Abstract
Leishmaniasis is one of the six major tropical diseases that is spreading geographically in the world, with no definitive cure. The aim of the present study was to investigate the anti-leishmanial effects of Olea europaea and Ficus carica extracts against Leishmania major in both in vitro and in vivo experimental models. The in vitro efficiency concentrations of 0.1–2 mg mL− 1 of O. europaea and F. carica extracts were effective against promastigote L. major at 48 h. In addition, the lesion size and parasite burden in BALB/c mice infected with promastigote of L. major were quantified for in vivo evaluation. Results showed that IC50 of O. europaea and F. carica extracts against promastigote were 1.5 and 1.2 mg mL− 1, respectively. In addition, results from in vivo assay revealed that the mean size ± SD of lesions significantly decreased to 3.46 ± 0.96 and 3.65 ± 0.9 mm2 in mice treated with O. europaea and F. carica extracts, respectively, compared with that in the untreated group (p = 0.001). Findings also indicated that O. europaea and F. carica extracts considerably lowered the parasite burden in lesions (p < 0.001), compared with the untreated group. Both extracts showed notable activity against L. major. However, further investigations are required to controlling CL and inhibiting the development of lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is a neglected disease caused by protozoan parasites of the genus Leishmania spp. and transmitted via the bite of female Phlebotomine sandflies (Torres-Guerrero et al. 2017). This clinical form of the disease ranges from self-healing cutaneous lesion to severe and non-healing disseminated cutaneous or visceral leishmaniasis (VL) (Ponte-Sucre et al. 2017). This species may be found in Asia, Africa, the Americas, and the Mediterranean region (Torres-Guerrero et al. 2017). In 2012, the World Health Organization (WHO) launched a project to report on the burden and distribution of leishmaniasis in 102 nations, regions, or territories throughout the world, with the goal of identifying CL and visceral leishmaniasis cases (VL). According to statistics collected till 2010, 90% of VL cases in the world happened in Bangladesh, Brazil, Ethiopia, India, South Sudan, and the Sudan, whereas roughly 70% of CL cases occurred in Afghanistan, Algeria, Brazil, Colombia, Costa Rica, Ethiopia, Iran, Sudan, and the Syrian Arab Republic (Torres-Guerrero et al. 2017).
On the other hand, 13 countries including Bangladesh, China, Ethiopia, Georgia, India, Kenya, Nepal, Paraguay, Somalia, South Sudan, Spain, Sudan, and Uganda out of the 25 nations studied have a high prevalence of VL, 11 have a high prevalence of CL (Afghanistan, Algeria, Colombia, Iran, Morocco, Pakistan, Peru, Saudi Arabia, Syria Arab Republic, Tunisia, and Turkey), whereas one (Brazil) has a high prevalence of both clinical types. Every year, roughly 2,000 autochthonous CL cases are documented in Turkey (WHO 2016). In this respect, CL is a public health concern. According to WHO’s report, 350 million people are living in regions with a high risk of infection and 12 million cases of leishmaniasis exist worldwide with an estimated number of 1.5–2 million new cases occurring annually (Hailu et al. 2016).
Although pentavalent antimony (Sodium stibogluconate (SSG) and meglumine antimoniate (MA)) is still the first-line treatment of CL, these drugs are expensive and associated with side effects such as cardiac toxicity and elevation in the levels of hepatic enzymes. Moreover, injections are painful, and sometimes there is a need for multiple injections as a result ineffective or low cure rates (Alvar et al. 2012). Alternatively, Amphotericin B, pentamidine, paromomycin, fluconazole, and miltefosine can be used as second choice drugs, however, these drugs also exhibit high toxicity in addition to being expensive (McGwire and Satoskar 2014). On the other hand, resistance to these drugs has increased all over the world (Mishra et al. 2007).
