Abstract
The study of host–parasite interactions is essential to understand the role of each host species in the parasitic transmission cycles in a given community. The use of ecological network highlights the patterns of interactions between hosts and parasites, allowing us to evaluate the underlying structural features and epidemiological roles of different species within this context. Through network analysis, we aimed to understand the epidemiological roles of mammalian hosts species (n = 67) and their parasites (n = 257) in the Pantanal biome. Our analysis revealed a modular pattern within the network, characterized by 14 distinct modules, as well as nestedness patterns within these modules. Some key nodes, such as the multi-host parasites Trypanosoma cruzi and T. evansi, connect different modules and species. These central nodes showed us that various hosts species, including those with high local abundances, contribute to parasite maintenance. Ectoparasites, such as ticks and fleas, exhibit connections that reflect their roles as vectors of certain parasites. Overall, our findings contribute to a comprehensive understanding of the structure of host–parasite interactions in the Pantanal ecosystem, highlighting the importance of network analysis as a tool to identifying the main transmission routes and maintenance of parasites pathways. Such insights are valuable for parasitic disease control and prevention strategies and shed light on the broader complexities of ecological communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of host–parasite interactions in tropical natural environments is extremely complex since different host species maintain different transmission cycles in the natural environment through a reservoir system formed by hosts species with different epidemiological competencies (e.g., maintenance host, amplifier host, dead-end host) (Herrera et al. 2011; Porfirio et al. 2018; de Macedo et al. 2022a, b). Determining the key species in transmission networks is essential to mitigate control strategies to prevent disease outbreaks (Júnior et al. 2020; Santos et al. 2021; Cardoso et al. 2021; Santos and Sano 2022a, b).
Network studies provide detailed information about ecological systems, with structural and mathematical information (Delmas et al. 2019; Mello et al. 2019; Queiroz et al. 2021; Santos and Sano 2022a, b). Different interactions play a crucial role in shaping the structure of networks, but studies continue to evolve. Initially, it was suggested that mutualistic networks were more nested, connected, and less modular, in contrast to host–parasite networks, which tended to be more modular and disconnected (Júnior et al. 2020; Santos and Sano 2022a, b). However, this structural distinction between mutualistic networks (nested/connected) and antagonistic networks (modular/disconnected) has been changing (Guimarães 2020). Frugivory networks, for instance, indeed tend towards nesting. Plant-pollinator networks, however, are often as modular or even more modular than host–parasite networks (Pinheiro et al. 2022). Defensive mutualisms between ants and myrmecophyte plants, on the other hand, tend to be extremely modular and disconnected. Intimacy is probably more important in defining network structure than whether the interaction is mutualistic or antagonistic (Hembry et al. 2018; Guimarães 2020; Pinheiro et al. 2022). Furthermore, network studies can help elucidate how biological communities are organized and how species are connected (Poulin 2007; Júnior et al. 2020; Alcantara et al. 2022; Bezerra and Bocchiglieri 2022).
Also, ecological network analysis allows us to investigate the patterns of interactions between hosts and their parasites (Delmas et al. 2019; Vitorino et al. 2022), providing important structural information on the role of different host species in the parasitic transmission cycles (Dallas et al. 2019; Cardoso et al. 2021; Santos and Sano 2022a; Alcantara et al. 2022; Bezerra and Bocchiglieri 2022). Although there are many studies involving host–parasite interactions, they generally do not provide a broad and contextualized view of the general structure of the different interactions between host and parasite. It is essential to consider all species as integral parts of ecological networks and not as isolated groups (Herrera et al. 2004, 2007, 2011; Rademaker et al. 2009; Santos et al. 2021; de Macedo et al. 2022a, b).
Therefore, the network approach can contribute to the general understanding of the host-parasitic system, allowing for the analysis of the community as a whole and modeling factors associated with the transmission and maintenance of parasites among different host species (Morris et al. 2014; Santos and Sano 2022a, b; Felix et al. 2022a, b; Alcantara et al. 2022). It also provides valuable information for understanding the epidemiological roles played by parasites, such as specificity related to their hosts in a given community (Poulin 2007). The patterns of the networks are related to the dynamics of the communities studied, and in the case of a parasitic network, we can identify the central host species that can be sources of many parasites for other host species, connecting different transmission cycles in the network (Cardoso et al. 2021; Santos and Sano 2022a; Alcantara et al. 2022). Thus, the present study aimed to evaluate the structure of the parasitic network in the Pantanal biome, hypothesizing that the parasitic network will present a modular pattern with internal nesting, and we will be capable of showing species of parasites and hosts that have greater importance in the network.
