Abstract
Conserving freshwaters in West Anatolia requires biomonitoring tools to assess the ecological status of surface water bodies threatened by anthropogenic pollution. This study was carried out to determine water quality of Demirci Stream based on biotic indices. In this seasonal study, a total of seven sampling stations were determined. Asterics software was used to determine biotic and diversity index scores. Pearson’s based correlations were applied in order to determine the proper biotic indices. Relationships between macrobenthic orders and physicochemical variables were revealed by Redundancy Analysis (RDA). Eleven taxonomic groups were found in Demirci Stream consisting of Hygrophlia, Arhynchobdellida, Isopoda, Tubificida, Amphipoda, Ephemeroptera, Plecoptera, Trichoptera, Diptera, Coleoptera and Hemiptera. As a result of this research, the sampling pints 1, 2, 3 and 4 were of good water quality class while sampling points 5, 6 and 7 were polluted. Arhynchobdellida, Tubificida and Diptera orders showed positive correlations with BOI5, EC, Cl−, TDS, ToC, NH4-N, NO2-N and NO3-N variables, while negatively correlated with DO, Sat-O2 and pH. SI, BMWP, ASPT and BBI were more proper than the FBI index to determine the water quality of Demirci Stream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid growth of the world population, migrations, urbanization, industrialization, food crisis and the desire to reach better living standards affect freshwater ecosystems in an irreversible way. (Zhang et al. 2019; Sabha et al. 2022). The European Union Water Framework Directive (EU WFD) is the primary legal regulation for the protection of water resources. The WFD proposes the strategy of assessing environmental and ecological changes with a biological monitoring approach based on bioindicator organisms (Council of European Commission 2000).
Rapid changes in water chemistry, water quality and physical habitat can cause the survival of the most resistant species and the extinction of the sensitive species over a period of time (Kazancı et al. 2014). Therefore, the composition of macroinvertebrate species in a water body forms the basis of biological monitoring in determining pollution or morphological deterioration in that water body (Mahler and Barber 2017).
Many biotic indices (BMWP, ASPT, BBI etc.) have been developed by European countries using macroinvertebrate groups (Yorulmaz et al. 2015). Various studies have been carried out to evaluate the water quality of the freshwaters in Turkey through these developed indices (Akyildiz and Duran 2021; Ertaş et al. 2021a, b; Ertaş and Yorulmaz 2021; Öztürk 2021, Odabaşı et al. 2022). However, comparisons of these methods is relatively limited (Yorulmaz et al. 2015; Zeybek et al. 2014; Zeybek 2017; Ertaş and Yorulmaz 2021; Yorulmaz and Ertaş 2021; Ertaş et al. 2021a, c, 2022).
The Gediz River Basin is one of the most important freshwater ecosystems of Turkey. The Gediz River is the second largest stream flowing into the Aegean Sea after the Great Meander River. The increasing population, agricultural and industrial activities of the settlements around the Gediz River Basin have negative effects on the water quality of the river (Ünlü 2013; Şenol et al. 2016). However, the pollution degree of the streams which drain to the Gediz River have not been researched yet. This study aims to assess the water quality of the Demirci Stream which is an important branch of the Gediz River Basin and to determine the comparative performance of biotic indices.
Materials and methods
Study area
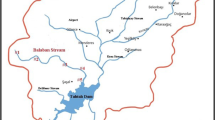
This study was carried out in the Demirci Stream in the Gediz River Basin of West Anatolia, Turkey. The length of the Demirci Stream is 50 km. The Demirci Stream emerges from the Demirci-Simav Mountain (1,475 m a.s.l.), northeast to the Gediz River (Fig. 1).
The Demirci Stream is exposed to the negative effects of polluting factors such as hydroelectric power plant, trout farm, carpet factory and domestic wastes. Sampling stations were chosen according to criteria for selecting operational monitoring sites given in WFD Annex V 1.3.2. (WFD 2000). This research was carried out seasonally (winter – December 2020, spring - April 2021, summer - July 2021, autumn - October 2021) over a year in seven sampling stations (Table 1).
