Abstract
Colonisation and weathering of freshly deglaciated granite are key processes in initial soil formation and development. We have obtained 438 isolates from granite sand covering glacial toe, 284 isolates at 22°C and 154 at 4°C incubation temperatures, respectively, to obtain cultures for the investigation of their weathering capabilities under laboratory conditions. The isolation of bacteria from granite sand was performed on rich-, intermediate- and low-nutrient-content solid media. Isolates were identified by 16S rRNA gene sequencing. According to the genera-associated weathering capabilities described in the literature and according to their abundance in our culture collection, we selected eight strains to analyse their effects on the weathering dynamics of granite sand during the batch culture experiment. Analysis of culturable bacteria showed higher species richness among isolates from 22°C than from 4°C incubations. In the R2A and 1/100 Ravan media, we observed the highest species richness of isolates obtained at 22°C and 4°C incubation temperatures, respectively. The obtained 16S rRNA sequences revealed the presence of alpha-, beta- and gamma-proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes. The most numerous group of isolates was distantly related to Collimonas representatives, and according to the sequences of the 16S rRNA genes, they can form a new genus. Isolates from this group had the capability of causing increased dissolution rates for Fe, W, Ni and Rb. In general, at each sampling during the 30-day experiment, every strain showed a unique weathering profile resulting from differential rates of the dissolution and the precipitation of different minerals in the batch culture. Consequently, the presence of different strains, their growth stage and changes in proportions of strains in the bacterial community can affect further soil development and the successive colonisation by plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global retreat of glaciers has received much attention recently because it is a consequence of climate change [1, 2]. When glaciers retreat, they expose barren substrates. These become colonised by organisms from air, clouds, snow, rain and runoff waters from the glacier body as well as by autochthonous microorganisms [2–5]. This is the beginning of the process of soil formation and primary succession [6–8]. As a result, the colonisation and activity of certain pioneer bacteria species on such barren substrates may greatly affect the subsequent succession of other organisms [7, 9–11]. Successful colonisation of new substrates by multicellular organisms can be affected by bacteria that interact with the barren substrate, release or precipitate minerals, fix atmospheric N2 and CO2, and consequently control the amount of available nutrients [7, 8]. Therefore, bacterial weathering activity can contribute to the better growth of plants, which results in a faster succession of vegetation [12, 13].
There are few data available on the microbial weathering effects in glacial environments. It is known that microbial mediation may increase the rate of sulphide oxidation under subglacial conditions [14]. Microbes also may catalyze the reduction of Fe(III) and the oxidation of FeS2 [15], which affects the subsequent chemical weathering of subglacial bedrock. Bacteria that are present in the glacier forefield are capable of dissolving K, Mg, Ca, Al, Fe, Mn and P from granite sand as a result of the exudation of oxalic acid or HCN, the formation of biofilms and the lowering of pH [16]. Recently, it was reported that chemolithotrophic iron and sulphur oxidising along with nitrogen-fixing bacteria establish the first steps in the development of fertile soils. This process is then followed by the colonisation of heterotrophic bacteria [17]. The contribution of heterotrophic bacteria to further soil development due to their weathering activities has not been extensively investigated.
To investigate specific weathering capabilities of different microorganisms present in the glacier forefield, it is necessary to obtain very diverse groups of isolates by applying cultivation methods using different media. A cultivation strategy using only nutrient-rich media is rather selective, favouring fast- over slow-growing bacterial species [18–20]. Consequently, it is very convenient to use nutrient dilution techniques to broaden the spectrum of isolated strains from the environment [2, 21–25]. Accordingly, in this study, we applied low-, medium- and rich-nutrient-content media to obtain a very diverse group of organisms from the environment.
Among the numerous reports on the isolation of bacteria from cold environments and media of diverse composition reviewed by Tindall [26], only a few studies describe isolates from glaciers in the Central Alps. For example, Edwards et al. [27] investigated the culturability of bacteria from the Damma glacier. They detected a culturable bacterial fraction between 0.2% and 3% from different periods of soil development after deglaciation. According to Edwards et al. [27], the culturability of bacteria depends mostly on the soil age after deglaciation, the media used for cultivation (R2A, TSA, CSEA) and on the spatial distribution (rhizospheric and interspatial).
Here, we present for the first time data of diverse bacterial isolates obtained from granite sand covering the glacier toe and its granite-weathering capabilities. The granite sand covering the glacier can provide a special niche for colonising bacteria. Such a niche offers a relatively stable environment with low amounts of organic nutrients. This niche might appear because the ice, which is covered with sand, melts more quickly than clear ice as a consequence of sand weight and lower albedo [2]. Elevated pressure on the ice caused by sand results in the transition of ice into water at lower temperatures. Water with dissolved nutrients is then transported due to capillary drag through the stones up to the surface [28, 29]. A very important reason for sampling granite sand is that microbes are exposed to the grains of high surface-to-volume ratio, and this results in the rapid mineral weathering and inorganic nutrient acquisition by microorganisms that are present at the site.
To determine variations in the weathering capabilities of microorganisms from such sites, we took five strains from our culture collection. These were selected on account of their phylogenetic similarity to strains isolated elsewhere, but that have particular weathering capabilities reported by others: Sphingomonas sp. [12], Collimonas sp. [12], Pseudomonas sp. [12, 30], Paenibacillus polymixa [13, 30–32] and Rhodococcus sp. [16, 30]. In addition, we investigated the weathering properties of the three most abundant strains in our culture collection: Janthinobacterium sp., Collimonas sp. and the undetermined Burkholderiaceae bacterium—“Collimonas sp. relatives”
Our aims were, (1) using selective media and media with different organic matter contents, cultivated at 4°C and 22°C, respectively, to determine the species richness of bacteria from a collection of various heterotrophic culturable bacteria isolated from granite sand covering the glacier toe and (2) to identify the variation in the mineral dissolution rates of selected isolates during the granite exposure experiment.
Material and Methods
Site Description and Sample Collection
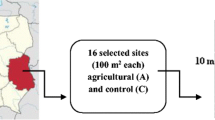
Samples of the fine rock material covering the glacier toe were collected on 15 September 2007 just behind the front side of the Damma glacier, at an altitude of approximately 2,100 m a.s.l., located in the alpine Aar massif in central Switzerland (46°38.177′ N, 008°27.677′ E). The local climate is characterized by an average precipitation rate of 2,400 mm per year water equivalents and a rather short vegetation period from approximately June to August. Currently, the front of the ice body is detached from the snow loading area of the glacier.
Although several varieties of metagranite are present, the bedrock mineral composition is relatively stable and is composed of coarse-grained metamorphic granite of the Aar massif with quartz, albite, microcline, muscovite, biotite, epidote and chlorite. Along with the bedrock mineral composition, the clay fraction is also characteristic of the granite, and as expected from the relatively recent surface exposure of bedrocks, there is no indication of formation of new clay minerals.
The sampling sites were free of mosses and higher plants. Samples are composed mainly of gravel- and sand-sized material (on average 80% of soil dry weight), minor amounts of silt (on average 17% of soil dry weight), and a clay fraction (on average 8% of soil dry weight) and contain very low amounts of dissolved organic carbon (<0.01 mg/g dry weight) [33]. The material is slightly acidic (pH 5.35) with low SO 2−4 (1.34 μg/g), NH +4 (0.79 μg/g), phosphorus (0.25 μg/g) and NO −3 (0.19 μg/g) levels [33, 34]. Among metal cations besides Si (27.35%), the elements Ca (1%), Fe (1.36%), Al (5.42%) and Mg (0.56%) are predominant [33].
Bacterial Isolation and Cultivation
Samples from the glacial toe of approximately 40 g were placed into sterile 50-ml centrifuge tubes and transported to the laboratory, where 30 ml of sterile Milli-Q water was added. The samples were shaken in Milli-Q water overnight at 100 rpm at 4°C to extract bacterial cells and to achive homogeneity. After this treatment, 1 ml subsamples of the supernatant water was used in a tenfold dillution up to 10−4. A 100-μl aliquot of liquid from each dilution was spread onto separate plates of solid media. The inoculation procedure was prepared in triplicate for each dilution and for each different medium.