Materials and methods
Network structure
The network was constructed using four groups of parasites (arthropods, bacteria, helminths, and protozoa) and their mammalian hosts in the Pantanal floodplain as reported by de Macedo et al. (2022b). The search was conducted to identify full-text articles reporting parasites in wild mammal species in the Brazilian Pantanal biome. The following databases were explored: (i) PubMed; (ii) SciELO; (iii) Jstor; (iv) Science Direct; and (v) Scholar Google. We used the terms “Arthropods, Bacteria, Helminths, and Protozoa” and “Pantanal” applied in the title, abstract, and keywords. We made the analysis only when the host species was well defined (genus identification was excluded), but we considered the identification of species and/or genus level for the parasite. We used these data in a matrix of interactions (A × B), in which the lines correspond to the node parasites (i) and the columns to the node hosts (j). Each cell in the Aij matrix contained interaction frequency values, that is, the number of times that a host species was reported to be parasitized by a species of parasite j. We describe the structure of the studied network using three network-level metrics that vary between 0 and 1: (i) network’s specialization—specialization (H2’) is a measure of niche divergence between species, where higher values of H2’ indicate higher specialization (Blüthgen 2010); (ii) network’s nestedness (WNODF metric—describes the aggregate pattern of parasitic-host infections (few hosts have many parasites, and most hosts have few species of parasites) (Almeida-Neto and Ulrich 2011; Mello et al. 2019; Santos and Sano 2022a), and (iii) we also tested the hierarchical compound topology in the studied network (Felix et al. 2022a, b; Pinheiro et al. 2022). A compound network can have a modular structure; however, its modules can present a different type of internal structure (Queiroz et al. 2021). We first calculate modular structure of the network to determine weighted modularity (Qw) assesses the extent to which species form subgroups with higher internal than external interaction density. The values of weighted modularity (Qw). Finally, we calculated the nestedness in the entire network (WNODA), between modules (WNODADM), and within its modules (WNODASM). A compound network is expected to exhibit higher nestedness within its modules than between its modules and the entire network. The significance of the network metrics was estimated using Monte Carlo procedures based on comparisons with randomized distributions generated with null models. We generated 1000 randomized matrices based on the original weighted matrix using the algorithm proposed by Vázquez et al. (2007) and Pinheiro et al. (2019). The network structure was considered to deviate significantly from the null model at p < 0.05.
Species roles
To assess the relative importance of each node to the structure we calculated the “species role” of each species of parasite and host through its “network functional role,” which classifies the node according to their position and importance in the network the each species assigned based on its position in a space defined by zi and Pi, as (i) ultraperipheral vertices (all interactions within their module (Pi ≤ 0.05)), (ii) peripheral vertices (most interactions within their module (0.05 < Pi ≤ 0.62)), (iii) non-hub connector vertices (many interactions to other modules (0.62 < Pi ≤ 0.80)), (iv) non-hub kinless vertices (interactions evenly distributed among all modules (Pi > 0.80)), (v) provincial hubs (most interactions within their module (zi ≥ 2.5 and Pi ≤ 0.30)), (vi) connector hubs (many interactions to most of the other modules (zi ≥ 2.5 and 0.30 < Pi ≤ 0.75)), and (vii) kinless hubs (interactions homogeneously distributed among all modules (zi ≥ 2.5 and Pi > 0.75)) (Mello et al. 2013; Queiroz et al. 2021; Santos and Sano 2022a).
In addition, to assess the relative importance of each host and parasite node in the network structure, we calculated a set of species-level metrics. The centrality of a species (parasite or host) was first measured using the normalized degree (nk). A node connected to a higher proportion of nodes has a greater influence on the structure and dynamics of a network (Freeman 1978; Martín González et al.2010; Santos and Sano 2022a). We also calculated the betweenness centrality (BC), that is, the proportion of shortest paths that pass through a node. Species positioned between several pairs are assumed to contribute more to connecting the different regions of the network (Freeman 1977; Mello et al. 2015; Santos and Sano b). The network was constructed with the packages “igraph” (Csardi and Nepusz 2006) and the incidence matrix of host–parasite interaction with the package “Bipartite” (Dormann et al. 2008; Dormann 2011). All data were analyzed using R 4.2.1 (R Core Team 2022).
