Abstract
In this study, agroactive and bioactive compound production by actinobacteria isolated from rhizospheres of different plants was screened. Six of the forty-two isolates showed the highest production of different compounds and were identified by using molecular methods. In the primary and secondary screening antimicrobial activities were determined by inhibition zones against six bacterial and three fungal pathogens. Streptomyces labedae CYP25 showed the highest antibacterial activity in screening with extracts obtained from Actinomycetes Isolation Broth (AIB) against Staphylococcus aureus (18 mm); Streptomyces sp. CYP30 showed the highest antifungal activities in screening with ethyl acetate extracts obtained from Glucose Soybean Meal Broth against Candida albicans (60 mm) and Alternaria sp. D21 (52 mm) and showed the highest antifungal activities in screening with extracts obtained from International Streptomyces Project-3 (ISP-3) Broth against Aspergillus niger (16 mm). The highest indole acetic acid (IAA) production was showed by Nocardiopsis sp. CYP39. This isolate produced the highest amount of IAA (76.5 µg/mL) in the presence of 0.9% L-tryptophan. Thirty-two of forty-two isolates were identified as siderophore producers. The best hydrogen cyanide producer was Nocardiopsis sp. CYP15. Streptomyces moderatus CYP35 and Streptomyces chartreusis CYP19 constitute the highest halo-zone diameter on phosphate solubilizing activity tests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants that are widely colonized by various microorganisms cause plant-microorganism interactions. Some of these interactions are beneficial for plants, while others are harmful. Plants are used by microorganisms as a food source or a habitat. In such a symbiotic interaction, it has been reported that the roots of many plants are infected with Rhizobia and actinobacteria (especially Streptomycetes) (Vurukonda et al. 2018).
There are bacteria in the rhizosphere of plants that accelerate their growth. In addition, these bacteria protect the plant from disease and abiotic stress on the root surface or depending on it (De Souza et al. 2015).
Rhizospheric bacteria and mycorrhizal fungi are among the microorganisms that show the highest efficiency in increasing plant growth and thus crop yield. Rhizospheric bacteria can increase the nutrient intake of the plants they colonize in the rhizosphere soil. Therefore, they can be considered efficient biofertilizers. Streptomyces spp., one of the important genus in actinobacteria also belongs to the rhizospheric microbial communities, but in recent studies, it has been more emphasized as plant growth promoter than other bacteria. These microorganisms, which have great potential as a renewable and environmentally friendly plant food source, can increase agricultural production and soil fertility (Vurukonda et al. 2018). Rhizospheric actinobacteria are predominant, with economic importance to humans because both agricultural and forest fields depend on their contributions to soil systems (Boukhatem et al. 2022).
Synthetic agrochemicals also pose serious risks to human health and the ecosystem, making it necessary to develop alternative approaches such as microbial bio-fermentors and biocontrol agents, the use of bioinoculants for disease control, and plant growth enhancement (Ningthoujam et al. 2016).
Nowadays, effective agricultural practices are based on the widespread use of chemical fertilizers to increase plant growth and yield. However, the cost, environmental concerns, and the resulting human health hazards arising from the introduction of these chemical fertilizers in the food chain are the main limiting factors. Microorganisms have been the source of important natural compounds with agroactive importance. The use of microbial consortia in the form of biofertilizers to reduce the use of chemical fertilizers, and pesticides is an important research area in agriculture, microbiology, and biotechnology today without sacrificing plant yield (Anwar et al. 2016).
Studies have shown that the discovery of new antimicrobial agents came mostly from natural sources. These new natural products have been found to have significant biological activities and produce a significant number of therapeutic agents around the world. They even act as templates for the synthesis of synthetic and semi-synthetic drugs (Adegboye and Babalola 2013). A lot of research is being done to control pathogens and identify new antimicrobial agents. Generally, microorganisms produce bioactive molecules that are useful for defense mechanisms but do not need them for their growth and development. Microorganisms isolated from the soil are the most important natural resources exhibiting strong biological activity against a wide range of pathogens (Ganesan et al. 2017). It is estimated that about two-thirds of naturally occurring antibiotics are derived from actinobacteria. Actinobacteria can be considered fertile sources of structurally diverse secondary metabolites; most of them have pharmaceutically relevant biological activities. Approximately 23,000 bioactive secondary metabolites have been reported from microorganisms, more than 10,000 of them are from actinobacteria and constitute 45% of all bioactive microbial metabolites. Thus, identifying new actinobacteria species has been an important component of natural product-based drug discovery in recent years (Sudha and Hemalatha 2015).
A good alternative to chemicals is microbial sources, which can have multifunctional and beneficial effects on plants. Actinobacteria are generally considered to be potent natural biocontrol agents in soil. They compete with pathogens through the production of different secondary metabolites and enzymes, and other biological control activities (Mitra et al. 2022).
New environmentally friendly approaches are required to improve plant biomass production. Beneficial plant growth-promoting actinobacteria can be used as excellent and efficient biotechnological tools to enhance plant growth in different environments such as stressful environments. We provide different mechanisms that improve and contribute to plant growth, development, and health. This study aims to isolate actinobacteria from rhizospheres of various plants, screen them in terms of their plant growth-promoting properties and determine their bioactive compound production potential. The actinobacteria isolated in this study are important for plant and human health due to their antifungal and antibacterial activity against both phytopathogens and human pathogens.
Materials and methods
Collection of rhizospheric soil samples and isolation of actinobacteria
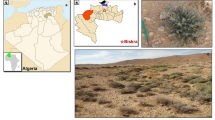
Rhizospheric soil samples were taken from the rhizosphere regions of 5 different terrestrials (Heracleum platytaenium (Cow parsnip), Morus alba (Mulberry), Cucumis sativus (Cucumber), Lycopersicon esculentum (Tomato)) and 1 aquatic plant (Fig. 1) aseptically, at a 5–15 cm depth and a distance of approximately 3 cm from the root (Kaur et al. 2013). One gram of soil sample was mixed in 9 mL of autoclaved dilution solutions (0.85% saline solution) and serially diluted to a final dilution of 10− 6. The 0.1 mL of each dilution was spread on Actinobacteria Isolation Agar (AIA) (Difco, 212,168). To prevent fungal growth, AIA was supplemented with a concentration of 50 mg/mL cycloheximide as an antifungal agent. After incubation at 28 °C for 4–7 days, different actinobacteria colonies were selected considering morphological features such as different colony shapes and colors. These actinobacteria colonies were purified on AIA and kept at + 4 °C for further studies (Gopalakrishnan et al. 2014).