Benthic macroinvertebrates were sampled by using a classic bottom kick net of a 50 × 30 cm size and 250 μm mesh according to the literature (AQEM Consortium 2002). Collected organisms were immediately fixed in 4% formalin in the field and then transferred to 70% ethyl alcohol. The benthic macroinvertebrates were first sorted out, then identified to the lowest possible taxon (species, genus or family) and counted under a stereomicroscope.
Stations 1 and 2 were selected in the headwaters of the stream which was formed by intercalation of siltstone and claystone formation. In these stations, the stream bed is narrow, the area around the bed is wooded and no contaminants were found. Station 3 was geologically in the same area as the first two sampling stations. In this station, the stream bed is narrow and the riparian zone around the stream is covered with bushes and dwarf trees. In stations 4 and 5, the stream bed is wide and the water flow of the stream is low. The stations 6 and 7 were geologically almost in the same area. Station 7 is located in the part of the drain of the Demirci Stream into the Gediz River.
.
Data analysis
Physicochemical parameters were measured by using CyberScan Series 600 Waterproof- Portable Meter and Oxi 315i/ SET WTW Oxygenmeter. The evaluation of water quality based on physicochemical parameters was made according to Klee’s method (Klee 1991).
Saprobe Index (SI), several modifications of Biological Monitoring Working Party (BMWP) [BMWP Original (BMWP-O), BMWP Spanish (BMWP-S), BMWP Hungarian (BMWP-H), BMWP Polish (BMWP-P), BMWP Czech (BMWP-C)], several modifications of Average Score Per Taxon (ASPT) [ASPT Original (ASPT-O), ASPT Hungarian (ASPT-H), ASPT Czech (ASPT-C)], Belgian Biotic Index (BBI), Family Biotic Index (FBI), Simpson’s (SDI), Shannon-Wiener (SWDI) and Margalef (MDI) diversity indices were applied on benthic macroinvertebrate data set by using ASTERICS Software (AQEM Consortium 2002). Pearson’s based correlation analyses between the biotic indices were performed by using SPSS version 20.0. Relationships between macrobenthic orders and physicochemical variables were revealed by Redundancy Analysis (RDA) on CANOCO-4.5 software. The compatibility of the RDA data has been tested with DCA (Detrended Correspondence Analysis) (ter Braak 1988).
Results and discussion
The range, mean and standard deviations of physicochemical variables are summarized in Table 2. According to the One Way ANOVA test, all physicochemical parameters differed significantly between stations (p < 0.05), but there was no significant difference between seasonal groups.
A total of 45 taxa and 7,444 specimens of benthic invertebrates were revealed in the stream (additional data are given in Online resource 1). Among all taxa, ten belonged to the order Ephemeroptera (Insecta), eight to the order Trichoptera (Insecta), five each to the orders Hygrophlia (Gastropoda) and Coleoptera (Insecta), six to the order Diptera (Insecta), four to the order Plecoptera (Insecta), two to the orders Tubificida (Oligochaeta) and Hemiptera (Insecta), and one each to the orders Arhynchobdellida (Hirudinea), Amphipoda (Malacostraca) and Isopoda (Malacostraca). The most dominant group was Insecta. Different researchers found similar results in different freshwater ecosystem (Guellaf and Kettani 2021; Kumari and Maiti 2020; Mehrjo et al. 2020; Sotomayor et al. 2020; Guellaf et al. 2021; Kabore et al. 2022). In spring. Trichoptera was the most dominant group (37.8%) and Amphipoda was the second dominant group (34.1%). On summer, Isopoda was the most dominant group (44.4%) and 0 Plecoptera was the second dominant group (27.3%). In autumn, Arhynchobdellida was the most dominant group (40.4%) and Hemiptera was second dominant group (39.2%). In winter, Plecoptera was the most dominant group (44.4%) and Diptera was the second dominant group (25.1%). The highest number of organisms was determined at the station 1 (1,396), and the lowest number at the station 7 (803) The lowest number of organisms was determined in winter, while the highest number was found in spring (Fig. 2). The maximum density of benthic fauna was observed during spring, this can be related to the availability of phytoplankton population in the form of food supply. Spring drifts that drive the vegetation were also factors affecting the abundance of benthic fauna. On the other hand, decline in the density of benthic fauna during winter may be due to the increased load of suspended solids, reduced transparency and increased water flow.