Results
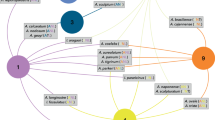
We compiled a network of different hosts and parasite groups. The parasite group with the highest number of nodes was that of helminths (n = 97), followed by hosts (n = 67), arthropods (n = 64), bacteria (n = 51), and protozoa (n = 45) (Supplementary 1 and 2). The network showed significant modular patterns, with 14 established modules (Qw modularity = 0.53, P = 0). The network did not show significant values for nestedness patterns or specialization (Table 1). However, there is an aggregation pattern (network’s nestedness) in the modules where the nesting within the modules was greater (WNODASM = 0.61, P = 0) than between modules (WNODADM = 0.01, P = 0) and across the entire network (WNODA = 0.10, P = 0) (Table 1) (Fig. 1).
Host–parasite networks of interactions between host (H) represented by a star shape and parasites (P) groups (being the arthropods represented as circle, bacteria as triangle, helminths as square, and protozoan as diamond) using the presence of parasite species in each host species. The color polygons around groups of nodes represent the interaction modules identified using the Beckett modularity detection algorithm
Most network nodes (85.50%) presented a functional role for ultraperipheral (n = 216, 66.67%) or peripheral vertices (n = 61, 18.83%). The remaining 14.51% (n = 47) were distributed in (i) non-hub connector vertices (n = 32, 9.85% – arthropods n = 04 (Amblyomma tigrinum – P10, Ornithodoros mimon – P32, Periglischrus herrerai – P39, P. torrealbai – P44), bacteria n = 07 (Anaplasma spp. – P68, Ehrlichia spp. – P78, Mycobacterium avium – P91, Rickettsia amblyommatis – P103, R. felis – P105, R. parkeri – P106, and R. rickettsii – P108), helminths n = 02 (Physaloptera spp. – P182 and Trichuris spp. – P208), protozoa n = 06 (Hepatozoon spp. – P233, H. felis – P231, Leishmania spp. – P238, Neospora caninum – P239, Toxoplasma gondii – P246, and Trypanosoma rangeli – P253), and host n = 13 (Cerdocyon thous – H9, Clyomys laticeps – H12, Dasyprocta azarae – H15, Euphractus sexcinctus – H22, Gracilinanus agilis – H25, Leopardus pardalis – H32, Myrmecophaga tridactyla – H40, Nasua nasua – H41, Panthera onca – H46, Phyllostomus hastatus – H50, Tamandua tetradactyla – H61, Tayassu pecari – H63, and Thrichomys fosteri – H64)); (ii) non-hub kinless vertices (n = 05, 1.55% – all arthropods (Amblyomma spp. – P9, A. cajennense – P2, A. parvum – P5, A. sculptum – P8, and Rhipicephalus (Boophilus) microplus – P47)); (iii) provincial hubs (n = 01, 0.31% – helminths (Wellcomia decorate – P211)), (iv) connector hubs (n = 03, 0.93 – bacteria n = 02 (R. rhipicephali – P107 and Leptospira interrogans – P88) and host n = 01 (Artibeus planirostris – H4)), and (v) kinless hubs (n = 06, 1.86% – arthropods n = 01 (A. ovale–P4); bacteria n = 03 (Leptospira spp. – P89, Rickettsia bellii – P104, and Brucella spp. – P72), and protozoa n = 02 (T. evansi – P252 and T. cruzi – P249)) (Figs. 2 and 3) (Table 2, Supplementary 1).
Host–parasite networks of species roles between host (H in the squares) and parasites (P in the circles) using the presence of parasite species in each host species. The color of nodes represent species roles following the ultraperipheral vertices (Pi ≤ 0.05); peripheral vertices (0.05 < Pi ≤ 0.62); non-hub connector vertices (0.62 < Pi ≤ 0.80); non-hub kinless vertices (Pi > 0.80); provincial hubs (zi ≥ 2.5 and Pi ≤ 0.30); connector hubs (zi ≥ 2.5 and 0.30 < Pi ≤ 0.75), and kinless hubs (zi ≥ 2.5 and Pi > 0.75)
Position and importance of the node (H for host and P for parasites) in the network according to zi and Pi space position. Ultraperipheral vertices (Pi ≤ 0.05); peripheral vertices (0.05 < Pi ≤ 0.62); non-hub connector vertices (0.62 < Pi ≤ 0.80); non-hub kinless vertices (Pi > 0.80); provincial hubs (zi ≥ 2.5 and Pi ≤ 0.30); connector hubs (zi ≥ 2.5 and 0.30 < Pi ≤ 0.75), and kinless hubs (zi ≥ 2.5 and Pi > 0.75)
Normalization values ranged from 0 to 0.36, with the three highest values being for two protozoa (T. evansi – P252 (nk = 0.35) and T. cruzi – P249 (nk = 0.34)) and one arthropod (Amblyomma spp. – P9 (nk = 0.30)). Regarding centrality, the values ranged from 0 to 0.18, with the three highest values being two protozoa (T. evansi – P252 (BC = 0.18) and T. cruzi – P249 (BC = 0.12)—we observed 29 hosts infected or co-infected by these parasites (Fig. 1)) and one host (A. planirostris – H4 (BC = 0.17)—host that is closely linked with other bat parasites and found infected by T. cruzi – P249 and Leishmania braziliensis – P237 (Fig. 1)).