Molecular identification and phylogenetic analysis of actinobacteria
DNA isolation was performed using the Zymo Research fungal/bacterial DNA isolation kit (Zymo Research, USA). Bacterial cells were taken into eppendorf tubes from 3-day cultures produced Actinomycetes Isolation Broth (AIB). The pellets were centrifuged for 10 min. at 10000 rpm to remove AIB residues. The supernatant was discarded, and pellets were washed with sterile distilled water by mixing up and down. The tubes were centrifuged at 10000 rpm for 10 min. and the supernatant was discarded. The DNA isolation procedure continued with the pellets remaining at the bottom of the tubes. The isolation process was completed according to the kit manual. The procedure corresponding to the kit content used was followed respectively. Q5 Hot Start High Fidelity DNA polymerase kit with bacterial specific universal primer sets 8–27 F (Forward, 5’- AGAGTTTGATCCTGGCTCAG) and 1492 − 1510 R (Reverse, 5’- GGCTACCTTGTTACGACTT) was used to perform the PCR reaction (Koçyiğit 2009). The PCR products were sequenced and obtained 16 S rRNA gene sequences were compared with others in the GenBank nucleotide library by a Basic Local Alignment Search Tool (BLAST) search through the National Center for Biotechnology Information (NCBI). The dendrogram of the phylogenetic relationship with other species were made using Mega 6 program (Tamura et al. 2007).
Screening for bioactive metabolite production by rhizospheric actinobacteria
Primary screening
Primary screening of the antibacterial activity was determined on Mueller Hinton Agar (MHA) by the cross-streak method (Chaudhary et al. 2013). The agar plug method was used as the primary screening method for determining antifungal activity (Mohanraj et al. 2011).
In antibacterial activity screenings, Bacillus cereus ATCC 7064, Enterococcus faecalis ATCC 29,212, Escherichia coli ATCC 29,998, Salmonella typhimurium, Pseudomonas aeruginosa ATCC 27,853, Staphylococcus aureus ATCC 6538/P were used as bacterial test organisms (Chaudhary et al. 2013). In antifungal activity screenings, Candida albicans, Aspergillus niger (Mohanraj et al. 2011), and Alternaria sp. D21 (Toker et al. 2021) was used as fungal test organisms.
Secondary screening
Actinobacteria isolates showing promising antimicrobial activity in primary screening were incubated at 30 °C with continuous agitation at 150 rpm using five different media (AIB, Glucose Soybean Meal Broth (GSB), Starch Casein Broth (SCB), International Streptomycetes Project 2 and 3 Broth (ISP-2 and ISP-3 Broth) for 7 days (Gopinath et al. 2013; Mohseni et al. 2013; Singh et al. 2014; Duddu and Guntuku 2016; Chandrakar and Gupta 2017). The antimicrobial activity of the cell-free supernatant of the isolates against the same test organisms used in primary screening was demonstrated using the disc diffusion method (Khamna et al. 2009). Bacterial test suspensions were adjusted to be equivalent to a 0.5 McFarland turbidity standard tube at 600 nm using a spectrophotometer. This is equal to 1.5 × 108 cfu/mL (Jobim et al. 2014). Fungal test organisms were counted with the help of Thoma cell counting chamber and adjusted to 3.0 × 107 cfu/mL. and 0.1 mL of each test organism was taken and spread to MHA. six mm diameter sterilized discs were added to the MHA containing the test organisms under aseptic conditions and 10 µL of the supernatant of each isolate was transferred onto these discs. The petri plates were then kept at room temperature for 30 min for antimicrobial diffusion and then incubated at 37 °C. At the end of 24 h, inhibition zones around the disc were measured (Rajan and Kannabiran 2014).
Extraction
Isolates selected as a result of screening with the supernatant were incubated in five different media for 7 days at 28 °C at 150 rpm in a shaking incubator (Gopinath et al. 2013; Mohseni et al. 2013; Singh et al. 2014; Duddu and Guntuku 2016; Chandrakar and Gupta 2017). The culture broth was then centrifuged for 20 min at a rotational speed of 5000 rpm. Bioactive metabolites produced in different liquid culture media are mixed with an equal volume of ethyl acetate (1:1) in a separation funnel twice for 15 min. It is extracted from the supernatant by shaking for a while. The solvent layer, one of the two layers formed as a result of this process, was separated and after this process, it was evaporated under vacuum in a water bath of 40 °C in the evaporator (Singh et al. 2014). Extracts were dissolved in ethyl acetate; the test organisms were used and the procedure was applied the same described above. Ethyl acetate, streptomycin, and cycloheximide were used as a negative and positive control for antibacterial and antifungal activity, respectively.
Screening for plant growth-promoting activities of rhizospheric actinobacteria
Indole acetic acid production
Actinobacteria isolates were grown on ISP-2 agar medium (g/L: yeast extract 4; malt extract 10; dextrose 4; peptone 5) at 30 °C for 7 days. Agar discs with a diameter of 1 cm were cut using sterile apparatus and inoculated into 100 mL ISP-2 broth containing 0.2% L-tryptophan. Isolates were incubated in this environment at 30 °C for 14 days at 150 rpm with continuous shaking. After incubation, samples were centrifuged at 11,000 rpm for 15 min and 1 mL of supernatant was mixed with 2 mL of Salkowski’s reagent. An uninoculated sterile medium was used as a blank. It was then incubated at 30 °C in the dark for 25 min. IAA production was observed as the development of a pink-red color (Passari et al. 2015).