Dominancy of benthic macroinvertebrate species at the stations are shown in Fig. 3. Baetis sp., Baetis rhodani (Pictet, 1843) and Simulium sp. were dominant in stations 1 and 2. Gammarus sp., Baetis sp. and Ibisia marginata (Fabricius, 1781) were dominant in station 3. Simulium sp., Gammarus sp. and Chironomus sp. were dominant in stations 4 and 5. Simulium sp., Limnodrilus hoffmeisteri (Claparede, 1862) and Tubifex tubifex (O. F. Müller, 1774) were dominant in station 6. Chironomus sp., L. hoffmeisteri and T. tubifex were dominant in station 7.
Ephemeroptera- Plecoptera- Trichoptera (EPT) taxa are the main indicator groups of unpolluted waters. The EPT fauna of the freshwaters showed completely parallel results with the water quality of the stations. The accordance between the water quality classes determined according to physicochemical measurements and the dominance of the EPT species found in these freshwaters are shown very clearly. It is indicated that Oligochaeta species are dominant in polluted waters (Ode et al. 2005; DeWalt et al. 2012; Kazancı et al. 2014; Öztürk et al. 2022). In our study, the highest EPT-taxa (%) values were obtained in upstream stations in all seasons, while the lowest EPT-taxa (%) values were obtained in downstream stations (Fig. 4).
Roline (1988), Clement et al. (1992), and Stoyanova et al. (2014) stated that Plecoptera and many Ephemeroptera species changed their habitat according to pollution The presence of EPT taxa in the upstream stations can be explained by the fact that agricultural and other anthropogenic activities around the Demirci Stream have not yet caused pollution pressure. Various researchers observed that EPT taxa were the most dominant in upstream sampling stations of their studies (Aazami et al. 2020; Mehrjo et al. 2020; Guellaf et al. 2021). Gammarus sp. was dominant in stations 3, 4 and 5 in the Demirci Stream. Gammarus sp., which belongs to the group of Amphipoda is found in low polluted river sections (Meyer 1987). Chironomus sp. was dominant in stations 4, 5, 6 and 7. Chironomus species are indicators for polysaprobic (heavy polluted) aquatic systems (Shaha and Pandit 2022; Shahidi-Hakak et al. 2022). According to Moisan and Pelletier (2008), the tolerance range of these organisms is high. They can be found at low or high DO (mg L− 1) concentrations, Sat O2 (%) and T (°C). The lowest benthic maroinvertebrate number was found in stations 6 and 7. Diptera was the most dominant taxon in stations 6 and 7, Chironomidae and Oligochaeta followed. Pollution tolerance ranges of these organisms are high (Popovic et al. 2022; Rosa et al. 2022). They can be found at low or high DO (mg L− 1) conentrations, Sat O2 (%) and T (°C). There is a trout farm around the stations 6 and 7 of the Demirci Stream. According to physicochemical variables, the organic matter is abundant there. Tubifex tubifex are abundant in these stations where the organic pollution pressures are high in the stream. Existing abundance of the organic matter is favorable for benthic macroinvertebrates such as Diptera and Oligochaeta (Brysiewicz et al. 2022).
Depending on changes in the chemical properties of the water, many parameters such as temperature, DO and pH can increase or decrease. The increase in water temperature is an environmental problem that speeds up the growth of aquatic plants and algae, increases the content of dissolved nutrients in the water, and increases the primary productivity of the ecosystem, generally leading to the depletion of oxygen by increasing abundance of bacteria. This situation causes eutrophication and deterioration in water quality and has negative consequences on many aquatic organisms (Pineda et al. 2022). As a result of these changes, many structural and distributional features of the macrobenthic fauna living in the aquatic system, such as their growth rates, metabolism and productivity, are affected. Although the tolerance of these living groups towards temperature varies according to the species, very few of them can show a high tolerance to temperature (Hussain and Pandit 2012).