Discussion
Our data showed the formation of modules with nestedness patterns within the modules, a structure that has already been discussed in host–parasite interaction networks (Santos and Sano 2022a, b). When analyzing the complete topology of the network, we observed a pattern of two large modules subdivided mainly by hosts, one for bats and another for other mammals’ species. In addition to these two large modules, our analysis showed the presence of 14 modules with a large pattern nested within the modules. This type of network architecture is called compound topology (Lewinsohn et al. 2006). These modules are formed due to the high degree of connection between the nodes present in the modules that belong in relation to other modules (Beckett 2016). Some empirical studies have shown the existence of this topology in pollination networks, seed dispersal, multilayer plant–animal interactions, and host–parasite interactions (Bezerra et al. 2009; Flores et al. 2013; Mello et al. 2019; Sarmento et al. 2014). Furthermore, theoretical studies have confirmed this topology in simulated host–parasite networks (Beckett 2016; Santos and Sano 2022a), as observed in our results.
The modular characteristics of networks formed by interspecific interactions (host–parasite) are the result of ecological and coevolutionary processes that shape different patterns (Morris et al. 2014; Júnior et al. 2020). The integrative specialization hypothesis (IHS), a mechanism by which these compound topologies in interaction networks can be explained, assumes that adaptations that improve a consumer’s performance in exploiting a given resource will also tend to help them to exploit similar resources (within modules), but will represent poor adaptations for exploiting different resources (outside their modules) (Cardoso et al. 2021). These factors are associated with greater stability at the community level with few variations in space and time (Júnior et al. 2020; Santos and Sano 2022a). In addition, other factors can structure host–parasite networks, such as the ability of parasites to locate multiple hosts or to deal with behavioral (spatial and/or temporal) or host immune responses (Morris et al. 2014; Júnior et al. 2020).
Although our network presents formations of modules, we observed key nodes that interact with many species present in other modules, as observed in other studies on host–parasite interactions (Santos and Sano 2022a, b; Alcantara et al. 2022). These modules were connected mainly by the multi-host parasites T. cruzi and T. evansi, confirming the important role of these species in the topology of the network—highest normalization and centrality values—by infecting several species of hosts. Indeed, species with a higher centrality play a more important role in networks (Mello et al. 2013). Among studied hosts species, A. planirostris plays a central role in the network, which presents specific bat parasites and infections by T. cruzi and L. braziliensis, connecting the two parts of the network. Bats play an important role in parasitic networks since these flying mammals have a wide geographic distribution, have high diversity, and are found in different habitats harboring a wide variety of parasites (Alcantara et al. 2022). Additionally, bats often live in large colonies, which can facilitate the transmission of parasites between them, such as A. planirostris (Alcantara et al. 2022; Urbieta et al. 2022). Another important metric for the transmission cycles is the normalized degree, and the species that showed a higher nk were the hosts that also had a high abundance in the Pantanal (e.g., T. fosteri, Hydrochoerus hydrochaeris, and Sus scrofa) (Desbiez et al. 2010; de Macedo et al. 2022a, b). More abundant species may have higher parasite encounter rates and contribute more to parasite spillover than hosts with low abundance (Cardoso et al. 2021).