To cultivate bacteria from gravel samples, we used nutrient-rich media (TSA [35] and NA [36], both from Fluka) which support the growth of copiotrophic bacteria, moderately nutrient-rich media (R2A [37], 1/4NA [37, 38], both from Fluka) and media that support the growth of oligotrophic bacteria (1/100Ravan [39], 1/160NA [19], both solidified with washed agar [19]). In addition, Pseudomonas isolation media and KingB media were used for the specific isolation of Pseudomonas spp. After plating on different solid media, we incubated inoculated plates at 22°C and 4°C separately in thermostated incubators for up to 4 weeks. After incubation, we counted the colony-forming units (CFU). Approximately 60% of all grown colonies on the solidified agar plates were subcultured and used for further genetic characterisation. The cultures were stored in Microbank bacterial storage system (Pro-Lab diagnostics) at −80°C.
Genetic Characterisation
Isolated strains were further analysed with restriction fragment length polymorphism (RFLP) of amplified 16S rRNA genes. Crude DNA prepared with Chelex 100 (Bio-Rad) was used according to the method described by Giraffa et al. [40]. Isolated DNA was transferred into the fresh tube and stored at −20°C prior to use. Ribosomal 16S genes were amplified as described below. PCR-amplified products were cut with HaeIII and HhaI restriction enzymes. Fragments of digested 16S rRNA genes were separated on 4% agarose gels. GelProAnalyser software (Media Cybernetics, Silver Spring, MD, USA) was used to determine fragment sizes by comparing their positions with the positions of fragments of the 100-bp size standard (Fermentas) observed on the agarose gels. The matrix based on the lengths of digested 16S rRNA genes was calculated and used to determine Jaccard index values for the comparison of band size patterns between strains. Jaccard coefficient values were calculated from the matrices of discrete characters based on the restriction patterns. The unrooted unweighted pair-group method using arithmetic averages (UPGMA) trees were constructed under the FreeTree software (http://www.natur.cuni.cz/∼flegr/programs/freetree) [41]. Separate UPGMA trees were constructed for each medium and incubation temperature. Groupings produced by the FreeTree UPGMA software were inspected by manual comparisons of the band patterns present on the pictures of agarose gels. From each group on the UPGMA trees, which were built for each medium and each temperature separately, one or two strains, sharing the same RFLP patterns and were isolated from the same medium, were selected for the sequencing of 16S rRNA genes. We sequenced overall more than three isolates per RFLP pattern because isolated strains from different media have the same RFLP patterns.
Bacteria-specific primers 27f, 1495rev or 1378r were used for the amplification of bacterial 16S rRNA genes from isolated strains [42, 43]. The PCR procedure started with 4 min of denaturation at 94°C, followed by 5 cycles of 30 s at 94°C, 30 s at 60°C and 4 min at 72°C; another 5 cycles of 30 s at 94°C, 30 s at 55°C and 4 min at 72°C; and 30 cycles of 30 s at 94°C, 30 s at 50°C and 4 min at 72°C.
Sequencing of PCR-Amplified Products
The 16S rRNA PCR products were cleaned on 96-well PCR cleaning Millipore plates and resuspended in sterile DNA- and nuclease-free water (Fluka). Subsequently, 2 μl of cleaned PCR product was transferred into a solution of 1:1 buffer and BigDye 3.1 terminator. For each 16S rRNA gene, we used four sequencing reactions with 0.16 μM primers—27f [42], 907r [44], 808f [45] and 1378r [43] or 1495r [42]—in order to obtain nearly full 16S rRNA sequences composed of four different contigs, two forward and two reverse. Conditions of the cycle sequencing reaction were as follows: initial denaturation of 1 min at 96°C and 25 cycles with 10 s at 96°C, 5 s at 50°C and 4 min at 60°C. The products of the cycle sequencing reactions were purified with Millipore plates, suspended in the injection solution (Millipore) and separated on an ABI 3730 sequencer.
Granite Dissolution Experiments
To investigate the effects of different isolated strains on mineral dissolution, we performed microcosm batch experiments which were each prepared in triplicate. Five grams of crushed granite (<63 μm fraction), which simulates the silt fraction in the mineral soil, was placed into acid-washed 500-ml Erlenmeyer flasks and autoclaved. Each microcosm received 200 ml of autoclaved MM9 medium [46] adjusted to pH 7.2. Strains were selected according to reported genera-associated weathering capabilities and according to the abundance in our culture collection. Then, 1 ml of cultures of eight selected strains incubated overnight in 50 ml MM9 media adjusted to pH 7.2 was added to each flask. All of the cultures had OD660 = 1.2, except Collimonas sp. and “Collimonas sp. relative” strains which had OD660 values of 0.6 and 0.5, respectively. In the experiment, we used three control batches: sterile MM9 medium (control for dissolution kinetics), MM9 citrate-amended medium (1 mM sodium citrate, pH 7.2—control for ligand-promoted dissolution) and MM9 adjusted to pH 4.6 with HCl (control for proton-promoted dissolution). The pH value of 4.6 for the sand was the lowest reported from sites at the Damma glacier. The triplicates of microcosm as well as control batches were incubated on the rotary shaker at an orbital speed of 195 rpm at 25°C for 30 days. Aliquots (10 ml) from each microcosm triplicate and control were sampled for chemical analysis after 3, 10, 17, 24 and 30 days.
Chemical Analyses
Specific mineral and elemental composition of samples of granite, which had been crushed in the laboratory and subsequently used in our experiments, was described by Frey et al. [16]. In our batch culture experiment, we analysed the elemental composition of the filtrate after filtration through the sterile 0.2-μm pore size filters at previously defined sampling time points. In the filtrate, we determined the concentrations of Al, As, B, Ba, Ca, Cd, Ce, Co, Cr, Cs, Cu, Fe, K, La, Mg, Mn, Mo, Na, Ni, P, Pb, Rb, Sn, Sr, Ti, V, W, Zn and Zr with inductively coupled plasma mass spectrometry (ICP-MS; Sciex Elan 6000, Perkin-Elmer, Boston, MA). In addition to ICP-MS measurements, we quantified Fe concentrations with the Viollier modified ferrozine method [47] because ICP-MS is relatively insensitive to Fe concentrations due to ArO+ interference. In the last time point samples, using the same ferrozine method, we also determined Fe(II)/Fe(III) ratios. At the beginning and end of the experiment, the pH and electrical conductivity of the solutions were determined with electrodes.
Data Analysis
The four sequences obtained for each 16S rRNA gene were assembled into one contig with Geneious software (Biomatters Ltd, Auckland, New Zealand) and the correctness of the sequences examined manually. Each of the nearly whole 16S rRNA sequences was compared with the five closest relatives in the RDP II 16S rRNA database. Our sequences and all five closest relatives from RDP II were aligned in the Clustal X software using default parameters [48]. Based on the alignment, first, the Kimura two-parameter distance matrix was calculated and, second, the phylogenetic tree was constructed using the neighbour joining algorithm in MEGA 4 software [49]. Interior branch positions were tested with the bootstrap procedure by means of 1,000 resamplings. Rarefaction analysis was performed with Rarefactwin software [50]. Rarefaction curves were based on the OTU, defined as the group of sequences which share 97% similarity with the sequences within the group and at the same time belong to the one branch on the RFLP-based UPGMA tree. All nucleotide sequences have been submitted to the GenBank database under accession numbers GU213297–GU213429.
All statistical calculations of the weathering experiment were performed with the R software (R Development Core Team 2005). Independent t tests were performed to determine differences between the dissolution of elements by different isolates and the control batch. Principal component analysis (PCA) of the dissolution data was performed using singular value decomposition of the centred and scaled data matrix according to the function prcomp from the R software package. With PCA, we compared the concentrations of dissolved elements at different sampling times in the media inoculated with different strains as well as in control batches.
Results
Enumeration and Characterization of Culturable Bacteria
Enumeration of Culturable Bacteria
In general, we observed the highest number of colonies on nutrient-poor media (1/100Ravan and 1/160NA) and the lowest on nutrient-rich media (TSA and NA; Fig. 1). When nutrient-poor media were incubated at 22°C, the CFU values were 3.3 (1/100Ravan) and 22 times (1/160NA) higher than when incubated at 4°C. On the nutrient-rich media, fewer CFU were detected when incubated at 22°C than at 4°C (15 times less on TSA and 88 times less on NA; Fig. 1). Similar to the nutrient-poor media, CFU on Pseudomonas spp.-specific media plates were higher when incubated at 22°C than at 4°C (3 times for KingB and 2.25 times for Pseudomonas spp. isolation plates). Among intermediate-nutrient content media, the differences were less, although slightly higher CFU values were observed at 4°C.