While it was observed that the variables related to pollution such as ToC, EC, Cl−, TDS, NH4-N, NO2-N, NO3-N, TN and TP increased noticeably at stations 6 and 7, it is seen that the variables that are indicators of clean conditions such as DO and Sat O2 have decreased. This is due to the close proximity of these stations to the downstream region and, accordingly, the changes in the chemical properties of the water (decreased flow rate, widening of the stream bed and increase of organic pollution due to the ingress of organic debris into the water). These changes were less common at other stations. According to Hynes (1994), presence of highly tolerant organisms usually indicates poor water quality. In stations 6 and 7, the macroinvertebrate samples consisted from Oligochaeta worms and Diptera, which were present in high abundances. They are known as tolerant to bad or poor water quality and can tolerate heavy to extreme pollution. According to Hachi et al. (2022), many species of Oligochaeta are tolerant to low oxygen concentration and can live in anoxic conditions.
The increased number of species in station 5 occured as the result of increased water level and flow velocity. Due to this improvement in environmental conditions in this station, the number of taxa further increased. In total 28 families were present, 10 belonging to the sensitive and semi-sensitive EPT group, and the rest consisted of semi tolerant-tolerant organisms (Baetidae, Chironomidae, Blephariceridae, Tubificidae, Athericidae, Tabanidae, Corixidae and Gerridae). In conclusion, the ecological conditions of the Demirci Stream indicate that the stream is disturbed by anthropogenic activities.
Table 3 shows the biological quality scores and quality classes in the Demirci Stream. According to Klee’s method, the water quality class in stations 1, 2 and 3 is oligo-betamesosaprob (Class I-II), in station 4 it is betamesosaprob (Class II), in station 5 beta-alphamesosaprob (Class II-III), in station 6 alphamesosaprob (Class III) and in station 7 polysaprob (Class IV) in summer and autumn. In winter and spring, the water quality class in stations 1 and 2 is oligosaprob (Class I), in station 3 oligo-betamesosaprob (Class I-II), in stations 4 and 5 betamesosaprob (Class II), in station 6 alphamesosaprob (Class III), and in station 7 it is alpha-polysaprob (Class III-IV).
All diversity indices showed the highest values in station 1, whereas the lowest values were registered in station 7. High species diversity at the upstream stations indicates unpolluted conditions whereas low species diversity in stations 6 and 7 indicates environmental stress. The sampling stations 6 and 7 are heavily disturbed due to many domestic wastes discharged in this part of the stream.
According to the BMWP- S, BMWP- H, BMWP- P and BMWP- C, the highest score values were found in the stations 1, 2 and 3. According to the BMWP- O, the highest score value was found in the station 5. According to all versions of ASPT, the highest score values were found in stations 1, 2 and 3, while the lowest score value was found in station 7. According to the BBI, the water quality class is unpolluted in stations 1, 2, 3, 4 and 5 (Class I) while stations 6 and 7 are lightly contaminated (Class II). According to the FBI, the water quality class is lightly contaminated in stations 1 and 2 (Class II) while stations 3, 4, 5, 6 and 7 are critically contaminated (Class II-III). According to the SI, the water quality class is oligosaprob/betamesosaprob in stations 1, 2 and 3 (Class I-II); betamesosaprob in stations 4 and 5 (Class II); α- mesosaprob in station 6 (Class III), and α- mesosaprob/ polysaprob in station 7 (Class III-IV). This index scores indicate that in the upstream stations, due to the distance with inhabited areas and lack of waste discharge, the water has a minimum human impact and is of high quality. In downstream stations, human activities become more intensive and they impact physical and chemical quality of the water in the Demirci Stream.
