Abstract
Dachigam-Dara catchment feeding the world-famous Dal Lake was assessed and evaluated for water quality and anthropogenic impacts using physico-chemical and biological data from 2016 to 2018. Seven sites belonging to Dachigam (DACZ) and Dara zone (DARZ) catchment, three sites from the confluence zone (WANZ), and two sites at the downstream end (TELZ) were selected characterized by varying degrees of anthropogenic pressures. Biological Monitoring Working Program, and Average Score Per Taxon at the upstream zones (DACZ, DARZ, and WANZ) recorded significantly higher scores with water quality indices falling within the good category than the downstream zone (TELZ). Taxa richness, and diversity indices of benthic macroinvertebrates recorded higher values at the upstream zones (DACZ, and DARZ), and confluence zone (WANZ), compared to the downstream zone (TELZ). Results revealed that phylum Arthropoda was most dominant contributing 37 invertebrate families (constituting 90% of the total macroinvertebrate community, including Crustacea and Arachnida) while phylum Mollusca and Annelida constitute 5% each. Macroinvertebrate families Baetidae, Erpobdellidae, Gammaridae, Chironomidae, and Heptagenidae contributed significantly to the similarity and dissimilarity between the sampling zones. The best subset of environmental variables (BIOENV) test revealed that the distribution of benthic macroinvertebrate assemblage in the Dachigam-Dara catchment is driven by pH, electrical conductivity, dissolved oxygen, and phosphate phosphorous. The upstream zones (DACZ, and DARZ) and confluence zone (WANZ), compared to the downstream zone (TELZ) suggest progressive shift of pollution sensitive macroinvertebrate taxa to pollution tolerant taxa in response to anthropogenic activities in the stream ecosystem over time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Multitude human pressures, such as pollution (Oliveira et al., 2017; Hamid et al., 2021), nutrient enrichment (Ferreira et al., 2020), damming, or overexploitation (de Paula et al., 2018; Sofi et al., 2022), have severely damaged freshwater ecosystems worldwide, and this has been more intense over the past five decades (Bhat et al., 2021a, b; Santos & Ferreira, 2020). Anthropogenic activities such as river regulation (Zhang et al., 2020; Sofi et al., 2020) and pollution (Landrigan et al., 2018) have a potentially negative cumulative impact on rivers and streams water quantity and quality (Hoang et al., 2018; Sabha et al., 2020; Musonge et al., 2020). Stream ecosystem assessment has increased substantially in recent years, to evaluate the effects of urban development (Paul & Meyer, 2001) and impacts on aquatic biota and other ecosystem services (Cuffney et al., 2000; Pitt, 2002). Topography, geology, and soil conditions along with human-driven agriculture, urbanization, and forestry activities determine the abiotic and biotic characteristics of streams draining the landscapes (Allan, 2004). Catchments with high populations have more pressure on the stream water body which exhibited altered water quality and macroinvertebrate assemblages (Edegbene et al., 2021; Newall & Walsh, 2005; Taylor et al., 2004; Walsh et al., 2005). Anthropogenic pressure alters the physical and chemical environment of rivers and streams (Alig et al., 2004; Allan, 2004; Bhat et al., 2021a, b). The demand for water for consumption, agriculture, and navigation development has led nations to recommend action defensible over long periods (Bagla, 2014; Brito & Magalhães, 2017). Aquatic organisms such as fish, macroinvertebrates, and diatoms (Hering et al., 2006; Negi & Singh, 2021; Tampo et al., 2021) indicate quite well the characteristics and degradation level of the catchment area (Wenger et al., 2009; Walsh & Wepner, 2009) and are used as the biomonitoring tools. In Himalayan lotic ecosystems, biomonitoring has started gaining pace to report the anthropogenic pressure and to know the status of water bodies, such kind of studies were carried out on streams such as Vishav, Jhelum, Lidder, and Dagwan (Hamid et al., 2016; Rashid and Romshoo, 2013; Sabha et al., 2019; Khanday et al., 2020; Sabha et al., 2020; Hamid et al., 2021). In the Himalayan region, the Dachigam-Dara catchment in the northwestern Kashmir Himalayas is an important tributary to the Dal Lake. As the region lies in the periphery of Srinagar city, it is subjected to an increase in the human population with an increase in water demand and thus substantially pressure on the water resources present (Fazal & Amin, 2011). It is crucial to identify, monitor, and analyze the effects of such stressors to provide a proper management policy to preserve, manage, and restore freshwater ecosystems. The present study investigates the relationship between the macroinvertebrate assemblages and physico-chemical characteristics in order to find out the best environmental factors explaining the variations S in composition and distribution of macroinvertebrates in the Dara-Dachigam catchment. We hypothesized that along the longitudinal gradient, water quality deterioration in response to anthropogenic impact leads to a progressive shift of macroinvertebrate taxa from pollution sensitive to pollution tolerant taxa in response to changing environmental conditions. Furthermore, we suggest that benthic macroinvertebrate assemblages vary seasonally and are location-dependent as well.
2 Methodology
2.1 Study Area
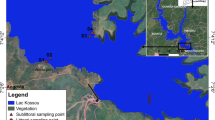
Dachigam-Dara catchment of the world-famous Dal Lake is located in the vicinity of Srinagar city towards the east in the North-Western Kashmir Himalaya, India. The study area is situated between geographical coordinates of 34° 5′ 20″ to 34° 13′ 40″ N latitude and 74° 48′ 35″ to 74′ 08′ 32″ E longitude, with altitude ranging from 1592 to 4250 m above mean sea level. Therefore, the gradient between the most upper site (1828 m) and lower site (1592 m) is more than 230 m. Telbal-Dachigam in the northeast is the largest sub-watershed (230 km2) of Dal catchment comprising nearly 70% of the watershed and divided into the Telbal-Dara (87 km2) and Dachigam National Wildlife Reserve (143 km2) sub-watersheds. The average annual rainfall in Srinagar is 650 mm, whereas in Dachigam it is 870 mm. During the summer season, snow thawing in the upper reaches of the watershed leads to maximum discharge in Dachigam and Dara Nallah (Shah et al., 2014). The Dara catchment is subjected to several biotic interferences, resulting in loose soil, and it is via this nallah that the Telbal stream gets enormous amounts of eroded sediments throughout the year (Pandit, 1999). Water bodies in the study area have been altered biologically and hydrologically as a result of significant anthropogenic pressures as a result of changing socioeconomic conditions in the region (Khan, 1993a, b). Because of the rise in % impervious surface area due to developmental activities and human settlements in the Dal catchment, there is a quick peak flow in the streams (mostly Telbal Nallah) feeding the Dal Lake after a short period of precipitation, which enhances the rate of erosion (Amin & Romshoo, 2007). Furthermore, deforestation, grazing, and agricultural activities in the Dal watershed have resulted in large sediment loads, and nutrient loads into the lake have accelerated eutrophication (Badar & Romshoo, 2007). Twelve study sites were selected for the present investigation with sites 1, 2, 3, and 4 from the Dara catchment (North-Western side) are the left bank tributaries and are grouped as Dara zone (DARAZ); sites 5, 6, and 7 from the Dachigam catchment (North-Eastern side) being right bank tributaries were grouped as Dachigam zone (DACZ). Sampling locations 5 and 6 fall within the Dachigam National park which is home to endemic Kashmiri stag “Cervus Elaphus Hanglu” which is a critically endangered species in an IUCN list. The habitat and other environmental characteristics make it mandatory to keep it under observation. The streams from both catchments meet at a confluence point known as Wangund, and the sampling sites chosen were sites 8, 9, and 10 which were grouped in the Wangund zone (WANZ). Finally, all the streams and channels enter the Dal Lake at Shanpora, and two points were chosen, sites 11 and 12, which were named as Telbal zone (TELZ) (Supplementary Table S1, Fig. 1). The present work addresses the water quality using biotic and abiotic factors of the Dara-Dachigam catchment draining into the Dal Lake of Kashmir Himalayas India.