Phylogenetic Characterisation of Isolates
From all media used in this study, we obtained a total of 284 isolates from 22°C and 154 isolates from 4°C incubations. During the growth and isolation procedure, we determined the morphology and pigmentation of colonies, observing that almost half of the isolates were pigmented (47% and 42% at 4°C and 22°C, respectively), whilst none of the isolates growing on the nutrient-poor media (1/100 Ravan and 1/160 NA) were pigmented.
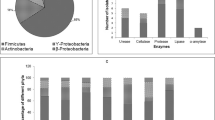
According to the number of different RFLP groups of all 438 isolates after HhaI and HaeIII digestions, we sequenced the 16S rRNA genes of selected isolates (see Table 1 and Electronic Supplementary Material (ESM) Tables 1a and 1b) to determine their phylogenetic assignment. In general, sequences of approximately half of our isolates (63.2% at 22°C and 50.3% at 4°C) had relatives among the sequences of isolates and clones which were obtained from cold environments and deposited in the RDPII database (see ESM Tables 1 and 2). When we examined the dependency of the occurrence of genera versus the number of isolates, we observed plateaux for the majority of the isolation media. This indicates that our culture collection consists of most of the culturable bacteria from the samples (Fig. 2a, b) and that accordingly, we were able to determine the parameters of our culture collection in terms of abundance, richness and media type as well as temperature preference of the strains for their isolation.
The incubation temperatures and media used during the isolation procedure affected the observed species richness and incidence of the different phylogenetic groups of closely related strains in our culture collection. According to the number of isolates belonging to the assigned genera (Table 1), we detected higher species richness among isolates from 22°C than from 4°C incubation, as can be seen from the shape of the rarefaction curves (Fig. 3). When comparing different media, TSA, NA and R2A were the best choices to isolate most diverse groups of bacteria from 22°C and R2A (Fig. 2b) and 1/100Ravan from 4°C (Table 2 and Fig. 2a) incubation temperatures, respectively. At 4°C, most of the rarefaction curves specific for each medium reached saturation with fewer OTU than when incubated at 22°C (Fig. 2a, b). At both temperatures, the most diverse groups of isolates were obtained when using 1/4NA and R2A media (Table 2). The lowest numbers of different OTU were obtained on KingB and Pseudomonas sp. isolation media at both incubation temperatures.
Whilst all isolates from both temperatures were distributed into four phyla (Table 1), we observed their temperature-dependant isolation on different media and noticed that the representatives of phylum Bacteroidetes and Alphaproteobacteria were absent from isolates obtained at 4°C and 22°C incubations. Strains belonging to the genera Pseudomonas, Arthrobacter and Janthinobacterium were isolated at both temperatures and on most media (see Table 2). Isolates obtained only after incubation at 4°C were classified in Paenibacillus, Cryobacterium, Sporosarcina and Herminiimonas groups (Table 2), and those only from the 22°C incubations were Sphingomonas, Polaromonas, Collimonas, unclassified Oxalobacteriaceae (distant relatives of Massilia), Variovorax, Pelomonas, Paucibacter, unclassified Pseudomonas, unclassified Microbacteriaceae, Frigoribacterium, Rhodococcus, Pedobacter and Staphylococcus groups (Table 2).
Isolates belonging into the Oxalobacteriaceae family were among the Proteobacteria most numerously represented at both temperature incubations (60% at 4°C and 47.6% at 22°C; Table 1 and ESM Fig. 1). In this family and likewise among all isolates, the representatives of the Janthinobacterium genus and “Collimonas sp. relatives” group were the most numerous, especially at the 4°C incubation (Janthinobacterium, 14.6% at 22°C and 24% at 4°C; “Collimonas sp. relatives”, 4.9% at 22°C and 20% at 4°C). The Pseudomonas sp. isolation medium, 1/4NA and R2A, supported the isolation of Janthinobacterium sp. at both temperatures (Table 2), and “Collimonas sp. relatives” isolates were obtained in all media except Pseudomonas sp. isolation medium and KingB at 4°C and 1/4NA and R2A at 22°C incubation temperatures (Fig. 4 and ESM Tables 1a and 1b).
Phylogenetic position of 17 isolates distantly related to the Collimonas spp. strains. Phylogenetic calculations are based on the common part of 1,214 bp of the 16S rRNA genes. The tree shows the position of isolates among the type strains of the Oxalobacteriaceae family. Stars show bootstrap values larger than 90. The tree is constructed with the neighbour joining method based on the Kimura two-parameter distances
Whilst Janthinobacterium isolates were clearly classified, the classification of the isolates assigned to the “Collimonas sp. relatives” group was less clear. This group is composed of isolates whose closest relatives are Collimonas fungivorans, Collimonas arenae and Collimonas pratensis. Although the isolates are a phylogenetically very coherent group (>99% similar 16S rRNA genes within the group), they share <96% of the 16S rRNA gene sequences with those three closest relatives, hence cannot be classified in the Collimonas genus. Besides the “Collimonas sp. relatives” strains, but in much smaller quantities, we also isolated other strains that could not be assigned to the known species or genera according to their 16S rRNA gene sequences. In general, these types of isolates were from families Oxalobacteriaceae and Incertae sedis 5 and Arthrobacter genus (Fig. 5 and ESM Fig. 1).
Phylogenetic position of 16S rRNA genes of isolates belonging to the Arthrobacter genus among the 16S rRNA genes of Arthrobacter type strains. The tree is constructed with the neighbour joining method based on the Kimura two-parameter distances. Stars indicate bootstrap values above 80. Isolates are presented with numbers. Isolates at 4°C are indicated by a number 4 preceding a slash; other isolates are from 22°C
Granite Dissolution
To determine the dissolution potentials of the different bacteria, eight strains from the various phyla and classes were selected. The bacterial strains were incubated for 30 days in MM9 media (pH 7.2) containing the granite powder. In the first 3 days, we did not measure the mineral dissolution kinetics because during this initial period the rate of abiotic mineral dissolution is the fastest. Hence, the differences in the contribution of biogenic from abiogenic dissolution of minerals could not be clearly distinguished. From the third day until the end of the experiment, abiogenic dissolution is less rapid. For instance, in the control batches, where we put only the sterile MM media (pH 7.2) and granite sand, we measured similar or below initial concentrations in the consecutive samplings for all elements except for Cr. In contrast, the control batches with 1 mM citrate (pH 7.2) and those with HCl (pH 4.6) showed significantly increased concentrations (Fig. 6a–d and ESM Table 2). Data from graphs for the citrate dissolution experiments were omitted since the dissolved elements were present in concentrations several orders of magnitude higher.
a Concentrations of Fe and Al in the batch reactors in the 30-day dissolution experiment. Cont. control, HCl pH 4.6, 29 R. erythropolis (strain 29), 17 S. aerolata (strain 17), 233 Pseudomonas sp. (strain 233), 55 Collimonas sp. (strain 55), 11 R. erythropolis (strain 11), 4–10 Paenibacillus sp. (strains 4–10), 4–24 Janthinobacterium sp. (strains 4–24), 256 Collimonas sp. relative (strain 256). b Concentrations of Co, Mo, Sn and Sr in the batch reactors during the 30-day dissolution experiment. Cont. control, HCl pH 4.6, 29 R. erythropolis (strain 29), 17 S. aerolata (strain 17), 233 Pseudomonas sp. (strain 233), 55 Collimonas sp. (strain 55), 11 R. erythropolis (strain 11), 4–10 Paenibacillus sp. (strains 4–10), 4–24 Janthinobacterium sp. (strains 4–24), 256 Collimonas sp. relative (strain 256), Sn min the detection limit for Sn. c Concentrations of Mn, Rb and V in the batch reactors during the 30-day dissolution experiment. Cont. control, HCl pH 4.6, 29 R. erythropolis (strain 29), 17 S. aerolata (strain 17), 233 Pseudomonas sp. (strain 233), 55 Collimonas sp. (strain 55), 11 R. erythropolis (strain 11), 4–10 Paenibacillus sp. (strains 4–10), 4–24 Janthinobacterium sp. (strains 4–24), 256 Collimonas sp. relative (strain 256). d Concentrations of Cr, Ni and W in the batch reactors during the 30-day dissolution experiment. Cont. control, HCl pH 4.6, 29 R. erythropolis (strain 29), 17 S. aerolata (strain 17), 233 Pseudomonas sp. (strain 233), 55 Collimonas sp. (strain 55), 11 R. erythropolis (strain 11), 4–10 Paenibacillus sp. (strains 4–10), 4–24 Janthinobacterium sp. (strains 4–24), 256 Collimonas sp. relative (strain 256), W min the detection limit for W
In all batches, we observed the highest concentrations of Fe and Al in the solution. Both elements exhibited similar dissolved concentrations at all sampling dates, except for the batch reactors inoculated with cultures that were able to dissolve Fe more efficiently (Fig. 6a). The highest Fe concentration in solutions was found in cultures of Sphingomonas aerolata (strain 17), Pseudomonas sp. (strain 233), Paenibacillus sp. (strain 4–10) and Janthinobacterium sp. (strains 4–24) at the first three sampling points. During further samplings at 24 and 30 days, the concentrations of Fe in the media of these cultures were lower except in the Paenibacillus sp.-inoculated batches. For instance, at the end of the experiment in cultures of S. aerolata (strain 17) and Pseudomonas sp. (strain 233), we detected five times and two times lower Fe concentration values, respectively, than peak concentrations. In the reactors inoculated with Paenibacillus sp. (strains 4–10) on the other hand, we observed an increase of the Fe concentration during all sampling dates indicated by a doubling of the concentration from the beginning until the end of the experiment (Fig. 6a). In batches inoculated with strains that were very efficient in dissolving Fe, we observed similar (Paenibacillus sp. and Janthinobacterium sp.) or lower (Pseudomonas sp., p < 0.05) Fe(II) to Fe(III) proportions than in the pH-lowered or control (pH 7.2) batches. The exception was S. aerolata where this proportion was above that observed in all control batches. Whilst the Fe concentrations in batches inoculated with strains less efficient in Fe dissolution were similar to the control batches (pH 7.2), the Fe(II) proportions were statistically higher (p < 0.05) in batches inoculated with Rhodococcus erythropolis strains (29 and 11) and “Collimonas sp. relative”. In both of the control (pH 7.2) and pH-lowered batches, we observed similar Fe(II) to Fe(III) proportions, but in the citrate amended batches, we could not detect any Fe(II) (data for citrate are not shown; Fig. 7).