Among the applied indices, positive correlations were observed between BMWP (Original, Spanish, Hungarian, Czech and Polish versions) and ASPT (Original, Hungarian and Czech versions) (Table 4). A highly significant correlation was observed between the BMWP- O and BMWP- S (r-value 1.000, p ˂ 0.01). Another highly significant correlation was determined between BMWP- O, BMWP- H and BMWP- C (r-value 0.998, p ˂ 0.01); BMWP-S, BMWP- H and BMWP- C (r-value 0.998, p ˂ 0.01). All versions of BMWP were positively and significantly correlated with each other (p ˂ 0.01). All versions of ASPT indices were also positively and significantly correlated with each other (p ˂ 0.01). All versions of BMWP were positively and significantly correlated with the BBI, while they did not correlate with FBI. ASPT- O and ASPT- H were positively and signifiantly correlated with the BBI while they negatively and signifiantly correlated with FBI. The SI showed significantly negative correlation with all indies except BMWP- P and MDI. All versions of BMWP showed positive significant correlations with all diversity indices.
According to DCA analysis, axis lengths were determined below three. This situation made it suitable for RDA analysis, which is a linear method. RDA analysis explained 75.2% of the variations between 11 sets and 13 ecological variables (Axis 2). Arhynchobdellida, Tubificida and Diptera orders showed positive correlations with BOI5, EC, Cl−, TDS, ToC, NH4-N, NO2-N and NO3-N variables, while negatively correlated with DO, Sa-O2 and pH (Fig. 5; Table 5). Although Tubificida includes different species living in clean and polluted environments, most of the species are found in environments with high organic pollution (Atanackovic et al. 2011). Taxa belonging to the order Diptera can be found in all environments from clean water to polluted water (Ertaş et al. 2022). Many species belonging to the order Arhynchobdellida are tolerant to organic pollution and can survive for a long time in oxygen-free environments (Cılız and Kırkağaç 2022). The distributions of Ephemeroptera- Plecoptera- Trichoptera (EPT) and Coleoptera species are positively correlated to DO, Sa- O2 and pH. It has been stated in some studies that the EPT group is found in the regions of rivers with high oxygen content (Wallace et al. 1996; Kazancı 2014; Serdar and Verep 2018).
Conclusion
The Demirci Stream was chosen as a study area since the Gediz River Basin served as one of the pilot basins for WFD applications in Turkey. Based on foregoing analyses, it was clearly shown that water quality of the Demirci Stream directly correlated with human influences and in turn affected the distribution of benthic macroinvertebrate community. The main findings of this study were as follows: (1) SI, BMWP, ASPT and BBI were more proper than FBI index to determine the water quality of the Demirci Stream. (2) In order to protect the water quality of the surface water resources of the Gediz River basin, intermittent biological monitoring studies should be carried out. Strategic planning of underground dams in the region should be started urgently in order to prevent water scarcity in the basin and to provide year-round water to agricultural lands. (3) Rainwater and sewage systems should be separated from each other in the settlements of the Gediz River basin.
References
Aazami J, Maghsodlo H, Mira SS, Valikhani H (2020) Health evaluation of riverine ecosystems using aquatic macroinvertebrates: a case study of the Mohammad–Abad River, Iran. Int J Environ Sci Technol 17:2637–2644. https://doi.org/10.1007/s13762-020-02658-4