2.2 Material and Methods
2.2.1 Macroinvertebrate Collection
Macroinvertebrate samples were collected by kicking and displacing the bottom substrate from 12 sites from four zones of Dachigam-Dara catchment area on a seasonal basis from Spring 2016 to Winter 2018. A D-net (0.25 m × 0.25 m size) with pore size 0.5 mm was held in the stream with a lower frame placed close to the bottom of the stream. The bottom substrate upstream of the D-net was disturbed for at least 1 min to dislodge the organisms, which then float to the net with the natural flow (Cuffney et al., 1993; Barbour et al., 1999; Ligeiro et al., 2020). The procedure was repeated four times within a 100-m stream reach covering different hydrological regimes and substrate types to get an area of 1 m2 (Ilmonen & Paasivirta, 2005; Malmqvist & Hoffsten, 2000). Collection of macroinvertebrates at TELZ (soft bottomed downstream sites) was carried out using Ekmans Dredge made up of a 6-in. square brass box fitted with spring opened closing jaws (15.2 × 15.2). In the laboratory, collected macroinvertebrate samples were washed and cleaned, and were fixed with 70% ethanol and 4% formalin. Macroinvertebrates were identified to the genus level and enumerated using a dissecting microscope (× 6 magnification) according to the standard keys (Edmondson, 1959; Pennak, 1978; McCafferty and Provonsha, 1998; Borror et al., 1989; Ward, 1992; Engblom & Lingdell, 1999; Dar et al., 2002, Bouchard Jr, 2004; Merritt & Cummins, 2006; Subramanian & Sivaramakrishnan, 2007; Bhagat, 2013; APHA, 2012). Several ecological characteristics of macroinvertebrate assemblages were calculated including Shannon diversity (H) (Shannon and Weiner, 1949), Simpson diversity (1_D) (-Simpson, 1949), richness (Duan et al., 2008; Moore, 2013), Equitability_J (Magurran, 2003), and dominance (D) (Camargo, 1992). Macroinvertebrate samples were collected in four replicas, and the average values were used in the final claculations.
2.2.2 Physicochemical Variables
To assess the water quality of streams traversing the world-famous Dachigam National Park before and after their confluence, a detailed characterization of physical and chemical characteristics of 12 sites belonging to four zones was carried out on a seasonal basis for a period of 2 years from Spring 2016 to Winter 2018. Physico-chemical variables including discharge (Dis, m3s−1), velocity (Vel, ms−1), and water temperature (WT, °C) were measured while pH, dissolved oxygen (mgL−1), conductivity (µScm−1), and TDS (mgL−1) were measured using a multi-parameter probe (Eutech PCSTEST35-01 × 441,506/Oakton 35,425–10) calibrated with standard solutions in the laboratory. Total alkalinity (TA, mgL−1), total hardness (TH, mgL−1), calcium hardness (CH, mgL−1), calcium content (Ca2+,mgL−1), magnesium hardness (MH, mgL−1), magnesium content (Mg2+, mgL−1), sulfate (SO42−,mgL−1), sodium (Na+, mgL−1), and potassium (K+, mgL−1) were measured using titrimetric methods (APHA, 2012). Nitrate-nitrogen (NO3-N, µgL−1), ammonical-nitrogen (NH3-N, µgL−1), phosphate-phosphorus(PO4-P, µgL−1), total phosphorus (TP, µgL−1), and dissolved silica (Si, mgL−1) were measured by colorimetric assays using double-beam UV–Visible spectrophotometer following standard methods (APHA, 2012). Water quality variables were analyzed in four replicates, and the average values were used for the final calculations. The bottom substrate composition of the stream ecosystem was divided into one of the five categories: boulders (> 256 mm), pebble (256–64 mm), stone (64–20 mm), gravel (20–2.0 mm), sand (2.0–0.06 mm), and silt (< 0.06 mm) (Kaufmann et al., 1999; Sharma et al., 2004). At each sampling site, percentages of the substrate type were determined using a 1-m2 area.
2.2.3 Data Analysis
Multivariate statistical techniques were employed to understand the relationship between environmental factors and macroinvertebrates: non-metric multidimensional scaling (nMDS), a robust ordination technique that generates an ordination based on similarity or dissimilarity among samples. Stress value depicts the goodness of fit between the estimated matrix of dissimilarities and the associated distance within an nMDS plot (Clarke & Warwick, 2001). Analysis of similarity (ANOSIM), a non-parametric technique was used to test if there is a significant difference between macroinvertebrate assemblages among the two or more groups of samples as observed from the nMDS plot. ANOSIM uses a dissimilarity matrix and its values range from − 1 to + 1 at α = 0.05 to calculate the distinction between two or more groups. Similarity percentages (SIMPER) analysis based on the Bray–Curtis dissimilarity metric was used to measure the individual variable contribution to overall group similarity or dissimilarity (Clarke & Gorley, 2006). Before SIMPER analysis, abundance-based datasets were log-transformed log (x + 1) and the cut-off limit for small contributions was set at 90%. Principal component analysis, a data reduction procedure that allows interpreting data in a more meaningful way was employed to large datasets of physicochemical variables. PCA analysis reduced a large number of variables to a few pairwise and interpretable correlations among the variables as a linear combination to consider. The best subset of environmental variables (BIOENV) test was employed to evaluate the impact of environmental variable(s) on the macroinvertebrate assemblage structure and composition (Clarke & Ainsworth, 1993). BIOENV is a dissimilarity and exploratory technique for identifying the best subset of a set of environmental variables whose euclidean distance matches that of the Bray–Curtis matrix of biological data based on abundance data. BIOENV employs the weighted Spearman rank correlation coefficient (ρ) between the environmental factors and macroinvertebrate assemblages. Before the multivariate statistical analysis, biological and physico-chemical factors were transformed to fulfill the normality assumption and to allow comparison at the same scale. The group with the highest ρ is considered best for driving and structuring the macroinvertebrate assemblages. ANOSIM, SIMPER analysis, nMDS, PCA (principal component analysis), and BIOENV were performed using Primer v 7 (Clarke & Warwick, 2001). ANOVA and diversity indices were calculated using SPSS (Statistical Package for Social Science) and PAST 3.20 (Paleontological Statistics Software Package for Education and Data Analysis, Øyvind Hammer Natural History Museum University of Oslo), and remaining graphs were prepared in Origin 8 software. The Biological Working Monitoring Program (BWMP) and Average Score Per Taxon (ASPT) pollution indices were calculated to determine the pollution status variation between the zones (Armitage et al., 1983; AQEM, 2002; Mason, 2002).