Ratio of Fe(II)/Fe(III) concentration in the solution at the end of the 30-day granite dissolution experiment. Contr. control, HCl pH 4.6, 29 R. erythropolis (strain 29), 17 S. aerolata (strain 17), 233 Pseudomonas sp. (strain 233), 55 Collimonas sp. (strain 55), 11 R. erythropolis (strain 11), 4–10 Paenibacillus sp. (strains 4–10), 4–24 Janthinobacterium sp. (strains 4–24), 256 Collimonas sp. relative (strain 256)
At the first two sampling dates, the concentrations of micronutrients such as Co, Mo (Fig. 6b) and V (Fig. 6c) were significantly lower than in the control (pH 7.2) batch. The dissolved concentrations of these three elements rose progressively during the following 3 weeks except in the cultures inoculated with R. erythropolis strains (Fig. 6b, c and ESM Table 2).
Ni and W were also released into the solution progressively, but the concentration of W was lower at the end of the experiment in most of the inoculated media (Fig. 6d). At the last sampling date, the most significant removal of W along with Ni from the solution was observed in the culture of Pseudomonas sp. (strain 233). However, the cultures of Collimonas sp. (strain 55), Janthinobacterium sp. (strains 4–24) and a “Collimonas sp. relative” bacterium (strain 256) were capable of dissolving W more efficiently than other strains, but only towards the end of the experiment.
In all batch experiments, except in those with 1 mM citrate, Cr showed similar dissolution behaviour (Fig. 6d). After 3 days only, R. erythropolis (strain 29) and Janthinobacterium sp. (strains 4–24) showed a statistically significant release of Cr from granite (see ESM Table 2). Whilst significantly different from the control, Mn concentrations in the solutions were always elevated, with exception of the batch culture inoculated with R. erythropolis (strain 11; Fig. 6c) in which the Mn concentration was lower at the last sampling date.
The dissolved concentration of Sn increased in the first 3 days in pH-lowered control batches and in all other inoculated batches, except in the batches inoculated with S. aerolata strain 17, Collimonas sp. (strain 55) and Janthinobacterium sp. (strains 4–24). In the next sampling times, dissolved Sn was below the limit of detection. In the control batch (pH 7.2), the dissolved concentration of Sn was below the detection limit in all sampling times. Sr dissolved concentrations were progressively decreasing with time in many batches, except in the HCl-amended batches where Sr concentrations were increasing and the Pseudomonas sp. (strain 233), Paenibacillus sp. (strains 4–10) and “Collimonas sp. relative” batches where Sr concentrations were similar across the sampling points (Fig. 6b). The elements Cs, Ce, Cd and Zr were in many cases not detected in the solution and are not shown in the figures. Due to the high K and P contents in the medium, these two elements exceeded the upper detection limit. The dissolution rates of As and Zn were almost the same as in the control (pH 7.2) batch.
In the batches with citrate, the measured concentrations of many elements (e.g. Fe, Al) were up to two orders of magnitude higher than in the other control or inoculated batches at the end of the experiment. But other elements like W, V, Zn, Ni and Mo were in the same concentration range or below the measured concentrations in the inoculated or both the other two control group batches. The simulation of pH of the glacier forefield (pH 4.6) also showed an increased release of many elements from the granite, although some precipitation occurred after 17 days. Lower pH showed an enhanced release of Mo, Co, Cu, Sr, Ca in all sampling dates and Cr in the first sampling date (p < 0.01, ESM Fig. 2a).
We performed PCA to assess statistically preferences for the dissolution of particular elements by individual strains and at different sampling points. The highest variability of the concentrations of dissolved elements was found to be on the 17th day of the experiment (see the highest dispersion), and it then becomes less correlated with the principal components (e.g. Pseudomonas sp. strain 233, S. aerolata strain 17 and R. erythropolis strain 11), more specialised in the release of element (strains Collimonas sp. (strain 55) and Collimonas sp. relative (strain 256)—W, Rb, Ni, V dissolution) or strongly negatively correlated (control batch and R. erythropolis (strain 29); ESM Fig. 2a–e). The PCA also showed a huge difference of the dissolution of minerals not only between different species but also between different strains (e.g. both R. erythropolis). Therefore, each strain in our experiments showed a unique pattern of dissolution rates during one time sampling point as well as between different sampling points (ESM Fig. 2a–e). In general, Pseudomonas sp., S. aerolata and Paenibacillus sp. dissolve Fe, Al, Zn and Mo preferentially, and Collimonas sp., Collimonas sp. relative and Janthinobacterium sp. strains dissolve W, V and Ni preferentially. For both R. erythropolis strains, we could not determine their preference because the concentrations of dissolved elements were very variable at each sampling point.
Discussion
According to our results, various heterotrophic aerobic bacterial strains isolated from granite sand deposited over the glacier toe have very diverse weathering efficiencies for each element, causing their higher (Fe, Al, Ni, W, Sn and Sr) or lower (Co and Mo) concentrations in solutes. Accordingly, strain diversity, abundance, growth phase and preference for dissolution of minerals, precipitation or uptake of different elements and their incorporation into the newly formed biomass can define how the initial soil development on the freshly deglaciated sand will proceed.
According to Borin et al. [17], soil development is triggered by the colonisation of Fe- and S-oxidising bacteria, which elevates cation exchange and water retention capacities. In parallel with Fe and S oxidation, the cyanobacteria with C and N fixation elevate organic nutrients in a specific niche. Such activities of both groups of bacteria establish conditions for the colonisation of heterotrophic bacteria. In contrast, it has been reported [6] that heterotrophic microorganisms are the first colonisers of barren rocks after glacier retreat. These microorganisms use organic carbon as a food source, which was either initially accumulated in the glacier body or deposited from the atmosphere. Regardless of whether they are present initially or in later stages of paedogenesis, heterotrophic bacteria appear to be important for soil development after deglaciation. Our results show that the variation in the release of micro- and macro-elements from granite by different strains isolated from deglaciated granite sand is very high, especially for Fe, Al, Ni, W, Sn, Co and Mo (see Fig. 6a–d). The changes in the abundance of particular bacterial strains in a heterotrophic bacterial community can therefore result in a patchy distribution of different elements, and this can contribute to the patchy processes of soil development followed by succession of vegetation [51].