Among the actinobacteria isolates, those showing red-pink color were measured at 535 nm using a spectrophotometer. The absorbance values are compared with the IAA standard curve (0-0.8 µg/mL) and the amount of IAA is expressed in µg/mL (Passari et al. 2015). The highest producer isolate was inoculated into ISP-2 medium prepared at L-tryptophan concentrations ranging from 0 to 1% to show the effect of incubation time and the amount of L-tryptophan on IAA production. The amount of IAA was measured for 14 days and expressed in µg/mL, using the method previously described (Abd-Alla et al. 2013; Patel and Patel 2014).
Siderophore production
Siderophore production of rhizospheric isolates was determined by the protocol using the indicator dye, chromium azurol S (CAS) described by Schwyn and Neilands. Isolates that exhibit a yellowish-orange halo-zone after 5 days of incubation at 28 °C were considered positive for siderophore production (Lakshmanan et al. 2015).
Hydrogen cyanide production
The production of hydrogen cyanide (HCN) by actinobacteria isolates has been evaluated by adapting the method of Lorck. Whatmann No.1 filter paper was soaked in 2% sodium carbonate in 0.5% picric acid for one minute. The filter papers soaked in this solution were then placed under the petri plates. Next, ISP-2 medium supplemented with 4.4 g/L glycine was evenly distributed in the petri plates and allowed to solidify. After the actinobacteria isolates were planted in this environment, the petri plates were covered with parafilm and left to incubation at 28 °C for 7–12 days. The red-brown color in the filter paper was indicative of HCN production (Anwar et al. 2016).
Phosphate solubilizing activity
Actinobacteria isolates were grown on Pikovskaya’s agar (PVKA) (g/L: yeast extract; 0.5; Dextrose 10; Ca3PO4 5; NH4SO4 0.5; KCl 0.2; MgSO4 0.1; MnSO4 0.0001; FeSO4 0.0001; agar 15) which is developed by Pikovskaya. The formation of a clear zone around the colony by dissolving Ca3(PO4) on the petri plate has been accepted as an indicator of phosphate solubility. While determining phosphate solubility, halo-zone and colony diameters were measured after 14 days of incubation at 27 °C. The halo-zone diameter was calculated by subtracting the colony diameter from the total diameter (Franco-Correa et al. 2010). After 14 days, isolates with a halo-zone diameter of 9 mm or greater were considered positive for phosphate solubility and were selected for quantitative screening. (Dastager and Damare 2013)
For quantitative measurement of soluble phosphate, 1 cm agar discs from 7-day old actinomycete cultures were inoculated into 100 mL Pikovskaya broth containing 0.1 g of tricalcium phosphate. The samples were incubated for 9 days in a shaking incubator at 28 °C at 150 rpm. Culture supernatants of 20 mL were taken from each flask and 30 min (15,000 rpm) and the amount of water-soluble phosphate released in the supernatant was determined on days 3, 5, 7, and 9 by the reduced chlorostannous molybdophosphoric blue acid method. 10 mL of supernatant, 10 mL of chloromolybdic acid, and 1 mL of chlorostannous acid were mixed and the volume was completed to 50 mL with distilled water. The pH values of the culture fluids taken on the same days were also recorded. An uninoculated sterile medium was used as a blank. The blue color formed was read at 600 nm with a spectrophotometer. The amount of soluble phosphate was calculated using the standard curve of KH2PO4 (Wani and Irene 2014; Kaur 2014).
Results
Isolation of rhizospheric actinobacteria
As a result of isolation from soil samples collected from rhizosphere regions of four terrestrial and one aquatic plant, forty-two actinobacteria colonies with different shapes and colors were selected and isolated from all dilution plates. The isolated actinobacteria and isolation areas are given in Table 1.
Molecular identification and phylogenetic analysis of actinobacteria
After the determination of the base sequences of the products obtained as a result of the polymerase chain reaction, comparisons were made with the other base sequences in the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The similarity rates and matching results of the isolates are given in Table 2. The phylogenetic dendrogram of the identified isolates is given in Fig. 2.
Screening for bioactive metabolite production by rhizospheric actinobacteria
As a result of primary and secondary screening of bioactive compound production, the isolates with the best antibacterial and antifungal activities were Streptomyces labedae CYP25 and Streptomyces sp. CYP30, respectively. These actinobacteria were selected for extraction steps. Streptomyces labedae CYP25 showed the highest antibacterial activity against S. aureus (18 mm) in AIB in the screening performed with ethyl acetate extracts (Table 3) (Fig. 3).
Streptomyces sp. CYP30 showed the highest antifungal activity against C. albicans (60 mm), Alternaria sp. D21 (52 mm) in GSB and A. niger (16 mm) in ISP-3 Broth (Table 4) (Fig. 4).
Screening for plant growth-promoting activities of rhizospheric actinobacteria
Indole acetic acid (IAA) production
As a result of the 14-day incubation in ISP-2 medium supplemented with tryptophan, twelve of the forty-two actinobacteria isolates produced IAA. These twelve isolates with positive results were selected for quantitative screening. The amount of IAA formed by the isolates ranges from 5.0 to 51.04 µg/mL. Streptomyces labedae CYP25 produced the lowest IAA with 5.0 µg/mL, while the highest production was performed by Nocardiopsis sp. CYP39 with 51.04 µg/mL. The highest producer Nocardiopsis sp. CYP39 was used to determine the effect of different L-tryptophan concentrations on IAA production.
IAA production was detected in very low amounts in medium without L-tryptophan. No IAA production was observed at any of the L-tryptophan concentrations during the first 4 days of the incubation period. IAA production process started on the 5th day and continued increasingly. Production amount varies between 4.7 and 76.5 µg/mL.
The lowest production (4.7 µg/mL) was on the 6th day in the medium containing 0.1% L-tryptophan, while the highest production (76.5 µg/mL) was on the 13th day in the medium containing 0.9% L-tryptophan. The earliest production was on day 5 in media containing 0.2% and 0.3% L-tryptophan. The latest production took place on the 11th day in a medium containing 1% L-tryptophan. Towards the end of the incubation period, the amount of production at all L-tryptophan concentrations stabilized or increased slightly.
Siderophore production
Of the forty-two actinobacteria isolates screened on CAS agar, thirty-four produced a siderophore. The highest halo-zone diameters were created by Streptomyces labedae CYP25 and Nocardiopsis sp. CYP15 (Fig. 5).