Since there is no effective vaccine for this infection, discovery and improvement of natural products, such as plant extracts may provide unlimited opportunities for new drug discoveries (Dias et al. 2012). Natural compounds of plant origin have been extensively investigated to find a better and safer alternative treatment (Akkol et al. 2021). Infectious disease is one of the top-most serious threats to human health globally, further exacerbated by antimicrobial resistance and lack of novel immunization options. In the study conducted by Hossain et al. (2021), Andrographis paniculata (Burm. f.) was introduced as a promising source of antimicrobial agents and safe treatment for infectious diseases. Within this context, the genus Olea is classified into the subfamily Oleideae, containing 35 species, which grow throughout southeast and southwest Europe, west Asia, and Africa (Chiappetta and Muzzalupo 2012). Olive tree (O. europaea) leaves are rich in phenolic compounds with strong antioxidant properties (Cardinali et al. 2010), glycosides, secoiridoid, flavonoids and, poly-unsaturated fatty acids. These compounds may exhibit numerous bioactivities such as antimicrobial, anitdiabetic, hypolipidemic, cardioprotective, antiviral effect, antiarrhythmic, spasmolytic, immune stimulant, hypotensive, and anti-inflammatory (Sahranavard et al. 2014; Ben Salem et al. 2015), scolicidal effects (Niazi et al. 2019), anti-atherogenic, and anticancer effects (Sahranavard et al. 2014). Published research suggested that leaves of the olive tree have been used in traditional medicine as a blood pressure-lowering medication, anti-atherosclerosis, and in the treatment of fever, urinary tract infections, and headache (Msomi and Simelane 2017; Benavente-Garcıa et al. 2000).
Moreover, olive tree contains a plethora of bioactive compounds including phenolic and flavonoid (Guo et al. 2018), lignans, volatile components (Keskin et al. 2012), tepenoids (Sato et al. 2007; Stiti and Hartmann. 2012), variety of aldehydes, alcohols, esters, ketones, norisoprenoids, and pyridines (Pérez-Bonilla et al. 2006; Guo et al. 2018) among others.
Furthermore, Ficus carica L. is an Asian species of flowering plant in the mulberry family (Moraceae), known as the common fig (Oliveira et al. 2009a). The common fig is one of the first plants that were cultivated by humans. Ficus carica is an important source of phenolic compounds such as proanthocyanidins. Fruits, root, and leaves of F. carica are recognized as a remedy with various therapeutic effects (Solomon et al. 2006; Oliveira et al. 2009). Ficus carica has been used in traditional medicine to treat various illnesses affecting the digestive, endocrine, reproductive, and respiratory systems. Traditional healers also used this plant to treat infections of the gastrointestinal system and urinary tract. It additionally contains organic acids (Oliveira et al. 2009), amino acids (Oliveira et al. 2010), fatty acids (Jeong and Lachance 2001), flavonoids (Vaya and Mahmood 2006), phenolic compounds (Oliveira et al. 2009b), phytosterols (Ostlund 2002), volatile compounds (Shiraishi et al. 1996), anthocyanin (Solomon et al. 2006), some enzymes (Sgariberi et al. 1964), and some nutritional contents fiber, copper, manganese, magnesium, potassium, calcium, and vitamin K (Badgujar et al. 2014).
Traditionally, haemorrhage is treated with F. carica fruit juice mixed with honey. Fruits are used as a mild laxative, expectorant, and diuretic in Indian medicine (Solomon et al. 2006). Furthermore, fruit paste is applied to swellings, tumors, and inflammation for relieving pain (Mawa et al. 2013). Pharmacologically, Fig demonstrated several activities in experimental model. It exhibits antioxidant (Solomon et al. 2006), anticancer (Yancheva et al. 2005), hepatoprotective (Gond and Khadabadi 2008), hypoglycemic (Asadi et al. 2006), antibacterial and antifungal (Aref et al. 2010), antipyretic (Patil Vikas et al. 2010), antituberculosis (Khadabadi et al. 2007), nematicidal (Liu et al. 2011), antispasmodic and antiplatelet (Mohamad et al. 2011), anti-HSV effect (Wang et al. 2004), antimutagenic (Agabeĭli and Kasimova 2005), and anthelmintic activities (de Amorin et al. 1999). On the basis of the previous discussion, the aim of the present study was to evaluate the antileishmanial activity of O. europaea and F. carica extracts against Leishmania major both in vitro and in BALB/c mouse model (in vivo) of cutaneous leishmaniasis.