In our inoculated batch reactors, we used pH-buffered media (pH 7.2) to keep proton-promoted dissolution minimal [52]. The pH-buffered media contained moderate organic and high Mg, P, K, Ca contents to lower the indirect effects of the microbial dissolution of minerals, including co-mobilisation and mineral destabilisation. For instance, an elevated concentration of K has two effects in our reactors. On the one hand, the uptake of K by microbes, reported to be one of the dissolution mechanisms [53], is not limited by slow dissolution from the granite minerals. On the other hand, the K bound in the minerals is not indirectly released as a consequence of lowering its concentration in solution by uptake into the bacterial cells. For instance, in the case of biotite, one of the mica minerals in the granite rocks, K binds interlayer spaces between Al, Fe, Mg and Si sheets. Lowering the K concentration in the solution results in a thermodynamically more favourable release of the K from these interlayers, but this makes the layers less stable [54].
The results of our dissolution experiment showed that some elements were depleted and others were elevated in the solution when the reactors were inoculated. Microelements such as Mo, Ni and Co were depleted from the solution during the first 2 weeks. This depletion can be a result of the active uptake of released elements by the bacterial cells (Fig. 6b–d). However, Fe was one of the elements that were particularly elevated during the dissolution experiment. Dissolution of Fe is a very complex process due to interaction with other elements and physicochemical factors, and we observed that the dissolution process of elements takes place in a certain order. For instance, in the isolate Pseudomonas sp. 233 strain, the major contribution of this isolate is the dissolution of Fe with the highest peak on the 17th day. The elements Al, Sr, Co, Ni, Mn and Rb follow these kinetics, but the elements Mo, Sn and Cr are dissolved independently. One possible reason for this is that siderophores are also capable of binding elements other than Fe, for instance Al [52]. Another possible explanation is that the released Fe could kinetically change the dissolution rate of these elements [55]. The mechanism of the release of Fe and Al, which are both preferentially released in acidified media [53], can be different in neutral media triggered by ligand-promoted mechanisms or by the local proton-promoted dissolution in the vicinity of cells.
The Pseudomonas sp. 233 strain is similar to other fluorescent Pseudomonas sp. producing siderophores. In addition, this strain produces large amounts of HCN in the early stationary phase (data not shown). Accordingly, high concentrations, as shown in Fig. 6a, could be caused by the fast dissolution of Fe in the first 17 days and the precipitation of HCN–Fe complexes at a later stage [56].
The most efficient strains in mineral dissolution were Pseudomonas sp. strain 233, Paenibacillus sp. strains 4–10, “Collimonas sp. relative” strain 256 and Collimonas sp. strain 55 (Fig. 6a–d). This is consistent with expectations as it is known that for all of these three strains, except for “Collimonas sp. relatives”, which has no defined phylogenetic position, their relatives can efficiently dissolve elements.
Although W is one of the less soluble elements under oxygenated conditions, three of our isolates preferentially released W from the granite (Fig. 6). W is in many cases interchanged with Mo in enzymes and might be biologically very relevant in those three bacterial strains by providing enzymatic function. Sometimes the enzymatic function is preserved [57], but in most cases, W inhibits cell growth [58]. To lower inhibition, bacteria can excrete catechol siderophores that could form complexes with W, thereby diminishing its availability for microorganisms [58]. In either case, the dissolution of W can be elevated.
The dissolution of elements from granite minerals is mediated by the interplay between biogenic and abiogenic factors. Hence, it is well known that the acidification of the silicates affects the dissolution rate by the proton-promoted dissolution mechanism [59]. In our experiments, lowered pH at values similar to the Damma glacier soil (pH 4.6) significantly increased the concentrations of Fe, Al, Co, Mo, Sr, Sn, Mn and Ni in solution than were observed at pH 7.2. The ligand-promoted dissolution at pH 7.2 by citrate was even more effective in the dissolution of elements than pH-lowered conditions. According to Hausrath et al. [60], citrate is the most effective ligand for Fe and other divalent and trivalent cations (M2+/M3+). Although citrate is a weak organic acid, it is completely deprotonated at values above pH 6.4, and accordingly, all of the sites on the molecules involved in the complexation can be fully available. The availability of the M2+/M3+ binding sites on the citrate molecule can be a key factor for significantly higher concentrations of dissolved ions in this citrate-amended control batch than in other controls as well as in the batches inoculated with strains. Both conditions, pH lowering and excretion of citrate by microorganisms, were observed to occur in the Damma glacier forefield [16].
In our experiments, the lowered pH had a less prominent effect on the Fe dissolution than expected (Fig. 6a) [61]. Given the high phosphate concentration in our media, the released Fe can be precipitated with phosphates [53]. Most of the Fe in the granite is in the Fe(II) redox state [62], and it would be expected that a majority of the Fe would be in the same form unless the conditions in the reactors are highly oxidative. Citrate can complex both Fe(II) and Fe(III) forms, but in our citrate-amended batch reactors, all detected Fe was in Fe(III) form, which indicates high oxidation of media, achieved by vigorous shaking. In the inoculated batches with cultures, we observed the presence of Fe(II) (see Fig. 7). The presence of Fe(II) in the solution could be as a result of the two most probable processes, the Fe(III) uptake [63] and the reduction of Fe(III) caused by a low dissolved O2 concentration due to culture respiration. Another less likely process is the excretion of Fe(II)-specific complexing substances (e.g. [64]).
According to known geochemical data of abiogenic transformation processes, the elements detected in our batch experiments can be grouped into elements preferentially dissolved in acidic environments (Co, Fe, Sr, Mn); those dissolved in low redox (E h) conditions (Sn); K-interacting elements (Rb); elements dissolved in oxic and acidic conditions (Ni, V, W); phosphate-interacting elements such as lanthanides; elements whose dissolution is impossible to predict (Mo); and elements which are very resistant to dissolution (e.g. Zr).
Conditions in our pH-lowered control reactors (pH 4.6), but not in others (pH > 7), caused the high solubility of Sr. It is known that Sr is highly soluble at pH values below 5.5 and at high E h values [65]. Similarly, Co and Mn concentrations were also higher in the pH-lowered control reactors than in the other reactors. Although both elements are at similar levels in our granite samples [16], it is expected that they would be in similar concentrations in the solution if they had similar dissolution rates. However, it seems that Mn is more soluble than Co at acidic pH (see Fig. 6b, c). In addition, higher concentrations of Mn in solution lower Co concentration due to precipitation. Although Mn is soluble under those conditions, it can also be precipitated by some metals (Ni, Mo, W, Zn and Cu) at low pH and high E h conditions at which Mn(OH)4 and MnO2 molecules are formed [66]. The levels of metals that precipitate Mn were detected in much lower concentrations in solutions than Mn itself, and this can be the reason for the measured high concentrations of Mn in our pH-lowered batch reactors (see Fig. 6b, d).
The stannous ion (Sn2+) is a strong reducing agent and is soluble under acid and low redox conditions. In addition, at high concentrations of Al and Fe hydroxides, Sn is precipitated on surfaces and is left in the weathering residues [67]. Accordingly, in our batch reactors, which were thoroughly oxidised, we observed a strong negative correlation between Sn and Fe/Al concentrations in most of the reactors, except in the reactors inoculated with Collimonas sp. strain 55 (Fig. 6a, b).
Our media had high levels of K. Despite normal low mobility of Rb due to stronger adsorption on the clay minerals than K, the high K concentrations in our batch reactors probably outcompete the binding sites on the surfaces of minerals and shift Rb from the precipitated to the dissolved form.
Judging by the approximately six times higher amount of V than Ni in the granite sample [16], Ni was much more soluble in our experiments. Both elements are highly soluble under acidic and oxic conditions and both are immobilised by phosphate complexes. The difference between V and Ni is that Ni is more soluble in the presence of organic compounds, especially at pH > 7 [68], and this could partially explain our results. However, in the control batch, we failed to observe any difference between soluble V and Ni concentrations (see Fig. 6d), and thus we speculate that the amount of Fe, phosphate and organic compounds and the release of Ni from dead cells in the late stationary phase serve to regulate the presence of both metals in the solution.
Although some of the lanthanides in our initial granite sample were at levels similar to those of Mn, V and Co [16], we could not detect this group of elements in the filtrates of batch reactors. According to the high phosphate concentration in media, which causes the precipitation of lanthanides, this result was expected.
Elements like Zr and W are normally not present in the solutions. Zr is not easily dissolved due to the high stability of the mineral zircon and Zn(OH)2 [69] and the solubility of W is highly suppressed by oxic conditions and the presence of divalent cations [70]. Accordingly, we observed a low amount of W in our control (pH 7.2) and acidified (pH 4.6) batches (Fig. 6d), but not in inoculated batches, and Zr was generally under the detection limit in all batch reactors.