3 Results and Discussion
3.1 Water Quality
The water quality variables measured from 12 sites belonging to four zones of Dachigam-Dara catchment indicated good status except at TELZ where a higher concentration of electrical conductivity and other key nutrients in comparison with other zones was recorded. The concentration of key plant nutrients (nitrogen and phosphorus) responsible for eutrophication at downstream zone was found crossing suggested trophic boundaries for streams (Dodds et al., 1998). The water quality index (WQI) calculated using BIS requirements rendered DARZ, DACZ, and WANZ as “clean water zones” and TELZ as “moderately polluted zone” (Table 1).
Repeated measure analysis of variance (RM-ANOVA) test was performed on BMWP and ASPT scores at four zones in the Dachigam-Dara catchment. BMWP (F3,7 = 15.009, p = 0.001) and ASPT (F3,7 = 8.04, p = 0.008) showed a significant difference between zones. The BMWP and ASPT findings show clean water conditions in the DARZ, DACZ, and WANZ zones, which were characterized by a higher number of taxa, pollution sensitive taxa, high dissolved oxygen, low electrical conductivity, and nutrients while TELZ was classified as moderately polluted with the lowest number of taxa, pollution tolerant taxa, low dissolved oxygen, high concentration of electrical conductivity, and nutrients. Therefore, based on calculated WQI and BMWP/ASPT scores, TELZ is not meeting the “Good Ecological Status” requirements expected in freshwater water ecosystems (Table 1 and Fig. 2).
3.2 Benthic Macroinvertebrate Assemblages
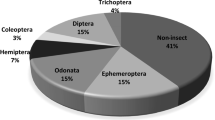
Sampling each of the 12 sites belonging to four different zones from Dachigam-Dara catchment of the Dal Lake resulted in a total of 5737 individuals representing 73 taxa belonging to 7 classes, 12 orders, and 41 families and spread over 3 phyla Mollusca (Gastropoda and Bivalvia), Annelida (Oligochaeta and Hirudinea), and Arthropoda (Insecta, Crustacea, and Arachnida). Class Insecta, was the most diverse group contributing 61 taxonomic forms belonging to 7 different orders (Hemiptera-1, Odonata-2, Plecoptera-6, Coleoptera-8, Trichoptera-14, Ephemeroptera-11, and Diptera-19) (Supplementary Table S2). The Arthropoda was the most dominant contributing 37 invertebrate families (constituting 90% of the total macroinvertebrate community including Crustacea and Arachnida) followed by Mollusca and Annelida constituting 5% each. Surprisingly, rivers and streams are found to support a large fraction of global biodiversity relative to their volume and aerial extent (McAllister et al., 1997). The presence of adequate benthic macroinvertebrate assemblages in the Dachigam-Dara catchment seems to be driven by favorable environmental conditions including water temperature, abundant food availability, habitat heterogeneity and stability, and hydraulic conditions (Brittain & Milner, 2001; Nicacio et al., 2020; Stanford et al., 2005).
The proportion of EPT (Ephemeroptera, Plecoptera, and Trichoptera) characterized mostly by pollution-sensitive taxa was significantly higher in DARZ, DARZ, and WANZ zones and became negligible in TELZ (Fig. 3). The higher proportion of EPT in DARZ, DARZ, and WANZ zones indicates their preference for stress-free environments (Mourier et al., 2019). EPT is considered pollution sensitive and prefers cold, oxygen-rich environs, and coarser substrate (help in retaining coarse particulate organic matter) (Jacobsen and Marín, 2008; Mourier et al., 2019; Pandiarajan et al., 2019). Furthermore, the occasional occurrence of Bibliocephala sp., Polycentropus sp. Corixidae sp., Agabus sp., and Cheumatopsyche sp. typically at the upper reaches belonging to DARZ and DACZ indicates a “healthy and natural” stream ecosystem condition (Ivol-Rigaut et al., 1997; Kaboré et al., 2016). There was a progressive shift in the benthic assemblages while moving down to the TELZ, marked by the higher proportion of Diptera, Opisthopora, Pharyngobdellida, Venerida, and Gastropoda which are mostly pollution tolerant (Bertaso et al., 2015). Increasing anthropogenic activities along the river continuum lead to water quality deterioration, subsequently leading to the progressive shift in benthic taxonomic composition from pollution-sensitive EPT to tolerant taxa (Harding et al., 1999). The TELZ zone characterized by deteriorated water quality as depicted by water quality assessment, BMWP/ASPT scores, and presence of fine-grained substrate (sand and silt) had less EPT and instead was dominated by Diptera, Opisthopora, Pharyngobdellida, Venerida, and Gastropoda (Bertaso et al., 2015). Slow-moving waters, with poor water quality (high electrical conductivity, nutrients), are unsuitable for rhithronic, rheophilus, and other sensitive species (Vander Laan et al., 2013, Jun et al., 2016a, b). Macroinvertebrate communities are highly adaptable to a variety of ecological factors, and they actively prefer suitable aquatic environments (Batzer et al., 2004).
The lower proportion of Diptera (comprising of pollution sensitive taxa and extremely pollution-tolerant taxa) at DARZ, DACZ, and WANZ zones and high proportion at TELZ zones (Fig. S1) display their potential to adapt in a variety of habitats (Bouchard Jr, 2004). In DARZ, DACZ, and WANZ zones, pollution-sensitive Dipterans representing natural conditions (Blephaceridae sp., Bibliocephala sp., Diamesinae sp., Atherix sp., Limonia sp., Antocha sp., Tipula sp., Simulium sp.) were progressively replaced with moderately to extremely pollution-tolerant taxa (Chrysops sp., Hexatoma sp., Tabanus sp., Chironomus sp., Psychodidae, Tanypodinae sp., Culex sp., and Procladius sp.) at TELZ zone indicating water quality deterioration (Barbour et al., 1999; Bouchard & Ferrington, 2011).