Hydrogen cyanide production
Color changes on filter papers were investigated after 12 days of incubation of isolates in ISP-2 agar medium supplemented with glycine. It was observed that 9 of 42 actinobacteria isolates produced HCN. It was determined that the best HCN producer is Nocardiopsis sp. CYP-15.
Phosphate solubilizing activities
It was determined that 15 of the 42 isolates used in this study performed the phosphate solubility activity. The halo-zone diameters of the isolates vary between 3 and 11 mm at the end of the 14th day. CYP7 isolate had the lowest halo-zone diameter and Streptomyces moderatus CYP35 had the highest halo-zone diameter (Fig. 6). At the end of day 14, two isolates (Streptomyces chartreusis CYP19 and Streptomyces moderatus CYP35) with a halo-zone diameter > 9 mm were selected for quantitative screening.
Both isolates, whose quantitative phosphate solubility screening was performed, achieved the highest soluble phosphate production on the 9th day of the incubation period. The isolate with the highest phosphate solubility activity was found to be Streptomyces moderatus CYP-35. Streptomyces chartreusis CYP19 produced 114.2 µg/mL, while Streptomyces moderatus CYP35 produced 196.49 µg/mL. With the increase in phosphate solubility, there was a decrease in the pH of the medium. Streptomyces moderatus CYP35 decreased pH from 7 to 3.82 and Streptomyces chartreusis CYP19 from 7 to 3.96.
Discussion
Microorganisms in the rhizosphere significantly support the growth of plants and help plant yield. Actinobacteria are one of the main components of rhizosphere microbial populations. In addition to its effects on the nutrient cycle in the soil, it also has very important effects on plant growth support. Actinobacteria produce secondary metabolites such as lytic enzymes, agroactive compounds, and antibiotics (Sreevidya et al. 2016).
As a result of secondary antibacterial activity studies, the type of isolate selected as promising was determined as Streptomyces labedae isolate CYP25 and was isolated from marine sources. In the trials with extracts, Streptomyces labedae isolate CYP25 exhibited antibacterial activity in all media used against all bacterial test organisms. However, this isolate formed the highest zone diameter at 18 mm against S. aureus in the AIB medium. Mohseni et al. (2013) reported an actinobacterial isolate of unidentified marine origin with the code of MN39 formed a zone diameter of 20.5 mm against S. aureus in AIB medium. Ganesan et al. (2017) reported the antibacterial activity experiment was carried out using the extract of the soil isolate Streptomyces rimosus FM-20 obtained by incubating in ISP-2 medium, using 10 times more extracts than the one used in this study, and this strain created a zone diameter of 16 mm against S. aureus. When all these results are evaluated, it is seen that the antibacterial activities of sea-originated or marine-adapted isolates against certain pathogens are higher than those of soil origin. Besides all these plants; actinobacteria play an important role as biocontrol agents through their antagonistic activities, while providing nutrients to actinobacteria in the rhizosphere with the secretions of carbohydrates, amino acids, organic acids, and other secretions (Ng and Amsaveni 2012).
As a result of secondary antifungal activity studies, the type of isolate selected as promising was determined as Streptomyces CYP30. Streptomyces sp. CYP30 showed the highest antifungal activity in GSB medium against all fungal pathogenic organisms used in the experiment. It has formed the highest zone diameter (60 mm) against C. albicans. In addition, the antifungal activities against Alternaria sp. D21 and Aspergillus niger with zone diameters of 52 mm and 16 mm were determined using the same medium. In a similar study, Singh and Rai (2012) reported GSB was chosen as the best medium among many and it was found that Streptomyces rimosus Y8 created a zone diameter of 28.6 mm against C. albicans, 37 mm against Alternaria alternata, and 32 mm against A. niger. When compared with this study, the zone diameter formed against C. albicans and Alternaria sp. D21 is significantly higher, but it displayed lower activity against A. niger. It is emphasized that the reason for this difference may be due to the specificity of the bioactive substance produced by each organism and the marked changes in its action mechanism.
Currently, the incidence of multidrug-resistant microorganisms is increasing and jeopardizing the treatment of a growing number of infectious diseases. Therefore, there is a need to develop new drugs that are effective against pathogens resistant to existing antimicrobials. Actinobacteria have been proven for many years as a potential source of bioactive compounds and rich secondary metabolites (Varalakshmi et al. 2014). In addition to the ongoing war with human pathogens in the field of health, it is of great importance to isolate and study actinobacteria that produce such high and perhaps new bioactive compounds from the rhizosphere to prevent various pathogenic microorganisms that cause problems in the agricultural field and may cause problems in the food field.
It has been shown that natural compounds are derived from microorganisms for various industries. Also, it is known that phytopathogenic fungi cause great losses in the yield of products obtained from plants and plant diseases. Pesticides are generally used in the control of plant diseases. In addition, the use of pesticides causes environmental pollution and adverse effects on naturally occurring organisms. However, the use of microorganisms as biological control agents has the potential to control plant pathogens and has no adverse effect on the environment or other naturally occurring organisms. There are reports that biocontrol agents can be used instead of agricultural chemicals (Khamna et al. 2009). In light of all this information, forty-two actinobacteria isolates; including indole acetic acid production, phosphate solubilizing capacity, siderophore production, HCN production, and ammonia production were investigated in terms of plant growth-promoting properties.
IAA ranges from 5.0 to 51.04 µg/mL in the presence of exogenous L-tryptophan. Aouar et al. (2016) reported IAA production ranging from 0.38 to 18.45 µg/mL of 6 actinobacteria isolated from rhizospheric soils. Mohandas et al. (2013) observed IAA production in actinobacteria ranging from 5.0 to 12 µg/mL. Rodrigues et al. (2018) reported that 9 of 12 isolates produced IAA ranging from 3.13 to 50.45 µg/mL. Damam et al. (2016) showed that there was IAA production in actinobacteria isolated from the rhizosphere of medicinal plants in amounts ranging from 0.008 to 2.50 µg/mL.