Materials and methods
Plants and extraction
Leaves of O. europaea and F. carica were purchased from a herbal shop and authenticated by the Herbal Medicine Research Center, School of Pharmacy, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Extraction was performed using the maceration method. In the first step, the leaves of O. europaea were dried at room temperature, ground, and then passed through a sieve. Then 50 g of the powdered material was poured into a sterile screw-capped glass container and mixed with 500 mL of ethyl alcohol (80%). The extraction was carried out for one week. Subsequently, the extract was concentrated by means of a rotary evaporator under reduced pressure at 45 °C and stored at − 20 °C until use. F. carica was extracted according to the same procedure used for O. europaea extraction.
Parasites and animals
Leishmania major (MRHO/IR/75/ER) parasites were cultured in RPMI 1640 media (Gibco, Life Technologies GmbH, Germany) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Germany), and 100 U mL− 1 penicillin and 100 µg mL− 1 streptomycin (Gibco, Germany). Cultures were incubated at 24 °C for promastigote proliferation and the infectivity of the parasites was maintained by the regular passage in susceptible BALB/c mice (Sudjana et al. 2009). For this purpose, forty BALB/c mice 4–6 weeks old, weighing 20–25 g were purchased from Pasteur Institute (Tehran, Iran). These animals were given free access to standard diet and water, and were kept under normal laboratory conditions (temperature: 22 ± 2 ºC, humidity: 50 ± 5%, and 12 h light/dark cycles). Experiments involving animals were conducted according to Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health and approved by the Ethical Committee of Shahid Sadoughi University of Medical Sciences (Yazd, Iran).
In vitro study
Promastigotes of L. major (1 × 106) were treated with O. europaea and F. carica extracts (0.1–2 mg mL− 1) in separate tubes and incubated for 48 h at 25 °C. After the incubation periods, 20 µL of each tube were mixed with the same volume of 0.4% Trypan blue to detect and count the viable promastigotes. The parasites mortality rate was calculated by counting the number of live (not stained) and dead (stained) promastigotes. Amphotericin B and dimethyl sulfoxide (DMSO) were used as controls, respectively. The IC50 values of the extracts were calculated as a concentration capable of inhibiting 50% growth of parasite. All tests were performed in duplicate and repeated at least three times.
In vivo study
BALB/c mice were randomly divided into four groups of ten mice each. These animals were infected with 0.1 mL of the promastigotes of L. major (2 × 106 cells mL− 1) by subcutaneous injection into the base of the tail. After four weeks post-infection when leishmanial lesions appeared, mice were then divided into four groups (n = 10) as follows: Group 1 included mice treated with intralesional injection of 30 mg/kg/day ethanol extract of O. europaea for 21 days. Group 2 included mice treated with intralesional injection of 24 mg/kg/day ethanol extract of F. carica for 21 days. Group 3 included mice treated with 4 mg/kg/day of amphotericin B through intraperitoneal injection for 21 days as a positive control group. Group 4 included infected but untreated mice as an untreated group. In the treatment period, the average lesion size was measured weekly as the differences obtained between lesions size using the caliper tool to assess the effects of treatments. Lesions were reported in square millimeters.
To analyze the parasite burden, smears were prepared from the margins of lesions, fixed with absolute methanol, stained with Giemsa, and observed microscopically for the presence of amastigotes or infected macrophages using a light microscope (Labomed, USA) before the initiation of treatments and three weeks after the treatment. The parasite burden was analyzed according to the WHO guidelines of 4+ (1–10 parasites/1 field), 3+ (1–10 parasites/10 fields), 2+ (1–10 parasites/100 fields), and 1+ (1–10 parasites/1000 fields).
Statistical analysis
Statistical differences and IC50 (50% inhibitory concentrations) of the products were determined using the Mann-Whitney test. In addition, results on lesion progression and parasite burden were treated by analysis of variance test, followed by a Post Hoc Test (LDS test or planned comparison). Data analysis was carried out using SPSS statistical package, version 17.0 (SPSS Inc., Chicago, IL, USA). Differences between the test and untreated groups were analyzed by t-test. Differences were considered significant at p ≤ 0.05.