3.3 Diversity Indices
The overall number of benthic macroinvertebrate taxa, as well as their richness and diversity indices (Shannon and Simpson) and equitability were substantially higher (p < 0.05) in DACZ followed by DARZ and lowest in TELZ (Fig. 3). However, dominance indices were significantly higher (p < 0.05) in TELZ. According to our findings, the DACZ and DARZ zones have significantly higher diversity and equitability values than the TELZ, indicating that the upper zones not only have a greater number of taxa, but also that the individuals in the macroinvertebrate community are distributed equally among the species (Begon et al., 1996; Rosenzweig, 1995). In the TELZ zone, there are fewer species, and individuals in the community are not distributed equally resulting in higher dominance values. Furthermore, the higher the dominance index, the lower is the diversity and vice versa (Ludwig & Reynolds, 1988). Therefore, diversity indices not only provide information about the species richness, but they also account for the rarity and commonality of species and levels of disturbance in the ecosystem (Begon et al., 1996; Rosenzweig, 1995). Upstream sites characterized by clean water conditions and habitat heterogeneity support a higher diversity of benthic macroinvertebrates (Azrina et al., 2006; Abhijna et al., 2013; Maneechan & Prommi, 2015) holds in the present study. Diversity indices based on richness and abundance indicates that the disturbance in the aquatic ecosystems under stress leads to the reduction in the diversity (De Pauw et al., 2006). Biodiversity indices clearly reflected the habitat degradation effectively with increase pollution taxa at the TELZ zone.
nMDS based on abundance data (Fig. 4) resulted in a clear distinction among the locations of the sites (stress value of 0.117) in two-dimensional space. The zonation between the sites showed that the Wangund zone was found to be scattered within the biplot whereas, the Telbal zone resulted in a separate cluster at the right bottom of the biplot. The Dara zone and the Dachigam zone are seen together in the extreme right. At each of the four zones, one-way nested ANOSIM revealed a substantial variation in macroinvertebrate assemblages (global test R = 0.372, p = 0.001). Pairwise ANOSIM test specified that the following zones were significantly different: DARZ and DACZ (global test R = 0.086, p = 0.064); DARZ and WANZ (global test R = 0.228, p = 0.001); DARZ and TELZ (global test R = 0.936, p = 0.001); DARZ and WANZ (global test R = 0.175, p = 0.001), DACZ and TELZ (global test R = 0.961, p = 0.001), and WANZ and TELZ (global test R = 0.277, p = 0.007). The results showed that the Telbal zone was significantly different from other zones.
To provide further insight, the SIMPER test was used to examine the macroinvertebrate assemblage, and the obtained results were compared with nMDS results. Within each zone, the SIMPER test revealed the highest average similarity (54.75%) in DARZ with the major contributing families Baetidae (11.75%), Erpobdellidae (11.32%), and Limnephillidae (6.85%). DACZ exhibited the second-highest average similarity (55.34%); the key contributing families were Baetidae (9.88%), Heptagenidae (8.45%), and Glossosomatidae (6.32%). The presence of Heptagenidae and Glossosomatidae in the upstream sites shows clean water conditions have been recorded by several other workers (Edema et al., 2002; Ikomi et al, 2005; Walsh et al, 2002). The average similarity of 22.78% was found at WANZ, and the contributing families were Batidae (17.80%), Gammaridae (15.90%), and Tipulidae (9.69%). The Telbal zone (TELZ) exhibited an average similarity score of 46.95% with major families contributing are Chironomidae (46.33%), Erpobdellidae (30.91%), and Gammaridae (9.22%). The higher proportion of Chironomidae and Erpobdellidae at downstream zone indicates the poor water quality in response to increasing anthropogenic conditions (Langdon et al., 2006) and possesses the ability to thrive in areas of low competition (Arimoro, 2009). A substantial proportion of Gammaridae downstream zone is in response to the considerable load of organic particles and seasonal improvement in water quality (Medupin, 2019; Miyake & Nakano, 2002). The present study depicted that Ephemeroptera dominated the macroinvertebrate assemblage and composition mainly by Baetidae at first three zones and Chironomidae at downstream zone, a characteristic feature of Asian streams (Jun et al., 2016a, 2016b). The SIMPER results showed the highest average dissimilarity between WANZ and TELZ (77.89%) followed by DACZ and TELZ (76.31%), DARZ and TELZ (71.08%), DACZ and WANZ (67.46%), DARZ and WANZ (64.87%); and least dissimilarity was exhibited in between DARZ and DACZ (46.95%).
3.4 Principal Component Analysis
Principal component 1 (PC1) accounted for 62.3% of the total variation and was dominated by altitude, boulders, pebble, stone, and gravel, whereas principal component 2 (PC2) accounted for 16.0% of the total variance and was dominated by velocity, pH, dissolved oxygen, and calcium hardness. To determine the physicochemical variables directly affecting the water quality of the Dachigam-Dara catchment under investigation, a backward elimination of highly weighted variables altitude, boulders, pebble, velocity, pH, and dissolved oxygen was executed. The new PC1 showed high values on discharge, ammonical-nitrogen, iron, sulfate, nitrate-nitrogen, nitrite-nitrogen, and stones which accounted for 67.3% overall variance, while PC2 accounted for about 10.1% of the overall variance. The results explain the difference between the different zones, i.e., Telbal zone forming the separate cluster in both the plots with higher water temperature, ammonical-nitrogen, electrical conductivity, sand, and silt (Fig. 5). The water quality variables measured in the DARZ, DACZ, and WANZ (upstream zones) had good water quality status while downstream zone (TELZ) moderately polluted status. Several studies have demonstrated the applicability of PCA to interpret and evaluate the relationship between benthic macroinvertebrates and water quality variables (Serpa et al., 2014; Tan and Beh, 2016). The outcome of the principal component analysis demonstrates that there are several potential macroinvertebrate taxa that could be used as bioindicators.
Principal components of physicochemical variables at different zones. (a) Variables included are Dis, Vel, WT, pH, EC, TDS, DO, FC, TA, Chl, TH, CH, Ca2+, MH, Mg2+, SO42−, NH3-N, NO2-N, NO3-N, PO4-P, TP, SI, Fe, Na+, K+, alt, boulder, pebble, gravel, stones, sand, and silt. (b) WT, DIS, FC, Mg2+, SO42−, NH3-N, NO2-N, NO3-N, TP, SI, and stones
3.5 Relation Between Physicochemical Variables and Macroinvertebrate Assemblages
The environmental parameters that significantly correlate with the macroinvertebrate assemblages were pH, electrical conductivity, dissolved oxygen, and phosphate-phosphorous with correlation coefficient (ρ) = 0.694 in BIOENV analysis (Table 2). Other physicochemical parameters were not recognized as having a significant impact on macroinvertebrate assemblages; the results suggest key factors that would influence macroinvertebrate assemblage patterns which are pH, electrical conductivity, dissolved oxygen, and phosphate-phosphorous. The BIOENV supported the results obtained from nMDS, thereby indicating that the downstream zone (TELZ) is characterized by higher values of EC, nutrients, and low dissolved oxygen and therefore suggests the impact of anthropogenic activities at these sites (WFD Uktag, 2013).