Although the dissolution processes of many detected elements in the reactors can be predicted somewhat, Mo is poorly predictable. In the pH range of 2–5, it forms HMoO 2−4 ions and can be co-precipitated with other cations such as Cu, Zn, Mn and Ca. This process is highly dependant on E h and the pH conditions [67].
According to our results, the granite sand, which covers the glacial ice, supports the survival and growth of heterotrophic bacteria belonging to the Proteobacteria, Actinobacteria, Firmicutes and Bacteroidetes phyla. At the genera level, the bacterial community structure of our isolates was very similar to the Finnish Lapland isolates obtained by Männisto and Häggblom [71]. They found low numbers of Firmicutes and a high number of Betaproteobacteria. According to the reports of Männisto and Häggblom as well as Schmidt et al. [72], it seems that in environments with extreme temperature fluctuations, Betaproteobacteria are predominant.
Liu et al. [73] compared these results with the results of Foght et al. [74] and postulated that the reason for the absence of the Betaproteobacteria from their Mount Everest East Rongbuk glacier samples is the surface exposure of the sampled material. However, they did not use oligotrophic media and incubation was at temperatures of 22°C; according to our results, the effect of such rich media incubated at temperatures close to 20°C (22°C or 18°C) is that the isolation of the majority of Betaproteobacteria from the glacial environment would not be supported (Fig. 5). According to our results, the condition of permanent darkness and isolation, both present in the subglacial environment (discussed in [73]), probably has a small effect on the amount of the isolated Betaproteobacteria. Therefore, we suggest that the choice of media and the temperature conditions for cultivation are the most important factors. For instance, our method of isolation was, in terms of the media used, very similar to the method of Foght et al. [74] who collected the samples from underneath the glacier body material. Accordingly, we obtained a large amount of Betaproteobacteria, even in our samples, from surface-exposed soil.
The most numerous isolates from the Betaproteobacteria in our culture collection belong to the family Oxalobacteriaceae. In this family, we obtained many similar isolates which were described by clone libraries from freshly deglaciated terrain: relatives to Polaromonas vacuolata, Duganella zoogloeoides and Janthinobacterium lividum [8]. There are distinct differences in the presence of Janthinobacterium and Duganella clones and isolates in alpine and Antarctic environments. The former are present only in Antarctic and the latter in alpine samples [75]. In our samples, most of the isolates that could be assigned to the Duganella genus were not phylogenetically clearly distinguishable from the Janthinobacterium relatives and were therefore included within the Janthinobacterium genus.
Our isolates belonging to the Bacteroidetes phylum were completely absent when the collected material was incubated at 4°C (Table 1). Bacteroidetes were also less abundant in glaciers [73, 76, 77], but were present among the Alaska glacier 16S rRNA clones [78]. In our culture collection, the Bacteroidetes isolates obtained at 22°C were related to Pedobacter sp. isolated from the alpine grassland at 2,970 m in China and German Westharz mountains below 1,000 m a.s.l.
Bacteria belonging to the Actionobacteria and Firmicutes are consistently detected in glacier bodies or alpine soil environments [45, 79–81]. The most frequently described genera among isolates as well as clone libraries are Arthrobacter and Rhodococcus [73, 74, 78, 82]. Representatives of both genera were isolated in the present study. A highly diverse group among our isolates was Arthrobacter, and based on the 16S rRNA genes, many new species could be potentially described (Fig. 6). Among Firmicutes isolates, we obtained representatives of Paenibacillus, but not of Bacillus. The Bacillus representatives have been described in many publications in which molecular techniques were applied [83, 84]. Their absence in our isolate collection might be due to the presence of spores in the environment and failure of germination recovery [85] or due to non-culturability.
Using our cultivation approach, higher species richness was observed among bacteria obtained from incubation at 22°C than that at 4°C. This could be, on the one hand, the consequence of the invasion of mesophilic bacteria from the lowland temperate environments transported by wind, rain or snow [5, 86, 87] and, on the other, the presence of the adapted microorganisms on the broad temperature range [88, 89]. In our investigation, we have shown that a higher percentage (25.9%) of bacterial isolates, which have no relatives among bacteria from glacier or other extremely cold environments, were detected when incubated at 22°C and a smaller percentage (only 10%) when incubated at 4°C. The highest species richness among isolates was also observed on 1/100 diluted Ravan medium at 4°C, but not at 22°C. This is in agreement with the adaptation of real inhabitants on freshly deglaciated sands to the content of lower organic nutrients and low temperatures. At 22°C incubation, we observed the lowest species richness on plates with low nutrients such as 1/160NA and 1/100Ravan. Probably even small differences in substrate utilization at different temperatures result in preferential growth at 4°C or 22°C, which we observed here as the differences in species distribution in media at 4°C or 22°C incubation temperatures.
At 4°C, we obtained the most abundant group of bacteria distantly related to the representatives of the genus Collimonas. These isolates are probably well adapted to low temperatures because only a few isolates were obtained at 22°C. Phylogenetically, these strains are only distantly related to all other Oxalobacteriaceae isolates (Fig. 4). The only similar strains were detected among clone libraries from alpine soils [90]. These isolates related to Collimonas at 95–96% similarity in 16S rRNA gene nucleotide sequences have not been described and might form a new genus according to the criteria based on 16S rRNA [91]. However, further investigation to describe these isolates properly is needed.
Interestingly, our isolates belong to all three physiological groups described by Liu et al. [73]: slow-growing (Microbacterium), intermediate- (Frigoribacterium) and fast-growing groups (Arthrobacter, Janthinobacterium). Fast-growing groups were isolated more frequently in our cultivation approach than the slow-growing ones, and this might be a result of the rather short incubation times.
In conclusion, the diversity of colonising microorganisms is important for the efficient establishment of new soil on the barren deglaciated granite. Our results suggest that species richness of microorganisms might affect variations in both quantity and quality of mineral dissolution. Each strain has intrinsic weathering and ion uptake capability at different growth stages. Just the right interplay between microbial diversity and environmental conditions where these microorganisms are growing probably differentiates sites of faster and slower granite weathering, which could result in more or less successful soil development after deglaciation.