Akyıldız GK, Duran M (2021) Evaluation of the impact of heterogeneous environmental pollutants on benthic macroinvertebrates and water quality by long-term monitoring of the Buyuk Menderes River basin. Environ Monit Assess 193:280. https://doi.org/10.1007/s10661-021-08981-8
Atanacković A, Jakovčev-Todorović D, Simić V, Tubić B, Vasiljević B, Gačić Z, Paunović M (2011) Oligochaeta community of the main serbian waterways. Water Resour Manage 1:47–54
AQEM Consortium (2002) Manual for the application of the Aqem system: a comprehenssive method to assess european streams using benthic macroinvertebrates, developed for the purpose of the Water Framework Directive. Version 1. www.aqem.de/mains/products.php
Brysiewicz A, Czerniejewski P, Dąbrowski J, Formicki K (2022) Characterisation of benthic macroinvertebrate communities in small watercourses of the european Central Plains Ecoregion and the effect of different environmental factors. Animals 12(5):606. https://doi.org/10.3390/ani12050606
Cılız S, Kırkağaç M (2022) The assessment of diversity of benthic macroinvertebrates of a stream from the eastern black sea basin, Turkey. Menba J Fisheries Fac 8(2):59–68
Clements W, Cherry D, Van Hassel J (1992) Assessment of the impact of heavy metals on benthic communities at the Clinch River (Virginia): evaluation of an index of community sensitivity. Can J Fish Aquat Sci 49:1686–1694
Council of European Communities (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Official J Eur Communities 327(1):72Water Framework Directive (WFD)
DeWalt RE, Cao Y, Tweddale T, Gruubs SA, Hinz L, Pessino M, Robinson JL (2012) Ohio USA stoneflies (Insecta, Plecoptera): species richness estimation, distribution of functional niche traits, drainage affiliations, and relationships to other states. ZooKeys 178:1–26. https://doi.org/10.3897/zookeys.178.2616
Ertaş A, Yorulmaz B (2021) Assessing water quality in the Kelebek Stream branch (Gediz River Basin, West Anatolia of Turkey) using physicochemical and macroinvertebrate-based indices. Aquat Res 4(3):260–278. https://doi.org/10.3153/AR21020
Ertaş A, Boz T, Tüney-Kızılkaya İ (2021a) Comparative analysis of benthic macroinvertebrate-based biotic and diversity indices used to evaluate the water quality of Kozluoluk Stream (West Anatolia of Turkey). Community Ecol 22:381–390. https://doi.org/10.1007/s42974-021-00061-8
Ertaş A, Boz T, Tüney-Kızılkaya İ (2021b) Determination of benthic macroinvertebrate fauna and some physicochemical properties of Balaban Lake (Menderes- Izmir). Aquat Sci Eng 36(3):116–125. https://doi.org/10.26650/ASE2020821658
Ertaş A, Yaşartürk M, Boz T, Tüney Kızılkaya İ (2021c) Evaluation of the water quality of Karabal Stream (Gediz River, Turkey) and comparative performance of the used indices. Acta Aquat Turc 17(3):334–349. https://doi.org/10.22392/actaquatr.819579
Ertaş A, Yorulmaz B, Sukatar A (2022) Comparative analysis of biotic indices for assessment of water quality of Balaban Stream in West Anatolia, Turkey. Biologia 77:721–730. https://doi.org/10.1007/s11756-021-00992-7
Guellaf A, El Alami M, Kassout J, Errochdi S, Khadri O, Kettani K (2021) Diversity and ecology of aquatic insects (Ephemeroptera, Plecoptera and Trichoptera) in the Martil basin (Northwestern Morocco). Commun Ecol 22:331–350. https://doi.org/10.1007/s42974-021-00058-3
Guellaf A, Kettani K (2021) Assessing the ecological status using physico-chemical, bacteriological parameters and biotic indices of the Oued Martil River basin in northwestern Morocco. Biologia 76:585–598. https://doi.org/10.2478/s11756-020-00560-5
Hachi T, Hachi M, Essabiri H, Belghyti D, Khaffou M, Merimi I, Alnane T, Abba E (2022) Assessment and relationship between organic pollution parameters and aquatic bio-indicators (Wadi Tighza. Morocco). Materials Today: Proceedings https://doi.org/10.1016/j.matpr.2022.09.511