4 Conclusion
The influence of anthropogenic activities on water quality, distribution, and diversity of benthic invertebrates has steadily increased. Pollution-tolerant taxa were noticed in the downstream zone of Dachigam-Dara catchment, which had poor and low water quality index, BMWP, and ASPT scores, compared to upstream zones. The higher abundance of Chironomidae and Erpobdellidae at TELZ zone indicates the poor water quality in response to increasing anthropogenic conditions, while sizeable presence of Gammaridae TELZ zone is in response to the considerable load of organic particles and seasonal improvement in water quality. The standardized statistical tests tested for the Dachigam-Dara catchment indicated that the macroinvertebrate metrics and diversity indices varied significantly across the four zones. Moreover, our results indicate that the TELZ zone stands out from other zones as revealed from nMDS, SIMPER, and ANOSIM in response to water quality and macroinvertebrate assemblage. The BIOENV technique was shown to be useful in explaining the key water quality parameters responsible for the observed macroinvertebrate assemblage differences between zones. The results obtained from this study support the use of certain macroinvertebrate taxa as potential bioindicators for evaluating water quality. The findings of this study could be used as a baseline for future research and to inform decision-makers and policymakers on how bioindicators can aid in-stream monitoring, management, and stream conservation in the area.
Data Availability
The data used/or analyzed that support the findings of this study are available in the main manuscript file and from the corresponding author on reasonable request.
References
Abhijna, U. G., Ratheesh, R., & Kumar, B. A. (2013). Distribution and diversity of aquatic insects of Vellayani lake in Kerala. Journal of Environmental Biology, 34(3), 605–611.
Alig, R. J., Kline, J. D., & Lichtenstein, M. (2004). Urbanization on the US landscape: Looking ahead the 21st century. Landscape and Urban Planning, 69, 219–234.
Allan, J D. (2004). Landscapes and riverscapes: The influence of land use on stream. https://doi.org/10.1146/annurev.ecolsys.35.120202.110122
Amin A., Romshoo S.A. (2007) Assessing the hydrologic characteristics of Dal Lake catchment using GIS. In: Proceedings of TAAL 2007: the 12th World Lake Conference (pp 659–667).
APHA. (2012). Standard methods for the examination of water and wastewater (22nd ed.). American Public Health Association.
AQEM Consortium. (2002). Manual for the application of the aqem system. A comprehensive method to assess european streams using benthic macroinvertebrates, developed for the purpose of the water framework directive. Version 1.0., 202 pp.
Arimoro, F. O. (2009). Impact of rubber effluent discharges on the water quality and macroinvertebrate community assemblages in a forest stream in the Niger Delta. Chemosphere, 77, 440–449.
Armitage, P. D., Moss, D., Wright, J. F., & Furse, M. T. (1983). The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running water sites. Water Research, 17, 333–347.
Azrina, M. Z., Yap, C. K., Rahim, I. A., & Tan, S. G. (2006). Anthropogenic impacts on the distribution and biodiversity of benthic macroinvertebrates and water quality of the Langat River. Peninsular Malaysia. Ecotoxicol Environ Saf., 64(3), 337–347. https://doi.org/10.1016/j.ecoenv.2005.04.003
Badar, B., & Romshoo, S. A. (2007). Modelling the non-point source pollution load in an urban watershed using remote sensing and GIS: A case study of Dal Lake. Journal of Himalayan Ecology & Sustainable Development, 2(1), 21–30.
Bagla, P. (2014). India plans the grandest of canal networks. Science, 345, 128. https://doi.org/10.1126/science.345.6193.128
Barbour, M. T., Gerritsen, J., Snyder, B. D., & Stribling, J. B. (1999). Rapid bioassessment protocols for use in streams and Wadeable rivers: Periphyton, benthic macroinvertebrates and fish (Vol. 339). US Environmental Protection Agency, Office of Water.
Batzer, D. P., Palik, B. J., & Buech, R. (2004). Relationships between environmental characteristics and macroinvertebrate communities in seasonal woodland ponds of Minnesota. Journal of the North American Benthological Society, 23, 50–68.
Begon, M., Harper, J. L., & Townsend, C. R. (1996). Ecology: Individuals, populations, and communities (3rd ed.). Blackwell Science Ltd.
Bertaso, T. R. N., Spies, M. R., Kotzian, C. B., & Flores, M. L. T. (2015). Effects of forest conversion on the assemblages’ structure of aquatic insects in subtropical regions. Revista Brasileira De Entomologia, 59, 43–49.
Bhagat R.C. (2013). Aquatic Beetles (Coleoptera: Insecta) of Jammu, Kashmir & Ladakh Region (North-West Himalaya): Inventory and biodiversity. Journal of Global Biosciences 2(4): 90–97. https://www.mutagens.co.in/jgb/vol.02/4/04.pdf
Bhat, S. U., Islam, S. T., Sabha, I., & Khanday, S. A. (2021b). Understanding the spatiotemporal pollution dynamics of highly fragile montane watersheds of Kashmir Himalaya, India. Environmental Pollution, 286, 117335. https://doi.org/10.1016/j.envpol.2021.117335
Bhat, S.U., Bhat, A.A. Jehangir, A, Hamid, A., Sabha,I., Qayoo, U. (2021a). Water quality characterization of marusudar river in chenab sub-basin of north-western Himalaya using multivariate statistical methods. Water, Air, & Soil Pollution, 449. https://doi.org/10.1007/s11270-021-05394-8
Borror, D. J., Triplehorn, C. A., & Johnson, N. F. (1989). An introduction to the study of insects (Ed. 6th ). Saunders college publishing.
Bouchard, R. W., & Ferrington, L. C. (2011). The effects of subsampling and sampling frequency on the use of surface-floating pupal exuviae to measure Chironomidae (Diptera) communities in Wadeable temperate streams. Environment Monit. Assess., 181(1–4), 205–223.
Bouchard Jr, R. W. (2004). Guide to aquatic invertebrates of the Upper Midwest: Identification manual for students, Citizen Scientist ‘s and Professionals. University of Minnesota. https://dep.wv.gov/WWE/getinvolved/sos/Pages/UMW.aspx
Brito, M. F. G., & Magalhães, A. L. B. (2017). Brazil’s development turns river into sea. Science, 358(6360), 179,1-179. https://doi.org/10.1126/science.aap9525
Brittain, J. E., & Milner, A. M. (2001). Ecology of glacier-fed rivers: Current status and concepts. Freshwater Biology, 46(12), 1571–1578. https://doi.org/10.1046/j.1365-2427.2001.00845.x
Camargo, J. A. (1992). Temporal and spatial variations in dominance, diversity and biotic indices along a limestone stream receiving a trout farm effluent. Water Air Soil Pollution, 63, 343–359. https://doi.org/10.1007/BF00475501
Clarke, K. R., & Ainsworth, M. (1993). A method of linking multivariate community structure to environmental variables. Marine Ecology Progress Series, 92, 205–219.
Clarke, K.R., Gorley, R.N. (2006) PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research). PRIMER-E, Plymouth.