References
Oerlemans J (2005) Extracting a climate signal from 169 glacier records. Science 308:675–677
Hodson A, Anesio A, Tranter M, Fountain A, Osborn M, Priscu J, Laybourn-Parry J, Sattler B (2008) Glacial ecosystems. Ecol Monogr 78:41–67
Segawa T, Miyamoto K, Ushida K, Agata K, Okada N, Kohshima S (2005) Seasonal change in bacterial flora and biomass in mountain snow from the Tateyama Mountains, Japan, analyzed by 16S rRNA gene sequencing and real-time PCR. Appl Environ Microbiol 71:123–130
Amato P, Parazols M, Sancelme M, Laj P, Mailhot G, Delort AM (2007) Microorganisms isolated from the water phase of tropospheric clouds at the Puy de Dome: major groups and growth abilities at low temperatures. FEMS Microbiol Ecol 59:242–254
Bauer H, Kasper-Giebl A, Löflund M, Giebl H, Hitzenberger R, Zibuschka F, Puxbaum H (2002) The contribution of bacteria and fungal spores to the organic carbon content of cloud water, precipitation and aerosols. Atmos Res 64:109–119
Bardgett RD, Richter A, Bol R, Garnett MH, Baumler R, Xu X, Lopez-Capel E, Manning DA, Hobbs PJ, Hartley IR, Wanek W (2007) Heterotrophic microbial communities use ancient carbon following glacial retreat. Biol Lett 3:487–490
Schmidt SK, Reed SC, Nemergut DR, Grandy AS, Cleveland CC, Weintraub MN, Hill AW, Costello EK, Meyer AF, Neff JC, Martin AM (2008) The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently deglaciated soils. Proc Biol Sci 275:2793–2802
Nemergut DR, Anderson SP, Cleveland CC, Martin AP, Miller AE, Seimon A, Schmidt SK (2007) Microbial community succession in an unvegetated, recently deglaciated soil. Microb Ecol 53:110–122
Sigler WV, Zeyer J (2002) Microbial diversity and activity along the forefields of two receding glaciers. Microb Ecol 43:397–407
Tscherko D, Rustemeier J, Richter A, Wanek W, Kandeler E (2003) Functional diversity of the soil microflora in primary succession across two glacier forelands in the Central Alps. Eur J Soil Sci 54:685–696
Schutte UME, Abdo Z, Bent SJ, Williams CJ, Schneider GM, Solheim B, Forney LJ (2009) Bacterial succession in a glacier foreland of the High Arctic. ISME J 3:1258–1268
Uroz S, Calvaruso C, Turpault MP, Pierrat JC, Mustin C, Frey-Klett P (2007) Effect of the mycorrhizosphere on the genotypic and metabolic diversity of the bacterial communities involved in mineral weathering in a forest soil. Appl Environ Microbiol 73:3019–3027
Uroz S, Calvaruso C, Turpault MP, Frey-Klett P (2009) Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol 17:378–387
Sharp M, Parkes J, Cragg B, Fairchild IJ, Lamb H, Tranter M (1999) Widespread bacterial populations at glacier beds and their relationship to rock weathering and carbon cycling. Geology 27:107–110
Tranter M, Sharp M, Lamb H, Brown G, Hubbard B, Willis I (2002) Geochemical weathering at the bed of Haut Glacier d’Arolla, Switzerland—a new model. Hydrol Process 16:959–993
Frey B, Rieder SR, Brunner I, Plotze M, Koetzsch S, Lapanje A, Brandl H, Furrer G (2010) Weathering-associated bacteria from the Damma glacier forefield: physiological capabilities and impact on granite dissolution. Appl Environ Microbiol 76:4788–4796
Borin S, Ventura S, Tambone F, Mapelli F, Schubotz F, Brusetti L, Scaglia B, D’Acqui LP, Solheim B, Turicchia S, Marasco R, Hinrichs KU, Baldi F, Adani F, Daffonchio D (2010) Rock weathering creates oases of life in a high Arctic desert. Environ Microbiol 12:293–303
Ferrari B, Binnerup S, Gillings M (2005) Microcolony cultivation on a soil substrate membrane system selects for previously uncultured soil bacteria. Appl Environ Microbiol 71:8714–8720
Janssen P, Yates P, Grinton B, Taylor P, Sait M (2002) Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl Environ Microbiol 68:2391–2396
Connon S, Giovannoni S (2002) High-throughput methods for culturing microorganisms in very-low-nutrient media yield diverse new marine isolates. Appl Environ Microbiol 68:3878–3885
Button D, Schut F, Quang P, Martin R, Robertson B (1993) Viability and isolation of marine bacteria by dilution culture: theory, procedures, and initial results. Appl Environ Microbiol 59:881–891
Goltekar R, Krishnan K, DeSouza M, Paropkari A, LokaBharathi P (2006) Effect of carbon source concentration and culture duration on retreivability of bacteria from certain estuarine, coastal and offshore areas around the peninsular India. Curr Sci 90:103–106p
Janssen P, Schuhmann A, Morschel E, Rainey F (1997) Novel anaerobic ultramicrobacteria belonging to the Verrucomicrobiales lineage of bacterial descent isolated by dilution culture from anoxic rice paddy soil. Appl Environ Microbiol 63:1382–1388
Goldberg J (2000) Pseudomonas: global bacteria. Trends Microbiol 8:55
Meyer AF, Lipson DA, Martin AP, Schadt CW, Schmidt SK (2004) Molecular and metabolic characterization of cold-tolerant alpine soil Pseudomonas sensu stricto. Appl Environ Microbiol 70:483–489
Tindall B (2004) Prokaryotic diversity in the Antarctic: the tip of the iceberg. Microb Ecol 47:271–283
Edwards I, Bürgmann H, Miniaci C, Zeyer J (2006) Variation in microbial community composition and culturability in the rhizosphere of Leucanthemopsis alpina (L.) Heywood and adjacent bare soil along an alpine chronosequence. Microb Ecol 52:679–692
Moreno F, Vilela S, Antunes Â, Alves C (2006) Capillary-rising salt pollution and granitic stone erosive decay in the parish church of Torre de Moncorvo (NE Portugal)—implications for conservation strategy. J Cult Herit 7:56–66
Franzen C, Mirwald P (2004) Moisture content of natural stone: static and dynamic equilibrium with atmospheric humidity. Environ Geol 46:391–401
Gorbushina AA (2007) Life on the rocks. Environ Microbiol 9:1613–1631
Deo N, Vasan S, Modak JM, Natarajan K (1999) Selective biodissolution of calcium and iron from bauxite in the presence of Bacillus polymyxa. Process Metall 9:463–472
Vasan S, Modak JM, Natarajan K (2001) Some recent advances in the bioprocessing of bauxite. Int J Miner Process 62:173–186
Lazzaro A, Abegg C, Zeyer J (2009) Bacterial community structure of glacier forefields on siliceous and calcareous bedrock. Eur J Soil Sci 60:860–870
Noll M, Wellinger M (2008) Changes of the soil ecosystem along a receding glacier: testing the correlation between environmental factors and bacterial community structure. Soil Biol Biochem 40:2611–2619
Taylor RH, Geldreich EE (1976) A new membrane filter procedure for bacterial counts in potable water and swimming pool samples. J Am Water Works Assoc 38:191
Greenberg AE (1981) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Reasoner D, Geldreich E (1985) A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49:1
Logan N, Moss M (1992) Identification of Chromobacterium, Janthinobacterium and Iodobacter species. Soc Appl Bacteriol Techn Ser 29:183–192
Watve M, Shejval V, Sonawane C, Rahalkar M, Matapurkar A, Shouche Y, Patole M, Phadnis N, Champhenkar A, Damle K (2000) The ‘K’ selected oligophilic bacteria: a key to uncultured diversity? Curr Sci 78:1535–1542
Giraffa G, Rossetti L, Neviani E (2000) An evaluation of chelex-based DNA purification protocols for the typing of lactic acid bacteria. J Microbiol Methods 42:175–184
Lapanje A, Zrimec A, Drobne D, Rupnik M (2010) Long-term Hg pollution-induced structural shifts of bacterial community in the terrestrial isopod (Porcellio scaber) gut. Environ Pollut 158:3186–3193
Bianciotto V, Bandi C, Minerdi D, Sironi M, Tichy HV, Bonfante P (1996) An obligately endosymbiotic mycorrhizal fungus itself harbors obligately intracellular bacteria. Appl Environ Microbiol 62:3005–3010
Heuer H, Krsek M, Baker P, Smalla K, Wellington EM (1997) Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 63:3233–3241
Teske A, Wawer C, Muyzer G, Ramsing NB (1996) Distribution of sulfate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most-probable-number counts and denaturing gradient gel electrophoresis of PCR-amplified ribosomal DNA fragments. Appl Environ Microbiol 62:1405–1415
Cheng S, Foght J (2007) Cultivation-independent and -dependent characterization of bacteria resident beneath John Evans Glacier. FEMS Microbiol Ecol 59:318–330
Liermann LJ, Kalinowski BE, Brantley SL, Ferry JG (2000) Role of bacterial siderophores in dissolution of hornblende. Geochim Cosmochim Acta 64:587–602
Viollier E, Inglett P, Hunter K, Roychoudhury A, Van Cappellen P (2000) The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl Geochem 15:785–790
Larkin M, Blackshields G, Brown N, Chenna R, McGettigan P, McWilliam H, Valentin F, Wallace I, Wilm A, Lopez R (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596
Holland S (1988) A rarefactwin program, version 1.2. http://www.uga.edu/strata/Software.html
Raffl C, Mallaun M, Mayer R, Erschbamer B (2006) Vegetation succession pattern and diversity changes in a glacier valley, Central Alps, Austria. Arct Antarct Alp Res 38:421–428