Most of the parasite and host has a species role of ultraperipheral and peripheral vertices, showing multiples connections with their modules, but less specificity in the network. The low specificity between parasite–host species could be due to the majority of multi-host parasites species. Their detection may be related to a set of biotic and abiotic characteristics specific for a given parasite to occur, as vegetation density, diversity and density of parasites, vectors and hosts, rainfall, wind, sunlight, humidity, heat, and cold (Santos et al. 2022). It may also be due the lack of studies to determine parasite richness in hosts in the Pantanal. The IHS hypothesis also proposes that specialization is driven by its costs and benefits. A specialist parasite can exploit resources that are unavailable to generalists and/or dispend less energy to access these resources; however, the specialist parasite is subject to a greater risk of extinction if the resource becomes scarce or if the host dies (Felix et al. 2022a, b). We observed that the hosts’ species that present a high degree of connection with other modules throughout the network, as observed with the parameters of normality, are species that are abundant in the Pantanal (de Macedo et al. 2022a, b; Desbiez et al. 2010), mainly the base hosts of the food chain (C. laticeps, D. azarae, T. fosteri, and T. pecari) for the main predators in the region (C. thous, L. pardalis, N. nasua, and P. onca) (Bianchi et al. 2014; Porfirio et al. 2017), indicating that these hosts species can quickly obtain and transmit parasites in the network. In fact, C. thous, L. pardalis, and N. nasua shared T. evansi and T. cruzi with some prey species (such as C. laticeps, D. azarae, and T. fosteri), and P. onca shared some parasite species (Leptospira spp., T. gondii, and Ehrlichia spp.) with C. laticeps, T. fosteri, and T. pecari, demonstrating a complex network that encompasses mainly oral transmission by predation. In fact, the spread of parasites from prey to predators through a mechanism known as trophic transmission has already been described in the T. evansi and T. cruzi transmission cycles in the Pantanal (Herrera et al. 2011). The loss of the hub host can generate secondary interaction losses, but this does not necessarily mean local extinctions, it just means that the lost species no longer participate in the interaction system. Furthermore, the loss of connectors can lead to changes in network structure, such as fragmentation, mainly in modular networks (Mello et al. 2013). Therefore, identifying connectors is as crucial as identifying hubs for understanding transmission cycles.
In addition, we observed many species of ectoparasites (eight ticks and two fleas) with lack of specificity in result of their ecological roles as hubs in the network. The observed interactions among all these modules can be directly linked to the epidemiological characteristics of some bacteria (Anaplasma spp., Ehrlichia spp., M. avium, R. amblyommatis, R. bellii, R. felis, R. parkeri, R. rhipicephali, and R. rickettsii) and protozoa (Hepatozoon spp. and H. felis), classified as connection hubs, that are transmitted by ticks (Demoner et al. 2013; de Sousa et al. 2017; Gonçalves et al. 2020, 2021; Ramírez-Hernández et al. 2020). Two other genera of multi-host bacteria were considered hubs in our analyses: Brucella and Leptospira. Brucella spp. are enzootic in the Pantanal, which is maintained by a complex reservoir system that includes domestic (cattle and dogs) and wildlife species such as Ozotoceros bezoarticus, S. scrofa, Tayassu peccari, N. nasua, C. thous, P. onca, Dasypus novemcintus, Cabassous unicinctus, E. sexcinctus, Priodontes maximus, M. tridactyla, and H. hydrochaeris (de Macedo et al. 2022a, b). Leptospira is also transmitted by environmental contamination, however is profusely eliminated by urine of infected rodents and carnivores.
Regarding helminths, we highlight three nodes of parasites: (i) W. decorate, classified as provincial hubs (most interactions within their module) because it was detected in H. hydrochaeris (that formed a module with exclusive parasites), and in Coendou prehensilis, a host that presented infection only by this parasite; (ii) Physaloptera spp. and (iii) Trichuris spp., both classified as non-hub connector vertices, with infections detected in modules other than their main modules, showing a more generalist characteristic. Host feeding is an important factor in increasing exposure to helminth infections (Dallas et al. 2019; Cardoso et al. 2021) and many helminths can be acquired through contact with the immature infectious stages present in the environment or through the consumption of parasitized intermediate hosts (Leung and Koprivnikar 2019; Cardoso et al. 2021).