Clarke K.R., Warwick R.M. (2001) Change in marine communities: An approach to statistical analysis and interpretation, 2nd edn. PRIMER-E Ltd Plymouth Marine
Cuffney T. F., Gurtz, M. E. , & Meador, M. R. (1993) Methods for collecting benthic invertebrate samples as part of the National Water-Quality Assessment Program. U.S. Geological Survey OpenFile Report 93-406. US Geological Survey.
Cuffney, T. F., Meador, M. R., Porter, S. D., & Gurtz, M. E. (2000). Responses of physical, chemical, and biological indicators of water quality to a gradient of agricultural land use in the Yakima River Basin, Washington. Environmental Monitoring and Assessment, 64, 259–270.
Dar G.H., Bhagat R.C., Khan M.A. (2002). Biodiversity of the Kashmir Himalaya. Valley Book House, Srinagar. 399 pp.
Dodds, W. K., Jones, J. R., & Welch, E. B. (1998). Suggested classification of stream trophic state: Distributions of temperate stream types by chlorophyll, total nitrogen, and phosphorus. Water Research, 32, 1455–1462.
Duan, X., Wang, Z., & Tian, S. (2008). Effect of streambed substrate on macroinvertebrate biodiversity. Frontiers of Environmental Science & Engineering China, 2(1), 122–128.
Edegbene, A. O., Odume, O. N., & Arimoro, F. O. (2021). Identifying and classifying macroinvertebrate indicator signature traits and ecological preferences along urban pollution gradient in the Niger Delta. Environmental Pollution, 281, 117076. https://doi.org/10.1016/j.envpol.2021.117076
Edema, C. U., Ayeni, J. O., & Aruoture, A. (2002). Some observations on the zooplankton and macrobenthos of the Okhuo River, Nigeria. Journal of Aquatic Sciences, 17(2), 145–149.
Edmondson, W.T. (1959). Fresh-Water Biology 2nd Ed. New York (NY) John Wiley and Sons, INC. pp. 1050–1056.
Engblom, E., & Lingdell, P. E. (1999). Analyses of benthic invertebrates. In L. Nyman (Ed.), River Jhelum, Kashmir Valley- Impacts on the aquatic environment (pp. 39–75). SWEDMAR.
Fazal, S., & Amin, A. (2011). Impact of urban land transformation on water bodies in Srinagar City. India Journal of Environmental Protection, 2, 142–153. https://doi.org/10.4236/jep.2011.22016
Ferreira, V., Elosegi, A., Tiegs, S.D., von Schiller, D. & Young, R. (2020). Organic matter decomposition and ecosystem metabolism as tools to assess the functional integrity of streams and rivers—a systematic review. Water 12, 3523. https://doi.org/10.3390/w12123523
Hamid, A., Dar, N. A., Bhat, S. U., & Pandit, A. K. (2016). Water quality index: A case study of Vishav stream, Kulgam, Kashmir. International Journal of Environment and Bioenergy., 5(2), 1–15.
Hamid, A., Bhat, S.U., Jehangir, A. (2021). Assessment of ecological characteristics of macroinvertebrate communities and their relationship with environmental factors in a stream ecosystem. Chemistry and Ecology, 1–21 https://doi.org/10.1080/02757540.2021.1987419
Harding, J. S., Young, R. G., Hayes, J. W., Shearer, K. A., & Stark, J. D. (1999). Changes in agricultural intensity and river health along arriver continuum. Freshwater Biology, 42, 345–357.
Hering, D., Feld, C. K., Moog, O., & Ofenbock, T. (2006). Cook book for the development of a Multimetric-Index for biological condition of aquatic ecosystems: Experiences from the European AQEM and STAR projects and related initiatives. Hydrobiologia, 566, 311–324.
Hoang, H.T.T., Duong, T.T., Nguyen, K.T., Le, Q.T.P., Luu, M.T.N., Trinh, D.A., Le, A.H., Ho C.T., Dang K.D., Némery J., Orange D., Klein J. (2018). Impact of anthropogenic activities on water quality and plankton communities in the Day River (Red River Delta, Vietnam). Environment Monitoring Assessment, 190. https://doi.org/10.1007/s10661-017-6435-z
Ikomi, R. B., Arimoro, F. O., & Odihirin, O. K. (2005). Composition, distribution, and abundance of macroinvertebrates of the upper reaches of River Ethiope, Delta State, Nigeria. The Zoologist, 3, 68–81.
Ilmonen, J., & Paasivirta, L. (2005). Benthic macro crustacean and insect assemblages in relation to spring habitat characteristics: Patterns in abundance and diversity. Hydrobiologia, 533(1–3), 99–113.
Ivol-Rigaut, J. M., Guinand, B., Richoux, P., & Tachet, H. (1997). Longitudinal changes in Trichoptera and Coleoptera assemblages and environmental conditions in the Loire River (France). Archiv für Hydrobiology, 138, 525–557.
Jacobsen, D., & Marín, R. (2008). Bolivian Altiplano streams with low richness of macroinvertebrates and large diel fluctuations in temperature and dissolved oxygen. Aquatic Ecology, 42, 643–656.
Jun, Y. C., Kim, N. Y., Kim, S. H., Park, Y. S., Kong, D. S., & Hwang, S. J. (2016a). Spatial distribution of benthic macroinvertebrate assemblages in relation to environmental variables in Korean nationwide streams. Water (switzerland), 8(1), 1–20.
Jun, Y.-C., Kim, N.-Y., Kim, S.-H., Park, Y.-S., Kong, D.-S., & Hwang, S.-J. (2016b). Spatial distribution of benthic macroinvertebrate assemblages in relation to environmental variables in Korean nationwide streams. Water, 8, 27. https://doi.org/10.3390/w8010027
Kaboré, I., Moog, O., Alp, M., et al. (2016). Using macroinvertebrates for ecosystem health assessment in semi-arid streams of Burkina Faso. Hydrobiologia, 766, 57–74. https://doi.org/10.1007/s10750-015-2443-6
Kaufmann P. R., Levine P., Peck D. V., Robison E. G., Seeliger C. (1999). Quantifying physical habitat in Wadeable streams (p. 149). USEPA (National Health and Environmental Effects Research Laboratory, Western Ecology Division). https://archive.epa.gov/emap/archive-emap/web/html/phyhab.html
Khan, M. A. (1993a). Occurrence of a rare euglenoid causing red-bloom in Dal Lake waters of the Kashmir Himalaya. Archiv Für Hydrobiologie, 127, 101–103.
Khan, M. A. (1993b). Euglenoid red bloom contributing the environmental pollution of Dal Lake, Kashmir Himalaya. Environment Conservation, 20, 352–356.
