacerda GMC, Almeida WO, Ribeiro SC (2021) First report of Raillietiella mottae (Pentastomida: Raillietiellidae) infecting Rhinella diptycha (Anura, Bufonidae) in the Brazilian Northeast. Braz J Biol 84:e247768. https://doi.org/10.1590/1519-6984.247768
Lins AGS (2016) Helmintofauna associada a Leptodactylus fuscus (Anura: Leptodactylidae) em regiões de Cerrado, Pantanal e Caatinga no Brasil. Dissertação, Universidade Estadual Paulista Júlio de Mesquita Filho
Lins AGS, Aguiar A, Morais DH, Silva LAF, Ávila RW, Silva RJ (2017) Helminth fauna of Leptodactylus syphax (Anura: Leptodactylidae) from Caatinga biome northeastern Brazil. Rev Bras Parasitol Vet 26(1):74–80. https://doi.org/10.1590/S1984-29612017013
Lopes VL, Costa FV, Rodrigues RA, Braga EM, Pichorim M, Moreira PA (2020) High fidelity defines the temporal consistency of host-parasite interactions in a tropical coastal ecosystem. Sci Rep 10:16839. https://doi.org/10.1038/s41598-020-73563-6
Luque JL, Martins A, Tavares L (2005) Community structure of metazoan parasites of the yellow Cururu toad, Bufo ictericus (Anura: Bufonidae) from Rio de Janeiro. Brazil Acta Parasitol 50(3):215–220
Machado HTS, Oliveira SS, Benício RA, Araújo KC, Ávila RW (2021) Helminths infecting sympatric congeneric treefrogs in Northeastern Brazil. Acta Parasitol 67(2):658–667. https://doi.org/10.1007/s11686-021-00497-y
Madelaire CB, Franceschini L, Morais DH, Gomes FR, da Silva RJ (2020) Helminth parasites of three anuran species during reproduction and drought in the Brazilian semiarid Caatinga region. J Parasitol 106(3):334–340. https://doi.org/10.1645/16-130
Magalhães FM, Loebmann D, Kokubum MNC, Haddad CFB, Garda AA (2014) A new species of Pseudopaludicola (Anura: Leptodactylidae: Leiuperinae) from Northeastern Brazil. Herpetologica 70(1):77–88. https://doi.org/10.1655/HERPETOLOGICA-D-13-00054
Mângia S, Koroiva R, Nunes PMS, Roberto IJ, Ávila RW, Santanna AC, Santana DJ, Garda AA (2018) A new species of Proceratophrys (Amphibia: Anura: Odontophrynidae) from the Araripe Plateau, Ceará State, northeastern Brazil. Herpetologica 74(3):255–268. https://doi.org/10.1655/Herpetologica-D-16-00084.1
Marques F (2019) Ciclo interrompido. In: Revista pesquisa fapesp, ed 275 https://revistapesquisa.fapesp.br/ciclo-interrompido. Accessed 14 July 2022
Martins-Sobrinho PM, Silva WGDO, Santos EGD, Moura GJB, Oliveira JB (2017) Helminths of some tree frogs of the families Hylidae and Phyllomedusidae in an Atlantic rainforest fragment. Brazil J Nat Hist 51(27–28):1639–1648. https://doi.org/10.1080/00222933.2017.1337945
Mascarenhas W, Oliveira CR, Benício RA, Ávila RW, Ribeiro SC (2021) Nematodes of Proceratophrys ararype (Anura: Odontophrynidae), an endemic frog from the Araripe Plateau, Northern Brazil. Biota Neotrop 21:e20201164. https://doi.org/10.1590/1676-0611-BN-2020-1164
Mello M (2010) Redes mutualistas: pequenos mundos de interações entre animais e plantas. Ciência Hoje 47(277):32–37
Mello MAR, Rodrigues FA, Costa LF, Kissling WD, Şekercioğlu ÇH, Marquitti FMD, Kalko EKV (2015) Keystone species in seed dispersal networks are mainly determined by dietary specialization. Oikos 124(8):1031–1039. https://doi.org/10.1111/oik.01613
Menezes L, Canedo C, Batalha-Filho H, Garda AA, Gehara M, Napoli MF (2016) Multilocus phylogeography of the treefrog Scinax eurydice (Anura, Hylidae) reveals a Plio-Pleistocene diversification in the Atlantic Forest. PLoS One 11(6):e0154626. https://doi.org/10.1371/journal.pone.0154626
Metz J, Calvo R, Seno ER, Romero RA, Liang Z (2007) Redes Complexas: conceitos e aplicações. Relatórios Técnicos do ICMC-USP, São Carlos
Morais DH, Müller MI, Melo FTV, Aguiar A, Willkens Y, de Sousa SC, Giese EG, Ávila RW, da Silva RJ (2020) A new species of Rhabdias (Nematoda: Rhabdiasidae), a lung parasite of Pseudopaludicola pocoto (Anura: Leptodactylidae) from North-Eastern Brazil: description and phylogenetic analyses. J Helminthol 3(94):e209. https://doi.org/10.1017/S0022149X20000929