Conclusions
Parasitic infections can be influenced by several factors including interactions between hosts, their parasites and the environment in which this system is inserted (Urbieta et al. 2022). Network studies can help to better understand these complex interactions, allowing the identification of patterns and interactions between different hosts and their parasite species, as they allow the analysis of the complexity of interactions and how these interactions affect the transmission (Santos and Sano 2022a, b; Alcantara et al. 2022). Thus, it is possible to identify which host species are most important in the transmission and maintenance of a given parasite, as well as to understand how changes in host populations and the environment can affect the dynamics of parasitic infections. In addition, these studies provide valuable information for the development of effective strategies for the control and prevention of parasitic diseases.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Alcantara DMC, Ikeda P, Souza CS et al (2022) Multilayer networks assisting to untangle direct and indirect pathogen transmission in bats. Microb Ecol. https://doi.org/10.1007/s00248-022-02108-3
Almeida-Neto M, Ulrich W (2011) A straightforward computational approach for measuring nestedness using quantitative matrices. Environ Model Softw 26:173–178. https://doi.org/10.1016/j.envsoft.2010.08.003
Beckett SJ (2016) Improved community detection in weighted bipartite networks. R Soc Open Sci 3:140536. https://doi.org/10.1098/rsos.140536
Bezerra ELS, Machado IC, Mello MAR (2009) Pollination networks of oil-flowers: a tiny world within the smallest of all worlds. J Anim Ecol 78:1096–1101. https://doi.org/10.1111/j.1365-2656.2009.01567.x
Bezerra RHS, Bocchiglieri A (2022) Bat–parasite interaction networks in urban green areas in northeastern Brazil. Parasitology 1–7. https://doi.org/10.1017/S0031182022001718
Bianchi RD, Campos RC, Xavier-Filho NL et al (2014) Intraspecific, interspecific, and seasonal differences in the diet of three mid-sized carnivores in a large neotropical wetland. Acta Theriol (warsz) 59:13–23. https://doi.org/10.1007/s13364-013-0137-x
Blüthgen N (2010) Why network analysis is often disconnected from community ecology: a critique and an ecologist’s guide. Basic Appl Ecol 11:185–195. https://doi.org/10.1016/j.baae.2010.01.001
Cardoso TS, de Andreazzi CS, Junior AM, Gentile R (2021) Functional traits shape small mammal-helminth network: patterns and processes in species interactions. Parasitology 148:947–955. https://doi.org/10.1017/S0031182021000640
Csardi G, Nepusz T (2006) The igraph software package for complex network research. InterJournal, Complex Systems 1695:1–9
Dallas TA, Han BA, Nunn CL et al (2019) Host traits associated with species roles in parasite sharing networks. Oikos 128:23–32. https://doi.org/10.1111/oik.05602
de Macedo GC, Herrera HM, de Oliveira Porfírio GE et al (2022a) Brucellosis in the Brazilian Pantanal wetland: threat to animal production and wildlife conservation. Braz J Microbiol 53:2287–2297. https://doi.org/10.1007/s42770-022-00831-0
de Macedo GC, Herrera HM, Jansen AM et al (2022b) Saúde e conservação dos animais silvestres na natureza. Boletim do Museu Paraense Emílio Goeldi - Ciências Naturais 16:459–526. https://doi.org/10.46357/bcnaturais.v16i3.806
de Sousa KCM, Calchi AC, Herrera HM et al (2017) Anaplasmataceae agents among wild mammals and ectoparasites in Brazil. Epidemiol Infect 145:3424–3437. https://doi.org/10.1017/S095026881700245X
Delmas E, Besson M, Brice M-H et al (2019) Analysing ecological networks of species interactions. Biol Rev 94:16–36. https://doi.org/10.1111/brv.12433
Demoner LC, Rubini AS, Paduan KS et al (2013) Investigation of tick vectors of Hepatozoon canis in Brazil. Ticks Tick Borne Dis 4:542–546. https://doi.org/10.1016/j.ttbdis.2013.07.006
Desbiez ALJ, Bodmer RE, Tomas WM (2010) Mammalian densities in a neotropical wetland subject to extreme climatic events. Biotropica 42:372–378. https://doi.org/10.1111/j.1744-7429.2009.00601.x
Dormann CF (2011) How to be a specialist? Quantifying specialisation in pollination networks. Network Biology 1:1–20
Dormann CF, Gruber B, Fründ J (2008) Introducing the bipartite package: analysing ecological networks. R News 8:8–11
Felix GM, Pinheiro RBP, Poulin R, et al (2022a) The compound topology of host–parasite networks is explained by the integrative hypothesis of specialization. Oikos 2022:. https://doi.org/10.1111/oik.08462