Hussain QA, Pandit AK (2012) Macroinvertebrates in streams: a review of some ecological factors. Int J Fish Aquac 4(7):114–123. https://doi.org/10.5897/IJFA11.045
Hynes H (1994) Historical perspective and future direction of biological monitoring of aquatic systems. In: Loeb SL, Spacie A (eds) Biological Monitoring of Aquatic Systems. Lewis Publishers, Boca Raton, pp 11–22
Kabore I, Oueda A, Moog O, Meulenbroek P, Tampo L, Bance V, Melcher AH (2022) A benthic invertebrates-based biotic index to assess the ecological status of West African Sahel Rivers, Burkina Faso. J Environ Manage 307:114503. https://doi.org/10.1016/j.jenvman.2022.114503
Kazancı N, Türkmen G, Başören Ö, Ekingen P, Bolat AH (2014) Assessment of the land use effects in Yeşilırmak River Basin by determining water quality and Yeşilırmak river-specific biotic index (Y-BMWP): II. Evaluation with biological methods by using benthic macroinvertebrates and updated Y-BMWP. Rev Hydrobiol 7(2):75–155
Klee O (1991) Angewandte Hydrobiologie. G. Theieme Verlag, 2. neubearbeitete und erweiterte Auflage, Stuttgart-New York
Kumari P, Maiti SK (2020) Bioassessment in the aquatic ecosystems of highly urbanized agglomeration in India: an application of physicochemical and macroinvertebrate-based indices. Ecol Indic 111:106053. https://doi.org/10.1016/j.ecolind.2019.106053
Mahler RL, Barber ME (2017) Using benthic macroinvertebrates to assess water quality in 15 watersheds in the Pacific Northwest, USA. Int J Sustain Dev Plan 12:51–60. https://doi.org/10.2495/SDP-V12-N1-51-60
Mehrjo F, Abdoli A, Hashemi SH, Hosseinabadi F (2020) Development of a multimetric index based on benthic macroinvertebrates for downstream Jajrud River in Tehran province, Iran. Iran J Fish Sci 19(1):286–296. https://doi.org/10.22092/ijfs.2019.118321
Meyer D (1987) Makroskopisch- Biologische Feldmethoden zur Wassergütebeurteilung von Fliegewässern, 3. Auflage, A.L.G., 6, 3000, Hannover
Ode PR, Rehn AC, May JT (2005) A quantitative tool for assessing the integrity of southern coastal California streams. Environ Manage 35:493–504. https://doi.org/10.1007/s00267-004-0035-8
Odabaşı DA, Odabaşı S, Ergül HA, Özkan N, Boyacı Y, Bayköse A, Kayal M, Ekmekçi F, Dağdeviren M, Güzel B, Canlı O, Dügel M (2022) Development of a macroinvertebrate–based multimetric index for biological assessment of streams in the Sakarya River Basin, Turkey. Biologia 77:1317–1326. https://doi.org/10.1007/s11756-022-01041-7
Öztürk S (2021) The use of Ephemeroptera (Insecta) group in monitoring studies according to water framework directive and ecological.evaluation: Seyhan, Ceyhan and East Mediterranean Basins. PhD Thesis, Nevşehir Hacı Bektaş Veli University, Grauate School of Natural and Applied Sciences
Öztürk S, Dügel M, Çiçek E (2022) Seasonal distribution of Ephemeroptera (Insecta) fauna and relationship among physicochemical parameters in the Ceyhan Basin. Aquat Sci Eng 37(2):105–118. https://doi.org/10.26650/ASE20221069026
Pineda JJ, Munoz-Rojas J, Morales-Garcia YE, Hernandez-Gomez JC, Sigarreta JM (2022) Biomathematical model for water quality assessment: macroinvertebrate population dynamics and dissolved oxygen. Water 14(18):2902. https://doi.org/10.3390/w14182902
Popovic N, Marinkovic N, Cerba D, Rakovic M, Buknic J, Paunovic M (2022) Diversity patterns and assemblage structure of non-biting midges (Diptera: Chironomidae) in urban waterbodies. Diversity 14(3):187. https://doi.org/10.3390/d14030187
Roline R (1988) The effects of heavy metals pollution of the upper Arkansas River on the distribution of aquatic macroinvertebrates. Hydrobiologia 160:3–8. https://doi.org/10.1007/BF00014273