Legault et al. (2011) showed that in vitro IAA production in S. scabiei EF-35 is dependent on the availability of L-tryptophan in the growth medium. In contrast, the capacity of rhizospheric actinomycetes to produce IAA in vitro in the absence of L-tryptophan has been demonstrated (De Oliveira et al. 2010). Because actinomycetes use an alternative pathway that does not depend on L-tryptophan as a precursor for IAA synthesis (Aouar et al. 2016). In this study, although in limited amounts, IAA production was detected in the absence of exogenous L-tryptophan. Considering the information in the literature and this study, actinomycetes isolated from the rhizosphere differ significantly from those isolated from other isolation regions in that they can produce IAA in the absence of L-tryptophan.
Most PGPR (Plant Growth Promoting Rhizobacteria) actinomycetes synthesize IAA, which is responsible for increasing the number of adventitious roots, which helps the plant uptake large amounts of nutrients and absorb water. PGPR can produce IAA, thus improving plant growth by increasing seed germination, root elongation, and root dry weight (Anwar et al. 2016; El-Tarabily 2008). In this case, it contributes to the production of agricultural products that are sustainable and use fewer chemicals.
Low molecular weight iron chelators produced by many microorganisms to chelate ferric iron with high affinity are known as siderophores. The resulting ferric-siderophore complexes are returned to the cells by active transport mechanisms (Duddu and Guntuku 2016). This property is also beneficial for plants as the siderophores secreted by rhizospheric actinobacteria can significantly support plant growth by increasing iron uptake (Patel and Patel 2014; Anwar et al. 2016). Thirty-two of forty-two rhizospheric actinobacteria isolates produced siderophore. Among these isolates, the siderophore producers belong to Nocardiopsis and Streptomyces genera (Nocardiopsis sp. CYP15, Nocardiopsis sp. CYP39, Streptomyces labedae CYP25, Streptomyces chartreusis CYP19). Bhosale and Kadam (2015) reported that only nine of the nineteen rhizospheric actinobacteria isolates were produce siderophore, and most of the siderophore producers belong to the Streptomyces genus, a member of the Nocardiopsis genus did not produce siderophore. Anwar et al. (2016) reported that 87.5% of rhizospheric actinobacteria isolates produce siderophore and the strains producing siderophore were members of the Streptomyces genus.
Members of Streptomycetes are known for their ability to produce multiple siderophores that are independently organized and act conditionally to compete more efficiently in the environment. Siderophores produced by bacteria living in the plant rhizosphere are important for their proposed role in the biological control of soil-borne plant pathogens and disease-suppressing soils (Wang et al. 2014). The discovery of rhizosphere bacteria resistant to heavy metals and capable of promoting plant growth provides the potential for phytoremediation of heavy metal contaminated soils. The use of metal-resistant siderophore-producing rhizosphere bacteria is particularly important because they can provide plants with nutrients, especially iron, and reduce the harmful effects of metal contamination (Wang et al. 2014).
In our study 9 of 42 actinobacteria isolates were able to produce HCN, which plays a role in suppressing plant diseases. Rodrigues et al. (2018) reported that none of the 12 isolates were able to generate Bhosale and Kadam (2015) reported that HCN production was detected in 40% of rhizospheric isolates. In our study, Nocardiopsis sp. CYP15 was determined as the highest HCN producer. Anwar et al. (2016) reported that all HCN-producing isolates belong to the Streptomyces genus.
It shows that the ecological function of HCN produced by rhizobacteria depends on its interference with the release of elements from the mineral substrate, due to its ability to form complexes with transition metals in the mineral substrate. Recent research on mineral weathering in natural environments has shown that HCN-producing bacteria promote the mobilization of elements from rock-forming minerals (Frey et al. 2010; Lapanje et al. 2012; Wongfun et al. 2014). It is thought that by affecting the solubility of these elements, HCN may also indirectly interfere with phosphorus availability. Complexation of phosphate by calcium in basic soils or by aluminum and iron in acidic soils leads to the formation of insoluble metal-phosphate complexes. In acidic soils, since HCN can interact with iron, the main contribution of biogenic HCN is not in the increased decomposition of iron but the sequestration of phosphate leading to increased availability. It is hypothesized that biogenic HCN may increase the availability of nutrients in the substrate, resulting in increased plant growth support (Rijavec and Lapanje 2016).
Phosphorus is one of the most important nutritional requirements for plants. Phosphorus in the soil is found mainly in insoluble forms inaccessible to plants. Phosphate dissolving ability is therefore an important feature of plant growth-promoting rhizobacteria to increase plant yield (Anwar et al. 2016). In our study, 15 of the 42 isolates exhibited phosphate solubility activity in qualitative scans on Pikovskaya’s agar (PVKA). Aouar et al. (2016) reported that in the qualitative screening of PVKA, phosphate solubility activity was detected in 5 of 20 actinobacteria isolates.
Phosphate solubilizing microorganisms use different mechanisms to convert insoluble inorganic phosphate forms into soluble forms (Farhat et al. 2014). The best-characterized mechanism involves the secretion of organic acids or siderophore-like substances with high chelating power (Chaudhary et al. 2013; Dastager and Damare 2013) reported a decrease in pH (from 7 to 3.6) in their study. In this study, the decrease in pH values (from 7 to 3.82) observed during phosphate solubility tests suggested that it was due to the production of organic acids. The information in the literature supports this situation. In addition, most of the isolates that showed phosphate solubility activity also produce siderophores, therefore the effect of secretion of siderophores or siderophores-like substances on phosphate solubility activity has also been proven.
Phosphorus is the second most essential macronutrient for plant growth, after nitrogen, and is required in higher amounts. High phosphorus input applied for better crop yield causes environmental problems such as eutrophication. Therefore, phosphorus-solubilizing bacteria are emphasized for using phosphorus fixed in soil layers (Namlı et al. 2017). In recent decades, several phosphate-solubilizing bacteria exhibiting both heavy metal detoxifying properties and plant growth-promoting activities have been investigated and associated with the phytoremediation of different soils (Ahemad 2015; Guo et al. 2021).