Results
In vitro and in vivo anti-leishmanial effects
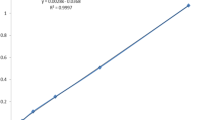
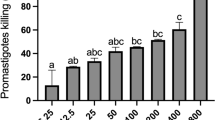
To evaluate the effect of O. europaea and F. carica extracts against L. major promastigotes, IC50 (µg mL− 1) values were determined. The IC50 values at 48 h for O. europaea and F. carica extracts were 1.5 mg mL− 1 and 1.2 mg mL− 1, respectively. In an in vivo assay, ulcer sizes were measured at day zero and during 3 weeks of the treatment course. Results showed that both the time (p < 0.001) and the different treatment regimens (p = 0.001) have significant effects on the size of ulcers. Results of the effect of O. europaea and F. carica extracts on the lesion size are shown in Table 1; Fig. 1. Our findings revealed that ulcer sizes have changed in mice treated with the extracts, but changes have not been the same in the four groups. A significant decrease in lesion size was observed in the groups treated with O. europaea, F. carica, and Amphotericin B (p < 0.001). However, there were no significant differences in treatment responses between the two first groups. In contrast, in the untreated group, with time, the ulcer size has increased significantly in comparison with the three other groups. One-Way ANOVA analysis indicated that in the first week of the treatment course, the mean size of the ulcers was not significantly different among the four groups (p = 0.5) but became significantly different at the end of the second and third weeks of treatment (p < 0.001).
Parasite burden assessment
Three weeks after the treatment, slides were prepared and carefully studied for the presence of amastigotes of L. major. Within a 21-day treatment period, mice in the untreated group presented numerous amastigotes inside macrophages at the lesions. However, in mice treated with 30 mg/kg/day extract of O. europaea and 24 mg/kg/day of F. carica, the number of amastigotes significantly decreased (p = 0.001) as compared with mice in the untreated group (Table 2; Fig. 2). In addition, in a group that received 4 mg kg− 1 Amphotericin B, few parasites were observed.
Discussion
Leishmaniasis is an important parasitic disease that affect millions of people worldwide (Saberi et al. 2018). The clinical manifestations of the disease vary from cutaneous (local or disseminated cutaneous), muco-cutaneous to visceral leishmaniasis (VL) and treatment of leishmaniasis ranges from local treatment of cutaneous lesions to systemic, often toxic, therapy for disseminated cutaneous, mucocutaneous and deadly VL. To reduce the side effect of synthetic drugs, high costs, and resistance in endemic areas, the use of medicinal plants as an alternative remedy has been gaining popularity in different regions of the world (Iwu et al. 1994). For the treatment of cutaneous leishmaniasis, it is best to use treatments that have lower systemic absorption and fewer side effects (Palumbo 2009). In order to find alternative therapies, various medicinal plants have been studied in in vitro and in vivo experiments (Soosaraei et al. 2017). In this study, our findings showed that extracts of O. europaea and F. carica exhibit significant in vitro antileishmanial activity against the promastigote L. major, and in vivo activity against lesion of mice and determine parasite burden. In vitro experiments, O. europaea extract showed anti-leishmanial activity against L. major promastigotes with IC50 of 1.5 mg mL− 1. On the other hand, F. carica extracts were somewhat less active against L. major promastigotes and showed an IC50 value of 1.2 mg mL− 1. These doses killed half of both forms of the promastigotes. These findings showed that the extracts exhibit potent action against L. major promastigotes.
In a similar fashion, our findings indicated that the ethanol leaf extracts of O. europaea and F. carica exhibit good effect against lesions caused by L. major and can be compared with chemical drugs in accelerating the healing of lesions. Results related to the lesion size in mice receiving daily treatment of O. europaea and F. carica extracts. These results demonstrate that the mean lesion sizes is reduced in groups receiving O. europaea and F. carica extracts compared with the untreated group. In addition, the parasite burden decreased in the O. europaea, F. carica, and Amphotericin B groups. In the current study, the association between lesion size and burden parasite was observed. These findings are in line with the analysis of the antileishmanial activity of Peganum harmala extract, which reduced parasite burden in CL infected mice caused by L. major (Khoshzaban et al. 2014).