Khanday, S. A., Bhat, S. U., Islam, T. S., & Sabha, I. (2020). Identifying lithogenic and anthropogenic factors responsible for spatio-seasonal patterns and quality evaluation of snow melt waters of the River Jhelum Basin in Kashmir Himalaya. CATENA, 196, 104853. https://doi.org/10.1016/j.catena.2020.104853
Landrigan, P. J., Fuller, R., Fisher, S., Suk, W. A., Sly, P., Chiles, T. C., et al. (2018). Pollution and children’s health. Science of Total Environment, 650(Pt 2), 2389–2394. https://doi.org/10.1016/j.scitotenv.2018.09.375
Langdon, P. G., Ruiz, Z., Brodersen, K. P., & Foster, I. D. L. (2006). Assessing lake eutrophication using chironomids: Understanding the nature of community response in different lake types. Freshwater Biology, 51, 562–577.
Ligeiro, R., Hughes, R. M., Kaufmann, P. R., Heino, J., Melo, A. S., & Callisto, M. (2020). Choice of field and laboratory methods affects the detection of anthropogenic disturbances using stream macroinvertebrate assemblages. Ecological Indicators, 115, 106382.
Ludwig, J. A., & Reynolds, J. F. (1988). Statistical Ecology. John Wiley.
Magurran, A. E. (2003). Measuring biological diversity. Oxford: Blackwell Science. 1–264. http://eu.wiley.com/WileyCDA/WileyTitle/productCd-0632056339.html. Accessed on 04-6-2020.
Malmqvist, B., & Hoffsten, P. O. (2000). Macroinvertebrate taxonomic richness, community structure and nestedness in Swedish streams. Archiv Für Hydrobiologie, 150(1), 29–54. https://doi.org/10.1127/archiv-hydrobiol/150/2000/29
Maneechan, W., & Prommi, T. O. (2015). Diversity and distribution of aquatic insects in stream of the Mae Klong watershed, western Thailand. Psyche: A Journal of Entomology, 2015(2), 1–7. https://doi.org/10.1155/2015/912451
Mason, C. F. (2002). Biology of freshwater pollution (4th ed.). Prentice Hall.
McAllister D. E., Hamilton A. L., & Harvey B. (1997). Global freshwater biodiversity: Striving for the integrity of freshwater ecosystems. Sea Wind, 11(3), 1 142. http://hdl.handle.net/10625/14024
McCafferty, W. P., & Provonsha, A. V. (1998). Aquatic entomology: The fishermen’s and Ecologists’ illustrated guide to insects and their relatives (p. 448). Jones and Bartlett Publishers.
Medupin, C. (2019). Distribution of benthic macroinvertebrate communities and assessment of water quality in a small UK river catchment. SN Applied Science, 1, 544. https://doi.org/10.1007/s42452-019-0464-x
Merritt, R. W., & Cummins, K. W. (2006). Trophic relationships. In Methods in stream ecology (2nd ed., pp 585–610). Academic Press. https://doi.org/10.1016/B978-0-12-416558-8.00020-2
Miyake, Y., & Nakano, S. (2002). Effects of substratum stability on diversity of stream invertebrates during baseflow at two spatial scales. Freshwater Biology, 47(2), 219–230. https://doi.org/10.1046/j.1365-2427.2002.00798.x
Moore J. C. (2013). Diversity, taxonomic versus functional. In Levin, S. A. (Ed.), Encyclopedia of biodiversity (2nd Ed., pp 648–656). Academic Press. https://doi.org/10.1016/B978-0-12-384719-5.00036-8
Mourier L., Bauer A., Newell C. (2019). Benthic macroinvertebrate and water quality characterization of the Yampa–Green Rivers. Ecogeomorphology, GEL 136 Final Paper. https://watershed.ucdavis.edu/education/classes/files/content/page/Ecogeo%20Bug%20Paper%20CN_AB_LM%20Final.pdf
Musonge, P. L. S., Boets, P., Lock, K., Ambarita, M. N. D., Forio, M. A. E., & Goethals, P. L. M. (2020). Rwenzori Score (RS): A benthic macroinvertebrate index for biomonitoring rivers and streams in the Rwenzori Region. Uganda. Sustainability, 12, 10473. https://doi.org/10.3390/su122410473
Negi, P., & Singh, D. (2021). Benthic macroinvertebrates diversity and quality of water in first-order streams of Badiyar Gad, lesser Himalaya, Uttarakhand. India. International Journal of Environmental Studies. https://doi.org/10.1080/00207233.2021.1992119
Newall, P., & Walsh, C. J. (2005). Response of epilithic diatom assemblages to urbanization influences. Hydrobiologia, 532, 53–67.
Nicacio, G., Cunha, E. J., Hamada, N., et al. (2020). How habitat filtering can affect taxonomic and functional composition of aquatic insect communities in small Amazonian streams. Neotropical Entomology, 49, 652–661. https://doi.org/10.1007/s13744-020-00780-z
Oliveira, V. A., de Mello, C. R., Viola, M. R., & Srinivasan, R. (2017). Assessment of climate change impacts on streamflow and hydropower potential in the headwater region of the Grande river basin, Southeastern Brazil. International Journal of Climatology, 37(15), 5005–5023. https://doi.org/10.1002/joc.5138
Pandiarajan, S., Thambiratnam, S., Rajasekaran, I., & Sivaruban, B. (2019). Bio-monitoring and detection of water quality using Ephemeroptera, Plecoptera and Trichoptera (EPT) complex in Karanthamalai Stream of Eastern Ghats. Indian. Journal of Ecology, 46(4), 818–822.
Pandit A.K. (1999) Trophic structure of plankton community in some typical wetlands of Kashmir, India. In: Mishra SR (ed) Limnological research in India. Daya Publishing House, Delhi-110035 p 190–224
Paul, M. J., & Meyer, J. L. (2001). Streams in the urban landscape. Annual Review of Ecology and Systematics, 32, 333–365.
de Paula, F. R., Gerhard, P., Ferraz, SFd. B., & Wenger, S. J. (2018). Multi-scale assessment of forest cover in an agricultural landscape of Southeastern Brazil: Implications for management and conservation of stream habitat and water quality. Ecological Indicators, 85, 1181–1191.
De Pauw, N., Gabriels, W., & Goethals, P. (2006). River monitoring and assessment methods based on macroinvertebrates. In Ziglio, G., Siligardi, M., & Flaim G. (Eds.), Biological monitoring of rivers: Applications and perspectives (pp 113–134). John Wiley & Sons. https://doi.org/10.1002/0470863781.ch7
Pennak, R. W. (1978). Freshwater invertebrates of United States. John Wiley and Sons.
Pitt R. (2002). Receiving water impacts associated with urban runoff. Pages 1–30 in D. Hoffman, B. Rattner. G.A. Burton, Jr. and J. Cairns Jr. Handbook of Ecotoxicology, 2 nd Edition. CRC - Lewis. Boca Raton Fl.
Rashid, I., & Romshoo, S. A. (2013). Impact of anthropogenic activities on water quality of Lidder River in Kashmir Himalayas. Environmental Monitoring and Assessment, 185(6), 4705–4719. https://doi.org/10.1007/s10661-012-2898-0
Rosenzweig, M. L. (1995). Species diversity in space and time. Cambridge University Press.