This study was aimed to screen bioactive and agroactive compounds from actinobacteria. It is important to detect these two important groups of compounds in the same organism. Because, within the scope of food safety, the presence of various organisms in foods is undesirable and if detected, it causes significant economic losses. Actinobacteria isolated by this study both support plant growth and protect plants from various pathogens with their bioactive compounds. These two mechanisms, which are provided naturally, are innovative in terms of preventing the use of harmful agricultural chemicals and being a step towards solving problems in food safety. Further studies will continue in the form of consortia of these organisms, ensuring their stability and applying them to selected plants.
Conclusions and future perspectives
Under changing environmental conditions, the need to produce an appropriate amount of plant biomass is an important challenge. Various microorganisms living in the rhizosphere, can have beneficial effects on plant growth, and health and increase plant biomass production. The potential of rhizobacteria to enhance health, growth, and development, predominantly as a result of antimicrobial activities to reduce pathogenic infections nutrient availability, and synthesis of phytohormones. The potential of microorganisms to support and enhance plant growth under different environmental conditions is still considered unimportant. Therefore, more study is needed to better understand the mechanisms of plant-microbe interactions under different stress conditions. New biotechnological products can be developed and innovative solutions can be offered that utilize plant beneficial actinobacteria for the biological control of plant diseases and promote plant growth for sustainable agricultural applications. The use of these actinobacteria for agricultural processes can be an alternative to the use of harmful chemicals that have a potential risk to living things and the environment.
There is a global demand for novel antimicrobial agents that are highly active, of low toxicity, and have an environmental impact. In addition to drug resistance in viruses, fungi, and bacteria, life-threatening microorganisms are more than before. This is due to misuse of drug administration dose and duration, increasing the need for new, highly potent compounds that help all aspects of the human condition. Actinobacteria have been isolated from different ecosystems including various bulk soils. As interest grows in bioactive molecules from drug formulations with minimal side effects, there is more opportunity to explore new sources and other biological properties of previously inaccessible natural products extracted from Actinobacteria.
Most of the publications in the literature have focused on thermophilic, alkaliphilic, and haloalkaliphilic actinobacteria and only on endophytic actinobacteria. They represent important and novel sources of many other biodiversities and multidisciplinary environments, potentially bioactive compounds. In conclusion, it seems that some new techniques and methodologies are required for a comprehensive investigation of bioactive natural compounds. This includes, for example, the discovery of new structure-activity relationships in nature that are becoming increasingly important for the synthesis inspiration of natural bioactive product compounds. This results in increased diversity with less complexity and good knowledge of isolation processes. To address the challenges of biodiversity and to promote the future sustainable use of natural resources, a multidisciplinary perspective is required to find, describe and communicate the wealth of nature. This is necessary to identify new bioactive chemicals from actinobacteria.
All the findings obtained in this study constitute an important preliminary study for novel bioactive compounds isolated from actinobacteria and their applications in industrial, agricultural, and environmental protection, pharmaceutical bioactive compounds, and pharmaceutically relevant biomolecules. This includes high-level metabolites that act as inhibitory or lethal agents against pathogens affecting humans and animals, including resistant bacteria, fungi, viruses, and several protozoa. Therefore, continuous selective isolation and screening studies are required for the characterization and identification of new potential bioactive compounds from actinobacteria. This is to create commercially viable, long-term, and cost-effective production methods. Their metabolic flexibility and abundance also provide a powerful new avenue for the bioremediation of organic wastes and pollutants.
Abbreviations
- IAA:
-
Indole acetic acid
- AIA:
-
Actinomycetes Isolation Agar
- AIB:
-
Actinomycetes Isolation Broth
- GSB:
-
Glucose Soybean Meal Broth
- SCB:
-
Starch Casein Broth
- ISP:
-
International Streptomycetes Project
- CAS:
-
Chromium Azurol S
- HCN:
-
Hydrogen cyanide
- PVKA:
-
Pkiovskaya’s Agar
- PGPR:
-
Plant Growth Promoting Rhizobacteria
- BLAST:
-
Basic Local Alignment Search Tool
- NCBI:
-
National Center for Biotechnology Information
References
Abd-Alla MH, El-Sayed EA, Rasmey AM (2013) Indole-3-acetic acid (IAA) production by Streptomyces atrovirens isolated from rhizospheric soil in Egypt. J Biology Earth Sci 3:182–193
Adegboye M, Babalola O (2013) Actinomycetes: A Yet Inexhaustive Source of Bioactive Secondary Metabolites. In: Mendez-Vilas A (ed) Microbial Pathogens and Strategies for Combating Them, Science, Technology and Education. Formatex, Badajoz, pp 786–795
Ahemad M (2015) Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: a review. Biotech 5:111–121. https://doi.org/10.1007/s13205-014-0206-0
Anwar S, Basharat A, Sajid I (2016) Screening of rhizospheric actinomycetes for various in vitro and in vivo plant growth promoting (PGP) traits and for agroactive compounds. Front Microbiol 7:1–11. https://doi.org/10.3389/fmicb.2016.01334