Moravec F, Kaiser H (1994) Brevimulticaecum sp. larvae (Nematoda: Anisakidae) from the frog Hyla minuta Peters in Trinidad. J Parasitol 80(1):154–156
Müller MI, Morais DH, Costa-Silva GJ, Aguiar A, Ávila RW, Silva RJ (2018) Diversity in the genus Rhabdias (Nematoda, Rhabdiasidae): Evidence for cryptic speciation. Zool Scr 47(5):595–607. https://doi.org/10.1111/zsc.12304
Nascimento LC, Gonçalves EC, Melo FT, Giese EG, Furtado AP, dos Santos JN (2013) Description of Rhabdias breviensis n. sp. (Rhabditoidea: Rhabdiasidae) in two Neotropical frog species. Syst Parasitol 86(1):69–75. https://doi.org/10.1007/s11230-013-9432-9
Oliveira SS, Machado HTS, Araújo KC, de Sousa Silva C, Avila RW (2024) Nematodes associated with Leptodactylus cf. mystaceus (Anura: Leptodactylidae) in agricultural landscapes of Ibiapaba plateau, Ceará state, Brazil. Caldasia 46(2). https://doi.org/10.15446/caldasia.v46n2.101535
Oliveira CR, Mascarenhas W, Batista-Oliveira D, de Castro AK, Ávila RW, Borges-Nojosa DM (2022) Endoparasite community of anurans from an altitudinal rainforest enclave in a Brazilian semiarid área. J Helminthol 96:e62. https://doi.org/10.1017/S0022149X22000499
Oliveira CR, Àvila RW, Morais DH (2019) Helminths associated with three Physalaemus species (Anura: Leptodactylidae) from Caatinga Biome, Brazil. Acta Parasitol 64:205–212. https://doi.org/10.2478/s11686-018-00022-8
Oliveira TAL (2012) Anurofauna em uma área de ecótono entre Cerrado e Floresta Estacional: diversidade, distribuição e a influência de características ambientais. Dissertação, Universidade Estadual Paulista
Orrico VGD, Dias IR, Marciano E Jr (2018) Another new species of Phyllodytes (Anura: Hylidae) from the Atlantic Forest of northeastern Brazil. Zootaxa 4407(1):101–110. https://doi.org/10.11646/zootaxa.4407.1.6
Pereira FB, Alves PV, Rochaf BM, Limat SS, Luque JL (2012) A new Physaloptera (Nematoda: Physalopteridae) parasite of Tupinambis merianae (Squamata: Teiidae) from Southeastern Brazil. J Parasitol 98(6):1227–1235. https://doi.org/10.1645/GE-3159.1
Pilosof S, Fortuna MA, Vinarski MV, Korallo-Vinarskaya NP, Krasnov BR (2013) Temporal dynamics of direct reciprocal and indirect effects in a host-parasite network. J Anim Ecol 82:987–996. https://doi.org/10.1111/1365-2656.12090
Poulin R (2018) Best practice guidelines for studies of parasite community ecology. J Helminthol 93(1):8–11. https://doi.org/10.1017/S0022149X18000767
Queiroz LP, Cardoso D, Fernandes M, Moro M (2017) Diversity and evolution of flowering plants of the Caatinga domain. In: da Silva JC, Leal I, Tabarelli M (eds) Caatinga: the largest tropical dry forest region in South America. Springer, Cham. https://doi.org/10.1007/978-3-319-68339-3_2
Razo-Mendivil UJ, León-Régagnon V, Pérez-Ponce de León G (2006) Monophyly and systematic position of Glypthelmins (Digenea), based on partial lsrDNA sequences and morphological evidence. Org Div Evol 6(4):308–320. https://doi.org/10.1016/j.ode.2005.12.005
Roberto IJ, Ribeiro SC, Loebmann D (2013) Amphibians of the state of Piauí, Northeastern Brazil: a preliminary assessment. Biota Neotrop 13(1):322–330. https://doi.org/10.1590/S1676-06032013000100031
Roberto IJ, Araújo-Vieira K, Carvalho-e-Silva SP, Ávila RW (2017) Uma nova espécie de Sphaenorhynchus (Anura: Hylidae) do Nordeste do Brasil. Herpetologica 73(2):148–161. https://doi.org/10.1655/HERPETOLOGICA-D-16-00021
Rodrigues FA, Peron RKDM, Peng J, Kurths J (2016) The Kuramoto model in complex networks. Phys Rep 610:1–98. https://doi.org/10.1016/j.physrep.2015.10.008
Runghen R, Poulin R, Monlleó-Borrull C, Llopis-Belenguer C (2021) Network analysis: Ten years shining light on host–parasite interactions. Trends Parasitol 37(5):445–455. https://doi.org/10.1016/j.pt.2021.01.005