Sabha, I., Bhat, S. U., Hamid, A., & Rather, J. A. (2019). Monitoring stream water quality of Dagwan Stream, an important tributary of Dal Lake. Kashmir Himalaya. Arabian Journal of Geosciences, 12, 273. https://doi.org/10.1007/s12517-019-4
Sabha, I., Khanday, S. A., Islam, S. T., & S.T., Bhat S.U. (2020). Longitudinal and temporal assemblage patterns of benthic macroinvertebrates in snow melt stream waters of the Jhelum River Basin of Kashmir Himalaya (India). Ecohydrology, 13(7), e2236.
Santos, J. M., & Ferreira, M. T. (2020). Use of aquatic biota to detect ecological changes in freshwater: Current status and future directions. Water, 12, 1611. https://doi.org/10.3390/w12061611
Serpa, D., Keizer, J. J., Cassidy, J., & Cuco, A. (2014). Assessment of river water quality using an integrated physicochemical, biological and ecotoxicological approach. Environmental Science: Processes & Impacts, 16, 1434–1444. https://doi.org/10.1039/C3EM00488K
Shah, A. H., Teli, P. A., & Bhat, M. S. (2014). Dynamics of land use/land cover change in Dal Lake watershed of Kashmir valley—a remote sensing and GIS approach. International Journal of Advanced Information Science and Technology. https://doi.org/10.15693/ijaist/2014.v3i12.1-9a
Shannon, C. E., & Weiner, W. (1949). The mathematical theory of communication (p. 144). University of Illinois Press, Urbana.
Sharma, R. C., Bhanot, G., & Singh, D. (2004). Aquatic macroinvertebrate diversity in Nanda Devi Biosphere Reserve. India. Environmentalist, 24(4), 211–221.
Simpson, E. H. (1949). Measurement of diversity. Nature, 163, 688.
Sofi, M. S., Bhat, S. U., Rashid, I., & Kuniyal, J. C. (2020). The natural flow regime: A master variable for maintaining river ecosystem health. Ecohydrology, 13(8). https://doi.org/10.1002/eco.2247
Sofi, M. S., Hamid, A., Bhat, S. U., Rashid, I., & Kuniyal, J. C. (2022). Biotic alteration of benthic macroinvertebrate communities based on multispatial-scale environmental variables in a regulated river system of Kashmir Himalaya. Ecological Engineering, 177, 106560. https://doi.org/10.1016/j.ecoleng.2022.106560
Stanford, J. A., Lorang, M. S., & Hauer, F. R. (2005). The shifting habitat mosaic of river ecosystems. SIL Proceedings, 29, 123–136. https://doi.org/10.1080/03680770.2005.11901979
Subramanian K. A., & Sivaramakrishnan K. G. (2007). Aquatic insects for biomonitoring freshwater ecosystems-A methodology manual. Ashoka Trust for Research in Ecology and Environment (ATREE), Bangalore, India. http://wgbis.ces.iisc.ernet.in/energy/water/paper/cistup_TR1/Indian_aqua_Insects.pdf. Accessed on 02-05-2018.
Tampo, L., Kaboré, I., Alhassan, E. H., Ouéda, A., Bawa, L. M., & Djaneye-Boundjou, G. (2021). Benthic macroinvertebrates as ecological indicators: Their sensitivity to the water quality and human disturbances in a tropical river. Frontiers in Water, 3, 662765. https://doi.org/10.3389/frwa.2021.662765
Tan, K. W., & Beh, W. C. (2016). Evaluation of water quality and benthic macroinvertebrates fauna relationship using principal component analysis (PCA): A case study of Cameron Highlands Malaysia. Environmental Management and Sustainable Development, 5, 1. https://doi.org/10.5296/emsd.v5i1.9399
Taylor, S. L., Robert, S. C., Walsh, C. J., & Hatt, B. E. (2004). Catchment urbanization and increased benthic algal biomass in streams: Linking mechanisms to management. Freshwater Biology, 49, 835–851.
Vander Laan, J. J., Hawkins, C. P., Olson, J. R., & Hill, R. A. (2013). Linking land use, in-stream stressors, and biological condition to infer causes of regional ecological impairment in streams. Freshwater Science, 32(3), 801–820. https://doi.org/10.1899/12-186.1
Walsh, C., Gooderham, J. P., Grace, M. R., Sdraulig, S., Rosyidi, M. I., & Lelono, A. (2002). The relative influence of diffuse and point-source disturbances on a small upland stream in East Java Indonesia: A preliminary investigation. Hydrobiologia, 487, 183–192.
Walsh, G., & Wepner, V. (2009). The influence of land use on water quality and diatom community structures in urban and agriculturally stressed rivers. Water SA, 35(5), 579–594. https://doi.org/10.4314/wsa.v35i5.49184
Walsh, J.C., Fletcher, T.D., Ladson, A.R. (2005). Stream restoration in urban catchments through redesigning storm water systems: Looking to the catchment to save the stream source: Journal of the North American Benthological Society, 24(3), 690–705. https://doi.org/10.1899/04-020.1. http://www.bioone.org/doi/full/10.1899/04-020.1
Ward, J. V. (1992). Aquatic insect ecology (p. 438). John Wiley.
Wenger, S. J., Roy, A., Jackson, C. R., & Walsh, J. C. (2009). Twenty-six key research questions in urban stream ecology: An assessment of the state of the science. Journal of the North American Benthological Society, 28(4), 1080–1098. https://doi.org/10.1899/08-186.1
WFD UKTAG. (2013) Water Framework Directive – United Kingdom Advisory Group (WFD-UKTAG). Environmental Standards River Basin Management 2015–2021. Wfd_Uktag, 2013
Zhang, J., Shang, Y., Liu, J., Fu, J., Wei, S. & Tong, L. (2020). Causes of variations in sediment yield in the Jinghe River Basin China. Scientific Reports, 10(1), 18054. https://doi.org/10.1038/s41598-020-74980-3
Acknowledgements
The authors are highly thankful to the Head, Department of Environmental Sciences, and the University of Kashmir for providing lab facilities and facilitating research work. Wildlife department, J&K, and Dachigam National Park officials are thanked for the permission to work in the Dachigam National Park.
Funding
This research work is funded and supported by SERB-DST having grant no. EMR/2016/000324 dated 24/03/2017.
Author information
Authors and Affiliations
Contributions
Inam Sabha: conceptualization, methodology, software, validation, visualization, roles/writing—original draft. Aadil Hamid: data curation, formal analysis, investigation, methodology, software; writing—original draft. Sami Ullah Bhat: conceptualization, supervision, validation, visualization, roles/writing—original draft, funding acquisition, project administration. Sheikh Tajamul Islam: formal analysis, investigation, methodology, writing—original draft.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sabha, I., Hamid, A., Bhat, S.U. et al. Water Quality and Anthropogenic Impact Assessment Using Macroinvertebrates as Bioindicators in a Stream Ecosystem. Water Air Soil Pollut 233, 387 (2022). https://doi.org/10.1007/s11270-022-05839-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05839-8