Aouar L, Lerat S, Boulahrouf A, Beaulieu C (2016) Bacterial antagonism and plant growth promoting traits of actinobacterial strains previously selected for their antifungal properties. Transylv Rev 24:2408–2417
Bhosale HJ, Kadam TA (2015) Generic diversity and a comparative account on plant growth promoting characteristics of actinomycetes in roots and rhizosphere of Saccharum officinarum. Int J Curr Microbiol Appl Sci 4:230–244
Boukhatem ZF, Merabet C, Tsaki H (2022) Plant Growth Promoting Actinobacteria, the Most Promising Candidates as Bioinoculants? Front. Agron 4:849911. https://doi.org/10.3389/fagro.2022.849911
Chandrakar S, Gupta AK (2017) Production and Characterization of Actinomycin D from Streptomyces parvulus Isolated from Aloe vera (l.) Burm. F. and its Antimicrobial Activity. International Journal of Pharmaceutical Sciences Review and Research 46: 169–175
Chaudhary HS, Yadav J, Shrivastava AR, Singh S, Singh AK, Gopalan N (2013) Antibacterial activity of actinomycetes isolated from different soil samples of Sheopur (A city of central India). J Adv Pharm Technol Res 4:118–123. https://doi.org/10.4103/2231-4040.111528
Damam M, Moinuddin MK, Kausar R (2016) Isolation and screening of plant growth promoting actinomycetes from rhizosphere of some forest medicinal plants. Int J ChemTech Res 9:521–528
Dastager SG, Damare S (2013) Marine Actinobacteria Showing Phosphate-Solubilizing Efficiency in Chorao Island, Goa, India. Curr Microbiol 66:421–427. https://doi.org/10.1007/s00284-012-0288-z
De Oliveira MF, Da Silva MG, Der Sand TV (2010) Antiphytopathogen potential of endophytic actinobacteria isolated from tomato plants (Lycopersicon esculentum) in southern Brazil, and characterization of Streptomyces sp. R18(6), a potential biocontrol agent. Res Microbiol 161:565–572. https://doi.org/10.1016/j.resmic.2010.05.008
De Souza R, Ambrosini A, Passaglia LMP (2015) Plant growth promoting bacteria as inoculants in agricultural soils. Genet Mol Biology 38:401–419. https://doi.org/10.1590/S1415-475738420150053
Duddu MK, Guntuku G (2016) Isolation, screening and characterization of antibiotic producing actinomycetes from Kapuluppada plastic waste dumping yard, Visakhapatnam. Int J Pharm Pharm Sci 8:221–229. https://doi.org/10.22159/ijpps.2016v8i11.10110
El-Tarabily KA (2008) Promotion of tomato (Lycopersicon esculentun Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase–producing Streptomycete actinomycetes. Plant Soil 308:161–174. https://doi.org/10.1007/s11104-008-9616-2
Farhat MF, Boukhris I, Chouayekh H (2014) Mineral phosphate solubilization by Streptomyces sp. CTM396 involves the excretion of gluconic acid and is stimulated by humic acids. FEMS Microbiol Lett 362:1–8. https://doi.org/10.1093/femsle/fnv008
Franco-Correa M, Quintana A, Duque C, Suarez C, Rodriguez MX, Barea JM (2010) Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl Soil Ecol 45:209–217. https://doi.org/10.1016/j.apsoil.2010.04.007
Frey B, Rieder SR, Brunner I, Plötze M, Koetzsch S, Lapanje A (2010) Weathering-associated bacteria from the Damma glacier forefield: physiological capabilities and impact on granite dissolution. Appl Environ Microbiol 76:4788–4796. https://doi.org/10.1128/AEM.00657-10
Ganesan P, Reegan AD, David RHA, Gandhi MR, Paulraj MG, Al- Dhabi N, Ignacimuthu S (2017) Antimicrobial activity of some actinomycetes from Western Ghats of Tamil Nadu, India. Alexandria J Med 53:101–110. https://doi.org/10.1016/j.ajme.2016.03.004
Gopalakrishnan S, Vadlamudi S, Bandikinda P, Sathya A, Vijayabharathi R, Rupela O, Kudapa H, Katta K, Varshney RK (2014) Evaluation of Streptomyces strains isolated from herbal vermicompost for their plant growth-promotion traits in rice. Microbiol Res 169:40–48. https://doi.org/10.1016/j.micres.2013.09.008
Gopinath BV, Vootla PK, Jyothi R, Redd KS (2013) Antimicrobial Activity of Actinomycetes Isolated From Coal Mine Soils of Godavari Belt Region, A.P, India. Asian J Exp Biol Sci 4:518–523
Guo S, Feng B, Xiao C, Wang Q, Chi R (2021) Phosphate-solubilizing microorganisms to enhance phytoremediation of excess phosphorus pollution in phosphate mining wasteland soil. Bioremediat J 25:271–281. https://doi.org/10.1080/10889868.2021.1884528
Jobim ML, Santos RCV, Alves CFS, Oliveira RM, Mostardeiro CP, Sagrillo MR, Filho OCS, Garcia LFM, Manica-Cattani MF, Ribeiroe EE, Da Cruz IBM (2014) Antimicrobial activity of Amazon Astrocaryum aculeatum extracts and its association to oxidative metabolism. Microbiol Res 169:314–323. https://doi.org/10.1016/j.micres.2013.06.006
Kaur G (2014) Studies on microbial phosphate solubilization and development of inoculum formulations. Dissertation, University of Thapar
Kaur T, Sharma D, Kaur A, Manhas KR (2013) Antagonistic and plant growth promoting activities of endophytic and soil actinomycetes. Archives of Phytopathology and Plant Protection 46:1756–1768. https://doi.org/10.1080/03235408.2013.777169
Khamna S, Yokota A, Lumyong S (2009) Actinomycetes isolated from medicinal plant rhizosphere soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J Microbiol Biotechnol 25:649–655. https://doi.org/10.1007/s11274-008-9933-x
Koçyiğit A (2009) Termofilik Bakterilerden Lipaz Üretimi ve Karakterizasyonu. Dissertation, Ege University
Lakshmanan V, Shantharaj D, Li G, Seyfferth AL, Sherrier DJ, Bais HP (2015) A natural rice rhizospheric bacterium abates arsenic accumulation in rice (Oryza sativa L.). Planta 242:1037–1050. https://doi.org/10.1007/s00425-015-2340-2
Lapanje A, Wimmersberger C, Furrer G, Brunner I, Frey B (2012) Pattern of elemental release during the granite dissolution can be changed by aerobic heterotrophic bacterial strains isolated from Damma glacier (Central Alps) deglaciated granite sand. Microb Ecol 63:865–882. https://doi.org/10.1007/s00248-011-9976-7