Kalinowski BE, Liermann LJ, Givens S, Brantley SL (2000) Rates of bacteria-promoted solubilization of Fe from minerals: a review of problems and approaches. Chem Geol 169:357–370
Banfield JF, Barker WW, Welch SA, Taunton A (1999) Biological impact on mineral dissolution: application of the lichen model to understanding mineral weathering in the rhizosphere. Proc Natl Acad Sci U S A 96:3404
Barshad I, Kishk FM (1968) Oxidation of ferrous iron in vermiculite and biotite alters fixation and replaceability of potassium. Science 162:1401
Murakami T, Utsunomiya S, Yokoyama T, Kasama T (2003) Biotite dissolution processes and mechanisms in the laboratory and in nature: early stage weathering environment and vermiculitization. Am Mineral 88:377
Faramarzi MA, Brandl H (2006) Formation of water soluble metal cyanide complexes from solid minerals by Pseudomonas plecoglossicida. FEMS Microbiol Lett 259:47–52
Kletzin A, Adams MWW (1996) Tungsten in biological systems. FEMS Microbiol Rev 18:5–63
Wichard T, Bellenger JP, Loison A, Kraepiel AML (2008) Catechol siderophores control tungsten uptake and toxicity in the nitrogen-fixing bacterium Azotobacter vinelandii. Environ Sci Technol 42:2408–2413
Wu L, Jacobson AD, Hausner M (2008) Characterization of elemental release during microbe–granite interactions at T = 28°C. Geochim Cosmochim Acta 72:1076–1095
Hausrath EM, Neaman A, Brantley SL (2009) Elemental release rates from dissolving basalt and granite with and without organic ligands. Am J Sci 309:633
Taunton AE, Welch SA, Banfield JF (2000) Microbial controls on phosphate and lanthanide distributions during granite weathering and soil formation. Chem Geol 169:371–382
Kiczka M, Wiederhold JG, Frommer J, Kraemer SM, Bourdon B, Kretzschmar R (2010) Iron isotope fractionation during proton- and ligand-promoted dissolution of primary phyllosilicates. Geochim Cosmochim Acta 74:3112–3128
Luu YS, Ramsay JA (2003) Review: microbial mechanisms of accessing insoluble Fe(III) as an energy source. World J Microbiol Biotechnol 19:215–225
Liu W, Fisher SM, Wells JS Jr, Ricca CS, Principe PA, Trejo WH, Bonner DP, Gougoutos JZ, Toeplitz BK, Sykes RB (1981) Siderochelin, a new ferrous-ion chelating agent produced by Nocardia. J Antibiot 34:791
Short NM (1961) Geochemical variations in four residual soils. J Geol 69:534–571
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants. CRC, Boca Raton
Kabata-Pendias A (2001) Trace elements in soils and plants. CRC Press, Inc., Boca Raton, Florida, 413 pp
Ashworth DJ, Alloway BJ (2008) Influence of dissolved organic matter on the solubility of heavy metals in sewage-sludge-amended soils. Commun Soil Sci Plan 39:538
Smith IC, Carson BL (1978) Trace metals in the environment. Volume 3 – Zirconium. Ann Arbor Science Publishers Inc., Ann Arbor, Michigan, 405 pp
Brookins DG (1988) Eh-pH diagrams for geochemistry. Springer-Verlag, Berlin-Heidelberg, 176 pp
Männisto MK, Häggblom MM (2006) Characterization of psychrotolerant heterotrophic bacteria from Finnish Lapland. Syst Appl Microbiol 29:229–243
Schmidt SK, Nemergut DR, Miller AE, Freeman KR, King AJ, Seimon A (2009) Microbial activity and diversity during extreme freeze–thaw cycles in periglacial soils, 5400 m elevation, Cordillera Vilcanota, Peru. Extremophiles 13:807–816
Liu Y, Yao T, Jiao N, Kang S, Huang S, Li Q, Wang K, Liu X (2009) Culturable bacteria in glacial meltwater at 6,350 m on the East Rongbuk Glacier, Mount Everest. Extremophiles 13:89–99
Foght J, Aislabie J, Turner S, Brown C, Ryburn J, Saul D, Lawson W (2004) Culturable bacteria in subglacial sediments and ice from two southern hemisphere glaciers. Microb Ecol 47:329–340
Lipson DA, Schmidt SK (2004) Seasonal changes in an alpine soil bacterial community in the Colorado Rocky Mountains. Appl Environ Microbiol 70:2867–2879
Zhang G, Niu F, Ma X, Liu W, Dong M, Feng H, An L, Cheng G (2007) Phylogenetic diversity of bacteria isolates from the Qinghai–Tibet Plateau permafrost region. Can J Microbiol 53:1000–1010
Brinkmeyer R, Knittel K, Jurgens J, Weyland H, Amann R, Helmke E (2003) Diversity and structure of bacterial communities in Arctic versus Antarctic pack ice. Appl Environ Microbiol 69:6610–6619
Skidmore M, Anderson SP, Sharp M, Foght J, Lanoil BD (2005) Comparison of microbial community compositions of two subglacial environments reveals a possible role for microbes in chemical weathering processes. Appl Environ Microbiol 71:6986–6997
Christner BC, Mosley-Thompson E, Thompson LG, Reeve JN (2003) Bacterial recovery from ancient glacial ice. Environ Microbiol 5:433–436
Miteva VI, Sheridan PP, Brenchley JE (2004) Phylogenetic and physiological diversity of microorganisms isolated from a deep Greenland glacier ice core. Appl Environ Microbiol 70:202–213
Miskin I, Rhodes G, Lawlor K, Saunders JR, Pickup RW (1998) Bacteria in post-glacial freshwater sediments. Microbiology 144(Pt 9):2427–2439
Mikucki JA, Priscu JC (2007) Bacterial diversity associated with Blood Falls, a subglacial outflow from the Taylor Glacier, Antarctica. Appl Environ Microbiol 73:4029–4039
Christner BC, Kvitko BH 2nd, Reeve JN (2003) Molecular identification of bacteria and Eukarya inhabiting an Antarctic cryoconite hole. Extremophiles 7:177–183
Zhang G, Ma X, Niu F, Dong M, Feng H, An L, Cheng G (2007) Diversity and distribution of alkaliphilic psychrotolerant bacteria in the Qinghai–Tibet Plateau permafrost region. Extremophiles 11:415–424
Black E, Wei J, Atluri S, Cortezzo D, Koziol-Dube K, Hoover D, Setlow P (2007) Analysis of factors influencing the rate of germination of spores of Bacillus subtilis by very high pressure. J Appl Microbiol 102:65–76
Gorbushina A, Kort R, Schulte A, Lazarus D, Schnetger B, Brumsack H, Broughton W, Favet J (2007) Life in Darwin’s dust: intercontinental transport and survival of microbes in the nineteenth century. Environ Microbiol 9:2911–2922
Sattler B, Puxbaum H, Psenner R (2001) Bacterial growth in supercooled cloud droplets. Geophys Res Lett 28:239–242
Steven B, Léveillé R, Pollard W, Whyte L (2006) Microbial ecology and biodiversity in permafrost. Extremophiles 10:259–267
Panikov N, Sizova M (2006) Growth kinetics of microorganisms isolated from Alaskan soil and permafrost in solid media frozen down to −35°C. FEMS Microbiol Ecol 59:500–512
Yuhana M (2005) Microbial diversity and community changes in high mountain habitats (2009) PhD thesis, ETH Zurich
Vandamme P, Pot B, Gillis M, De Vos P, Kersters K, Swings J (1996) Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Mol Biol Rev 60:407
Acknowledgements
Financial support for this study was provided by the BigLink project of the Competence Center Environment and Sustainability (CCES) of the ETH Domain. This study was also supported by the Genetic Diversity Centre of ETH Zurich (GDC). Helmut Brandl (University of Zurich) and Daniela Steiner (WSL) are acknowledged for the valuable scientific and technical support.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplement Figure 1
Phylogenetic position of the 16S rRNA genes of isolates. Here are selected isolates that have lower than 98% similarities among the RDPII database isolates and type strains of Burkholderiales. Collimonas relatives are here excluded. Numbers on the branches represent bootstrap values. Isolates are presented in bold numbers. Black-filled circles represent 4°C isolates. Tree is constructed with a neighbour joining method based on Kimura two-parameter distance values (DOC 507 kb)
Supplement Figure 2
Isolate preferences in dissolving different elements from the granite sand. Figures show principal component analysis (PCA) of the concentrations of elements in each batch reactor. Vectors specify multidimensional space determined by the variations in elements concentrations in batch reactors. Yellow numbers in blue circles show the mean scores of three batches per isolate (DOC 10472 kb)
Supplement Table 1a
Characteristics of isolates obtained at 22°C (DOC 772 kb)
Supplement Table 2
Dissolution rates of elements from the granite sand during the 30-day experiment. Red-coloured elements with arrows pointing up show the increase and blue-coloured elements with down-pointing arrows the decrease of dissolution rate according to the control. *90% confidence interval, **95% confidence interval, ***99% confidence interval in pairwise Student’s t tests (DOC 57 kb)
Rights and permissions
About this article
Cite this article
Lapanje, A., Wimmersberger, C., Furrer, G. et al. Pattern of Elemental Release During the Granite Dissolution Can Be Changed by Aerobic Heterotrophic Bacterial Strains Isolated from Damma Glacier (Central Alps) Deglaciated Granite Sand. Microb Ecol 63, 865–882 (2012). https://doi.org/10.1007/s00248-011-9976-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9976-7