Felix GM, Pinheiro RBP, Jorge LR, et al (2022b). A framework for hierarchical compound topologies in species interaction networks. Oikos e09538, https://doi.org/10.1111/oik.09538
Flores CO, Valverde S, Weitz JS (2013) Multi-scale structure and geographic drivers of cross-infection within marine bacteria and phages. ISME J 7:520–532. https://doi.org/10.1038/ismej.2012.135
Freeman LC (1977) A set of measures of centrality based on betweenness. Sociometry 40:35. https://doi.org/10.2307/3033543
Freeman LC (1978) Centrality in social networks conceptual clarification. Soc Networks 1:215–239. https://doi.org/10.1016/0378-8733(78)90021-7
Gonçalves LR, Herrera HM, Nantes WAG et al (2020) Genetic diversity and lack of molecular evidence for hemoplasma cross-species transmission between wild and synanthropic mammals from Central-Western Brazil. Acta Trop 203:105303. https://doi.org/10.1016/j.actatropica.2019.105303
Gonçalves LR, Paludo G, Bisol TB et al (2021) Molecular detection of piroplasmids in synanthropic rodents, marsupials, and associated ticks from Brazil, with phylogenetic inference of a putative novel Babesia sp. from white-eared opossum (Didelphis albiventris). Parasitol Res 120:3537–3546. https://doi.org/10.1007/s00436-021-07284-8
Guimarães PR (2020) The structure of ecological networks across levels of organization. Annu Rev Ecol Evol Syst 51:433–460. https://doi.org/10.1146/annurev-ecolsys-012220-120819
Hembry DH, Raimundo RLG, Newman EA et al (2018) Does biological intimacy shape ecological network structure? A test using a brood pollination mutualism on continental and oceanic islands. J Anim Ecol 87:1160–1171. https://doi.org/10.1111/1365-2656.12841
Herrera HM, Dávila AMR, Norek A et al (2004) Enzootiology of Trypanosoma evansi in Pantanal, Brazil. Vet Parasitol 125:263–275. https://doi.org/10.1016/j.vetpar.2004.07.013
Herrera HM, Rademaker V, Abreu UGP et al (2007) Variables that modulate the spatial distribution of Trypanosoma cruzi and Trypanosoma evansi in the Brazilian Pantanal. Acta Trop 102:55–62. https://doi.org/10.1016/j.actatropica.2007.03.001
Herrera HM, Rocha FL, Lisboa CV et al (2011) Food web connections and the transmission cycles of Trypanosoma cruzi and Trypanosoma evansi (Kinetoplastida, Trypanosomatidae) in the Pantanal Region, Brazil. Trans R Soc Trop Med Hyg 105:380–387. https://doi.org/10.1016/j.trstmh.2011.04.008
Júnior LDF, De Araújo WS, Falcão LAD (2020) Structure of the interaction networks between bats (Mammalia: Chiroptera) and ectoparasite flies (Diptera: Streblidae, Nycteribiidae) on a latitudinal gradient. Acta Chiropt 22:187. https://doi.org/10.3161/15081109ACC2020.22.1.018
Leung TLF, Koprivnikar J (2019) Your infections are what you eat: how host ecology shapes the helminth parasite communities of lizards. J Anim Ecol 88:416–426. https://doi.org/10.1111/1365-2656.12934
Lewinsohn TM, Inácio Prado P, Jordano P et al (2006) Structure in plant-animal interaction assemblages. Oikos 113:174–184. https://doi.org/10.1111/j.0030-1299.2006.14583.x
Martín González AM, Dalsgaard B, Olesen JM (2010) Centrality measures and the importance of generalist species in pollination networks. Ecol Complex 7:36–43. https://doi.org/10.1016/j.ecocom.2009.03.008
Mello MAR, Bezerra ELS, Machado IC (2013) Functional roles of Centridini oil bees and Malpighiaceae oil flowers in biome-wide pollination networks. Biotropica 45:45–53. https://doi.org/10.1111/j.1744-7429.2012.00899.x
Mello MAR, Rodrigues FA, Costa LDF et al (2015) Keystone species in seed dispersal networks are mainly determined by dietary specialization. Oikos 124:1031–1039. https://doi.org/10.1111/oik.01613
Mello MAR, Felix GM, Pinheiro RBP et al (2019) Insights into the assembly rules of a continent-wide multilayer network. Nat Ecol Evol 3:1525–1532. https://doi.org/10.1038/s41559-019-1002-3
Morris RJ, Gripenberg S, Lewis OT, Roslin T (2014) Antagonistic interaction networks are structured independently of latitude and host guild. Ecol Lett 17:340–349. https://doi.org/10.1111/ele.12235
Pinheiro RBP, Felix GMF, Dormann CF, Mello MAR (2019) A new model explaining the origin of different topologies in interaction networks. Ecology 100:. https://doi.org/10.1002/ecy.2796
Pinheiro RBP, Felix GMF, Lewinsohn TM (2022) Hierarchical compound topology uncovers complex structure of species interaction networks. J Anim Ecol 91:2248–2260. https://doi.org/10.1111/1365-2656.13806
Porfirio G, Sarmento P, Foster V, Fonseca C (2017) Activity patterns of jaguars and pumas and their relationship to those of their potential prey in the Brazilian Pantanal. Mammalia 81:401–404. https://doi.org/10.1515/mammalia-2015-0175