Rosa J, de Oliveira FR, Pereira LF, de Melo Silva M, Bueno-Krawczyk ACD (2022) Temporal variation in Oligochaeta species composition in an anthropized stretch of a neotropical urban river. Ann Limnol Int J Limnol 58:6. https://doi.org/10.1051/limn/2022006
Sabha I, Hamid A, Bhat SU, Islam ST (2022) Water quality and anthropogenic impact assessment using macroinvertebrates as bioindicators in a stream ecosystem. Water Air Soil Pollut 233:387. https://doi.org/10.1007/s11270-022-05839-8
Serdar O, Verep B (2018) The investigation of water quality of İyidere and Çiftekavak streams using physico-chemical and biotic indexes. Int J Pure Appl Sci 4(1):61–71. https://doi.org/10.29132/ijpas.398725
Shaha CM, Pandit RS (2022) Bio-based versus synthetic: comparative study of plasticizers mediated stress on Chironomus circumdatus (Diptera–Chironomidae). Ecotoxicology 31:385–395. https://doi.org/10.1007/s10646-021-02516-0
Shahidi-Hakak F, Amid-motlagh MH, Khosravani M (2022) A quick review of the family Chironomidae (Order: Diptera) with effect on the environments. Trends Med Sci 2(2):e129263. https://doi.org/10.5812/tms-129263
Sotomayor G, Hampel H, Vazquez RF, Goethals PLM (2020) Multivariate-statistics based selection of a benthic macroinvertebrate index for assessing water quality in the Paute River basin (Ecuador). Ecol Indic 111:106037. https://doi.org/10.1016/j.ecolind.2019.106037
Stoyanova T, Vidinova Y, Yaneva I, Tyufekchieva V, Parvanov D, Traykov I, Bogoev V (2014) Ephemeroptera, Plecoptera and Trichoptera as indicators for ecological quality of the Luda Reka River, Southwest Bulgaria. Acta Zool Bulg 66(2):255–260
Şenol M, Gül T, Atış E, Salalı HE (2016) Gediz Havzası’nda su Kirliliğinin Önlenmesinde Tarımın Rolü, XII. Ulusal Tarım Ekonomisi Kongresi, 25–27 Mayıs 2016, pp 2251–2252
Ter Braak CJF (1988) Partial canonical correspondence analysis. In: Bock HH (ed) Classification and related methods of data analysis. North- Holland, Amsterdam, pp 551–558
Ünlü M (2013) Biologic features and fishing of the Gediz River Basin.Marmara Coğrafya Dergisi(1):309–323
Wallace JB, Grubaugh JW, Whiles MR (1996) Biotic indices and stream ecosystem processes: results from an experimental study. Ecol Appl 6(1):140–151
Yorulmaz B, Sukatar A, Barlas M (2015) Comparative analysis of biotic indices for evaluation of water quality of Esen River in South-West Anatolia, Turkey. Fresenius Environ Bull 24(1):188–194
Zeybek M, Kalyoncu H, Karakuş B, Özgül S (2014) The use of BMWP and ASPT indices for evaluation of water quality according to macroinvertebrates in Değirmendere Stream (Isparta, Turkey). Turk J Zool 38:603–613. https://doi.org/10.3906/zoo-1310-9
Zeybek M (2017) Macroinvertebrate-based biotic indices for evaluating the water quality of Kargı Stream (Antalya, Turkey). Turk J Zool 41:476–448. https://doi.org/10.3906/zoo-1602-10
Zhang J, Siyue L, Ruozhu D, Changsheng J, Maofei N (2019) Influences of land use metrics at multi-spatial scales on seasonal water quality: a case study of river systems in the three Gorges Reservoir Area. China. J Clean Prod 206:76–85. https://doi.org/10.1016/j.jclepro.2018.09.179
Author information
Authors and Affiliations
Contributions
Methodology, AE and BY; formal analysis, AE; investigation, AE and MY; resources, AE and SÖ; data curation, AE and SÖ; writing—original draft preparation, AE, SÖ and BY; supervision, BY. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
Authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ertaş, A., Öztürk, S., Yaşartürk, M. et al. Biological assessment of Demirci Stream in Gediz River Basin (West Anatolia of Turkey) and comparative performance of benthic macroinvertebrate-based metrics. Biologia 78, 1103–1112 (2023). https://doi.org/10.1007/s11756-023-01329-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01329-2