Sá AA, Fonseca MG (2014) Helmintofauna associada à Proceratophrys sp. (Cycloramphidae) procedentes do semiárido brasileiro. In: XI Semana nacional de ciência e tecnologia, Picos, pp 1–407
Sampaio NKS, Nascimento JM, Camilo IS, Silva EG, Teixeira AAM, Almeida WO, Brito SV (2020) Proceratophrys aridus endoparasites. Herpetol Rev 51(2):302
Schowalter TD (2011) Insect ecology: an ecosystem approach, 3rd edn. Elsevier Academic Press, San Diego, California
Schreiber F (2008) Graph Theory. In: Junker BH, Schreiber F (eds) Analysis of biological networks. Wiley Interscience, Hoboken, pp 15–28
Segalla MV, Berneck B, Canedo C, Caramaschi U, Cruz CAG, Garcia PC, Grant T, Haddad CFB, Lourenco ACC, Mângia S, Mott T, Nascimento LB, Toledo LF, Werneck FP, Langone JA (2021) List of Brazilian amphibians. Herpetol Brasil 10(1):1–99. https://doi.org/10.5281/zenodo.4716176
Sena PA, Conceição BM, Silva PF, Silva WG, Ferreira WB, Júnior VAS, Oliveira JB (2018) Helminth communities of Pithecopus nordestinus (Anura: Phyllomedusidae) in forest remnants, Brazil. Herpetol Notes 11:565–572
Silva CS, Alcantara EP, Silva RJ, Ávila RW, Morais DH (2019) Helminths parasites of the frog Proceratophrys aridus Cruz, Nunes, and Juncá, 2012 (Anura: Odontophrynidae) in a semiarid region, Brazil. Neotrop Helminthol 13:169–179. https://doi.org/10.24039/rnh2019132638
Silva CS, Ávila RW, Morais DH (2018) Helminth community dynamics in a population of Pseudopaludicola pocoto (Leptodactylidae: Leiuperinae) from Northeast-Brazilian. Helminthologia 55(4):292–305. https://doi.org/10.2478/helm-2018-0032
Silva JMC, Tabarelli M, Fonseca MT, Lins LV (2004) Biodiversidade da Caatinga: áreas ações prioritárias para a conservação. Ministério do Meio Ambiente, Brasília
Silva-Neta AF, Alcantara EP; Oliveira CR, Fernandes CEF, Morais DH, Silva RJ, Ávila RW (2020) Helmintos asociados con 15 especies de anuros de la Meseta de Ibiapaba, en el Noredeste de Brasil. Neotrop Helminthol 14(2):207–216. https://doi.org/10.24039/rnh2020142795
Silvano DL and Segalla MV (2005) Conservação de anfíbios no Brasil. Megadiversidade 1(1):79–86
Sluys MV, Schittini GM, Marra RV, Azevedo ARM, Vicente JJ, Vrcibradic D (2006) Body size, diet and endoparasites of the microhylid frog Chiasmocleis capixaba in Atlantic Forest area of Southern Bahia state, Brazil. Braz J Biol 66(1):167–173. https://doi.org/10.1590/s1519-69842006000100021
Soares PBC, Passos DC, Anjos LA, Wachlevski M (2020) Helminth’s assemblage of a small frog in the Brazilian semiarid: parasite-host-environment relationships. Iheringia Sér Zool 112:e2022016. https://doi.org/10.1590/1678-4766e2022016
Souza JR, Alves KM, Vieira KM, Oliveira AP, Becker IS, Costa AN (2015) Herbivoria foliar no estrato lenhoso dentro do gradiente de fisionomias vegetais em uma área de savana Neotropical. Enciclopédia Biosfera, Goiânia 11(22). https://conhecer.org.br/ojs/index.php/biosfera/article/view/1346
Svensson-Coelho M, Ellis V, Loiselle BA, John G, Blake and Ricklefs RE (2014) Reciprocal specialization in multihost malaria parasite communities of birds: A temperate-tropical comparison. Am Nat 184(5):624–635.https://doi.org/10.1086/678126
Teles DA, Brito SV, Araújo-Filho JA, Ribeiro SC, Teixeira AAM, Mesquita DO, Almeida WO (2018a) Nematodes of the Rhinella granulosa Spix, 1824 (Anura: Bufonidae) from the Semiarid Northeastern Caatinga Region of Brazil. Comp Parasitol 85(2):208–211. https://doi.org/10.1654/1525-2647-85.2.208
Teles DA, Rodrigues JK, Teixeira AAM, Araújo-Filho JA, Sousa JGG, Ribeiro SC (2018b) Diet of Leptodactylus macrosternum Miranda-Ribeiro 1926 (Anura: Leptodactylidae) in the Caatinga domain, Northeastern Brazil, Neotropical Region. Herpetol Notes 11:223–226