Porfirio GEO, Santos FM, de Macedo GC et al (2018) Maintenance of Trypanosoma cruzi. T evansi and Leishmania spp by domestic dogs and wild mammals in a rural settlement in Brazil-Bolivian border. Int J Parasitol Parasites Wildl 7:398–404
Poulin R (2007) Evolutionary ecology of parasites. Princetin University, New Jersey, p 342
Queiroz JA, Diniz UM, Vázquez DP et al (2021) Bats and hawkmoths form mixed modules with flowering plants in a nocturnal interaction network. Biotropica 53:596–607. https://doi.org/10.1111/btp.12902
R Core Team (2022) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria
Rademaker V, Herrera HM, Raffel TR et al (2009) What is the role of small rodents in the transmission cycle of Trypanosoma cruzi and Trypanosoma evansi (Kinetoplastida Trypanosomatidae)? A study case in the Brazilian Pantanal. Acta Trop 111:102–107. https://doi.org/10.1016/j.actatropica.2009.02.006
Ramírez-Hernández A, Uchoa F, de Azevedo Serpa MC (2020) Capybaras (Hydrochoerus hydrochaeris) as amplifying hosts of Rickettsia rickettsii to Amblyomma sculptum ticks: evaluation during primary and subsequent exposures to R. rickettsii infection. Ticks Tick Borne Dis 11:101463. https://doi.org/10.1016/j.ttbdis.2020.101463
Santos FM, Sano NY (2022a) Functional role of small mammals in protozoan transmission networks in Brazilian biomes. Hystrix, the Italian Journal of Mammalogy 33:107–112. https://doi.org/10.4404/hystrix-00495-2021
Santos FM, Sano NY (2022b) Parasite association in non-volant small mammals in Brazil. Community Ecol 23:129–136. https://doi.org/10.1007/s42974-022-00080-z
Santos FM, de Sousa KCM, Sano NY et al (2021) Relationships between vector-borne parasites and free-living mammals at the Brazilian Pantanal. Parasitol Res 120:1003–1010. https://doi.org/10.1007/s00436-020-07028-0
Santos FM, Sano NY, Liberal SC et al (2022) The influence of abiotic and biotic variables on the patent parasitemias of Trypanosoma spp. in Thrichomys fosteri (Rodentia: Echimyidae) in the southern Pantanal. Parasitol Res 121:1719–1724. https://doi.org/10.1007/s00436-022-07522-7
Sarmento R, Alves-Costa CP, Ayub A, Mello MAR (2014) Partitioning of seed dispersal services between birds and bats in a fragment of the Brazilian Atlantic Forest. Zoologia (curitiba) 31:245–255. https://doi.org/10.1590/S1984-46702014000300006
Urbieta GL, Graciolli G, da Cunha TV (2022) Review of studies about bat-fly interactions inside roosts, with observations on partnership patterns for publications. Parasitol Res 121:3051–3061. https://doi.org/10.1007/s00436-022-07635-z
Vázquez DP, Melián CJ, Williams NM et al (2007) Species abundance and asymmetric interaction strength in ecological networks. Oikos 116:1120–1127. https://doi.org/10.1111/j.2007.0030-1299.15828.x
Vitorino BD, da Frota AVB, Maruyama PK et al (2022) Influence of sampling methods on the description of a neotropical seed dispersal network. Acta Oecologica 114:103805. https://doi.org/10.1016/j.actao.2021.103805
Acknowledgements
We thank the institutions that provide the structure to carried out our work: Federal University of Mato Grosso do Sul, Dom Bosco Catholic University and Homem Pantaneiro Institution.
Funding
This work was supported by the following Brazilian research agencies: Coordenação de Aperfeiçoamento de Nível Superior (CAPES – Finance code 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT). FMS received a fellowship from CAPES (88887.369261/2019–00). NYS received a fellowship from Project Rede Pantanal from the Ministry of Science, Technology and Innovations of Brazil (FINEP Grant No. 01.20.0201.00). HMH is a fellowship researcher from CNPq (Productivity Scholarship-Level 2).
Author information
Authors and Affiliations
Contributions
The conceptualization started by NYS, HMH, and FMS, while data curation were carried out by GEOP and GCM. Formal analysis was made by NYS and FMS, where Fig. 1 was made by FMS, and tables were made and formatted by NYS, with some advises of HMH and GEOP. HMH was responsible for the funding acquisition. The main manuscript was written by NYS, with editing of FMS. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Elizabeth Warburton.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sano, N.Y., Herrera, H.M., Porfirio, G.E.d. et al. Exploring interactions between parasites and their hosts in the Pantanal floodplain using an ecological network approach. Parasitol Res 123, 128 (2024). https://doi.org/10.1007/s00436-024-08140-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-024-08140-1