Legault G, Lerat S, Nicolas P, Beaulieu C (2011) Tryptophan regulates thaxtomin A and indole-3-acetic acid production in Streptomyces scabiei and modifies its interactions with radish seedlings. Phytopathol 101:1045–1051. https://doi.org/10.1094/PHYTO-03-11-0064
Mitra D, Mondal R, Khoshru B, Senapati A, Radha TK, Mahakur B, Uniyal N, Myo EM, Boutaj H, Sierra BEG, Panneerselvam P, Ganeshamurthy AN, Elković SA, Vasić T, Rani A, Dutta S, Mohapatra PKD (2022) Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: Advances in soil, plant, and microbial multifactorial interactions. Pedosphere 32:149–170. https://doi.org/10.1016/S1002-0160(21)60042-5
Mohandas S, Poovarasan S, Panneerselvam P, Saritha B, Upreti KK, Kamala R, Sita T (2013) Guava (Psidium guajava L.) rhizosphere Glomus mosseae spores harbor actinomycetes with growth promoting and antifungal attributes. Sci Hort 150:371–376. https://doi.org/10.1016/j.scienta.2012.11.019
Mohanraj D, Bharathi S, Radhakrishnan M, Balagurunathan R (2011) Bioprospecting of actinobacteria from Yelagiri hills with special reference to antibacterial activity. J Chem Pharm Res 3:439–446
Mohseni M, Norouzi H, Hamedi J, Roohi A (2013) Screening of Antibacterial Producing Actinomycetes from Sediments of the Caspian Sea. Int J Mol Cell Med 2:64–71
Namlı A, Mahmood A, Sevilir B, Özkır E (2017) Effect of phosphorus solubilizing bacteria on some soil properties, wheat yield and nutrient contents. Eurasian J Soil Sci 6:249–258. https://doi.org/10.18393/ejss.293157
Ng YZ, Amsaveni S (2012) Isolation, Screening and Characterization of Antibiotic-Producing Actinomycetes from Rhizosphere Region of Different Plants from a Farm of Sungai Ramal Luar, Malaysia. J Adv Biomedical Pathobiology 2:96–107
Ningthoujam DS, Chanu SB, Tamreihao K, Lynda R, Devi KA, Jeeniita N (2016) Plant Growth Promotion and Biocontrol Potential of a Streptomyces sp. strain N3-3b isolated from the rhizosphere of Chakhao, a Black Rice Variety of Manipur, India. Br Microbiol Res J 16:1–11
Passari AK, Mishra VK, Gupta VK, Yadav MK, Saikia R, Singh BP (2015) In Vitro and In Vivo Plant Growth Promoting Activities and DNA Fingerprinting of Antagonistic Endophytic Actinomycetes Associates with Medicinal Plants. PLoS ONE 10:1–18. https://doi.org/10.1371/journal.pone.0139468
Patel MV, Patel RK (2014) Indole-3-acetic acid (IAA) production by endophytic bacteria isolated from saline dessert, The Little Runn of Kutch. CIBTech J Microbiol 3:17–28
Rajan BM, Kannabiran K (2014) Extraction and Identification of Antibacterial Secondary Metabolites from Marine Streptomyces sp. VITBRK2. Int J Mol Cell Med 3:130–137
Rijavec T, Lapanje A (2016) Hydrogen Cyanide in the Rhizosphere: Not Suppressing Plant Pathogens, but Rather Regulating Availability of Phosphate. Front Microbiol 7:1785. https://doi.org/10.3389/fmicb.2016.01785
Rodrigues AA, Araujo MVF, Soares RS, De Oliveira BFR, Sibov TD, Vieira JDG (2018) Isolation and Screening for Multi-trait Plant Growth Promoting Actinobacteria From Organic Sugarcane Rhizosphere. Int J Microbiol Res 10:1193–1198. https://doi.org/10.4161/cib.27683
Singh LS, Sharma H, Talukdar NC (2014) Production of potent antimicrobial agent by actinomycete, Streptomyces sannanensis strain SU118 isolated from phoomdi in Loktak Lake of Manipur, India. BMC Microbiol 14:1–13. https://doi.org/10.1186/s12866-014-0278-3
Singh N, Rai V (2012) Optimization of cultural parameters for antifungal and antibacterial metabolite from microbial isolate; Streptomyces rimosus MTCC 10792 from soil of Chhattisgarh. Int J Pharm Pharm Sci 4:94–101
Sreevidya M, Gopalakrishnan S, Kudapa H, Varshney RK (2016) Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Brazilian J Microbiol 47:85–95. https://doi.org/10.1016/j.bjm.2015.11.030
Sudha SSK, Hemalatha R (2015) Isolation and screening of antibiotic producing actinomycetes from garden soil of Sathyabama University, Chennai. Asian J Pharm Clin Res 8:1210–1140
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4,0. Mol Biol Evol 24:1596–1599. https://doi.org/10.1093/molbev/msm092
Toker SK, Evlat H, Koçyi̇ği̇t A (2021) Screening of newly isolated marine-derived fungi for their laccase production and decolorization of different dye types. Reg Stud Mar Sci 45:1–12. https://doi.org/10.1016/j.rsma.2021.101837
Varalakshmi T, Sekhar KM, Charyulu PBB (2014) Taxonomic studies and phylogenetic characterization of potential and pigmented antibiotic producing actinomycetes isolated from rhizosphere soils. Int J Pharm Pharm Sci 6:511–519
Vurukonda SSKP, Giovanardi D, Stefani E (2018) Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int J Mol Sci 19:1–26. https://doi.org/10.3390/ijms19040952
Wang W, Qiu Z, Tan H, Cao L (2014) Siderophore production by actinobacteria. Biometals 27:623–631. https://doi.org/10.1007/s10534-014-9739-2
Wani AP, Irene OI (2014) Screening of microbes for their metal, antibiotic resistance and plant growth promoting activity. Currrent Res Bacteriol 7:22–31. https://doi.org/10.3923/pjbs.2014.206.212
Wongfun N, Plötze M, Furrer G, Brand H (2014) Weathering of granite from the Damma glacier area: the contribution of cyanogenic bacteria. Geomicrobiol J 31:93–10. https://doi.org/10.1080/01490451.2013.802396
Author information
Authors and Affiliations
Contributions
This study was performed based on a Master of Science (MSc) thesis addressed to Hüseyin EVLAT. All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Hüseyin EVLAT, Sultan Kübra TOKER and Ali KOÇYİĞİT. Ali KOÇYİĞİT supervised the study.The first draft of the manuscript was written by Hüseyin EVLAT and all authors commented and make corrections on all versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Statements and declarations
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Evlat, H., Toker, S.K. & Koçyiğit, A. Screening for agroactive and bioactive metabolites production by actinobacteria isolated from rhizospheric soils. Biologia 78, 187–200 (2023). https://doi.org/10.1007/s11756-022-01226-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01226-0