Teles DA, Araújo-Filho JA, Teixeira AAM, Lima VF, Pereira AMA (2017a) First record of Foleyella convoluta (Nematoda: Onchocercidae) parasitizing Leptodactylus macrosternum (Anura: Leptodactylidae) from Brazil. Herpetol Notes 10:617–618
Teles DA, Brito SV, Araújo-Filho JA, Teixeira AAM, Ribeiro SC, Mesquita DO, Almeida WO (2017b) Nematode parasites of Proceratophrys aridus (Anura: Odontophrynidae), an endemic frog of the Caatinga domain of the Neotropical Region in Brazil. Herpetol Notes 10:525–527
Teles DA, Teixeira AAM, Araújo-Filho JA, Rodrigues JK (2016) Leptodactylus vastus (Northeastern Pepper Frog) endoparasites. Herpetol Rev 47(4):642–643
Teles DA, Sousa JGG, Teixeira AAM, Silva MC, Oliveira RH, Silva MRM, Ávila RW (2015) Helminths of the frog Pleurodema diplolister (Anura, Leiuperidae) from the Caatinga in Pernambuco State, Northeast Brazil. Braz J Biol 75(1):251–253. https://doi.org/10.1590/1519-6984.08513
Teles DA, Cabral MES, Araújo-Filho JA, Dias DQ, Ávila RW, Almeida WO (2014) Helminths of Leptodactylus vastus (Anura: Leptodactylidae) in an area of the Caatinga, Brazil. Herpetol Notes 7:355–356
Tkach VV, Kuzmin Y, Scott B, Snyder D (2014) Molecular insight into systematics, host associations, life cycles and geographic distribution of the nematode family Rhabdiasidae. Int J Parasitol 44(5):273–284. https://doi.org/10.1016/j.ijpara.2013.12.005
Toledo LF, Martins IA, Bruschi DP, Passos MA, Alexandre C, Haddad CFB (2014) The anuran calling repertoire in the light of social contexto. Acta Ethol 1–13. https://doi.org/10.1007/s10211-014-0194-4
Vázquez DP, Morris WF, Jordano P (2005) Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol Let 8(10):1088–1094. https://doi.org/10.1111/j.1461-0248.2005.00810.x
Vázquez DP, Bluthgen N, Cagnolo L, Chacoff NP (2009) Uniting pattern and process in plant-animal mutualistic networks: a review. Ann Bot 103:1445–1457
Vicente JJ, Rodrigues HO, Gomes DC, Pinto RM (1991) Nematóides do Brasil 2ª Parte: Nematóides de Anfíbios [Nematodes of Brazil 2nd part: Nematodes of Amphibians]. Rev Bras Zool 7(4):549–626. https://doi.org/10.1590/S0101-81751990000400015
Vizentin-Bugoni J, Maruyama PK, Debastiani VJ, Duarte LS, Dalsgaard B, Sazima M (2016) Influences of sampling effort on detected patterns and structuring processes of a Neotropical plant–hummingbird network. J Anim Ecol 85(1):262–272. https://doi.org/10.1111/1365-2656.12459
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES. [88887.888289/2023–00; 88887.738154/2022–00].
Author information
Authors and Affiliations
Contributions
The manuscript in question was developed from the monograph of Ednalva da Silva Santos, supervised by Charles de Sousa Silva and co-supervised by Drausio Honorio Morais. All people who meet criteria of article authorship are listed as authors, and all authors are sufficiently at work to assume responsibility for its content, including participation in the concept, analysis, writing or revision of the manuscript, as detailed below.
Conceptualization and study design: Ednalva da Silva Santos and Charles de Sousa Silva; Methodology: Ednalva da Silva Santos; Acquisition and compilation of data: Ednalva da Silva Santos; Analysis, interpretation of data: Ednalva da Silva Santos, Isabela Hevily Silva Torquato and Charles de Sousa Silva; Construction of images: Ednalva da Silva Santos and Isabela Hevily Silva Torquato; Writing the manuscript: Ednalva da Silva Santos, Isabela Hevily Silva Torquato, Drausio Honorio Morais and Charles de Sousa Silva; Critical revision of paper: Paulo Cascon and Charles de Sousa Silva.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva Santos, E., Torquato, I.H.S., Morais, D.H. et al. What is the diversity and pattern of network interactions parasite-host in amphibians (Anura) from Caatinga domain? – A meta-analysis. Biologia 79, 2401–2421 (2024). https://doi.org/10.1007/s11756-024-01717-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-024-01717-2