Similarly, results of an investigation conducted by Badirzadeh et al. (2020) showed that Urtica dioica extract was effective against L. major in both in vitro and in vivo experiments and exhibited an inhibitory effect against the parasite. In addition, these researchers believed that U. dioica was one of the most effective herbal plants against L. major parasite and could be strongly considered for the treatment of cutaneous leishmaniasis. On the other hand, Akhlaghi et al. (2014) evaluated the effect of Hyssopus officinalis, Tussilago farfara, Carum copticum extracts on mice infected with L. major. Results indicated that plants ointments were effective in reducing ulcer size and burden parasite in the spleen (Maleki et al. 2017). In addition, results of a study conducted by Taran et al. (2010) showed that P. atlantica var. kurdica decreased skin lesion size in BALB/c mice infected with L. major (p < 0.05). In another study, finding showed that plants ointments were effective in reduction of ulcer size and burden parasite (Akhlaghi et al. 2014).
Phytochemicals found in olive tree leaves and fruit exhibit free-radical scavenging activities along with antibacterial properties, making them beneficial to human health. Along this line, nonpolar oleuropein and ligstroside caffeic acid, verbascoside, oleuropein, luteolin 7-O-glucoside, apigenin 7-O-glucoside, and luteolin 4′-O-glucoside, as well as polar hydroxytyrosol, rutin, and tyrosol, are the most prevalent phenols in olive leaves (Saija and Uccella 2000; El and Karakaya 2009). Phenolic substances derived from olive fruit and leaves, such as oleuropein, tyrosol, hydroxytyrosol, caffeic acid, gallic acid, syringic acid, p-coumaric acid, and luteolin, have demonstrated activity against viruses, retroviruses, bacteria, yeasts, fungus, and parasites, suggesting that they might be beneficial as a food additives (Yigit et al. 2001; Pereira et al. 2007). Therefore, it should be possible for olive tree extract to show antiparasite activity in experimental model due to its bioactive compounds.
Because leaves of F. carica, have irritating properties, they may be tested for parasite infestation and ovicidal action. In this respect, the bulk of pharmacological research on F. carica has been done with uncharacterized crude extracts, making it difficult to reproduce the findings of these studies and identify the bioactive compounds. However, phytochemical research on F. carica led to the isolation of a few classes of plant metabolites. Furthermore, the majority of phytochemical studies on F. carica have focused on the leaves and fruits, with little information on the stem and root profiles (Mawa et al. 2013).
Taken all together, our findings demonstrated that olive leaf extract exerts immunomodulatory effects. These findings agree with those obtained by other researchers (Kheirandish et al. 2018). In this regard, Kheirandish et al. (2018) determined the levels of transforming growth factor-β (TGFβ), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNFα) when the infected and/or not infected macrophages with L. major promastigotes were affected by different concentrations of olive leaf extract. These researchers found that production of IFNγ and TNFα. significantly increased when the infected and/or not infected macrophages with L. major promastigotes were treated with by different concentrations of olive leaf extract. On the other hand, the production of TGFβ was significantly lowered under the same conditions (Kheirandish et al. 2018).
Conclusions
In conclusion, findings from this investigation highlight the therapeutic effect of O. europaea and F. carica extracts against L. major in both in vitro and in vivo experiments. These extracts exhibited significant activities against the parasite. However, further investigations are required to control cutaneous leishmaniasis and inhibit the development of lesions in clinical trials. In addition, further studies are required to establish the safety, efficacy, and active constituents of the extracts.
Abbreviations
- ANOVA:
-
Analysis of variance
- °C:
-
Celsius degree
- DMSO:
-
dimethyl sulfoxide
- IC50 :
-
50% inhibitory concentrations
- IFN-γ:
-
interferon-gamma
- kg:
-
kilogram
- mg:
-
milligram
- mL:
-
milliliters
- MA:
-
meglumine antimoniate
- SD:
-
Standard deviation
- SSG:
-
Sodium stibogluconate
- TGFβ:
-
transforming growth factor-β
- TNFα:
-
tumor necrosis factor-alpha
- USA:
-
United States
- VL:
-
visceral leishmaniasis
- WHO:
-
World Health Organization
References
Agabeĭli RA, Kasimova TE (2005) Antimutagenic activity of Armoracia rusticana, Zea mays and Ficus carica plant extracts and their mixture. Tsitol Genet 39:75–79
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7:e35671. https://doi.org/10.1371/journal.pone.0035671
Akhlaghi L, Yeganeh M, Maleki F, Golestani M, Noori M, Ghelman M, Tabatabaie F (2014) Antileishmanial activity of Hyssopus officinalis, Tussilago farfara, Carum copticum extracts in mice infected with Leishmania major. Int J Herb Med 2(2):142–145
Akkol EK, Çankaya IT, Karatoprak G, Carpar E, Sobarzo-Sánchez E, Capasso R (2021) Natural compounds as medical strategies in the prevention and treatment of psychiatric disorders seen in neurological diseases. Front Pharmacol 12:669638. https://doi.org/10.3389/fphar.2021.669638
Amorin A, Borba HR, Carauta JP, Lopes D, Kaplan MA (1999) Anthelmintic activity of the latex of Ficus species. J Ethnopharmacol 64:255–258. https://doi.org/10.1016/S0378-8741(98)00139-1
Aref HL, Salah KBH, Chaumont JP, Fekih A, Aouni M, Said K (2010) In vitro antimicrobial activity of four Ficus carica latex fractions against resistant human pathogens (antimicrobial activity of Ficus carica latex). Pak J Pharm Sci 23:53–58
Asadi F, Pourkabir M, Maclaren R, Shahriari A (2006) Alterations to lipid parameters in response to fig tree (Ficus carica) leaf extract in chicken liver slices. Turk J Vet Anim Sci 30:315–318
Badgujar SB, Patel VV, Bandivdekar AH, Mahajan RT (2014) Traditional uses, phytochemistry and pharmacology of Ficus carica: a review. Pharm Biol 52:1487–1503. https://doi.org/10.3109/13880209.2014.892515
Badirzadeh A, Heidari-Kharaji M, Fallah-Omrani V, Dabiri H, Araghi A, Salimi Chirani A (2020) Antileishmanial activity of Urtica dioica extract against zoonotic cutaneous leishmaniasis. PLoS Negl Trop Dis 14:e0007843. https://doi.org/10.1371/journal.pntd.0007843
Ben Salem M, Affes H, Ksouda K, Sahnoun Z, Zeghal KM, Hammami S (2015) Pharmacological activities and Olea europaea leaf. J Food Process Preserv 39:3128–3136. https://doi.org/10.1111/jfpp.12341
Benavente-Garcıa O, Castillo J, Lorente J, Ortuño AD, Del Rio JA (2000) Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem 68(99):457–462. https://doi.org/10.1016/S0308-8146
Cardinali A, Cicco N, Linsalata V, Minervini F, Pati S, Pieralice M, Tursi N, Lattanzio V (2010) Biological activity of high molecular weight phenolics from olive mill wastewater. J Agric Food Chem 58:8585–8590. https://doi.org/10.1021/jf101437c
Chiappetta A, Muzzalupo I (2012) Botanical Description. In: Muzzalupo I (ed) Olive Germplasm - The Olive Cultivation, Table Olive and Olive Oil Industry in Italy. InTech Open Book series, London, pp 78–112. https://doi.org/10.5772/51933
Dias DA, Urban S, Roessner U (2012) A historical overview of natural products in drug discovery. Metabolites 2:303–336. https://doi.org/10.3390/metabo2020303
Gond NY, Khadabadi SS (2008) Hepatoprotective activity of Ficus carica leaf extract on rifampicin-induced hepatic damage in rats. Indian J Pharm Sci 70:364–366. https://doi.org/10.4103/0250-474X.43003
Guo Z, Jia X, Zheng Z, Lu X, Zheng Y, Zheng B, Xiao J (2018) Chemical composition and nutritional function of olive (Olea europaea L.): A review. Phytochem Rev 17:1091–1110. https://doi.org/10.1007/s11101-017-9526-0
Hailu A, Dagne DA, Boelaert M (2016) Leishmaniasis. In: Gyapong J, Boatin B. (eds) Neglected tropical diseases - sub-Saharan Africa. Neglected Tropical Diseases. Springer, Berlin, pp 114–134. https://doi.org/10.1007/978-3-319-25471-5_5
Hossain S, Urbi Z, Karuniawati H, Mohiuddin RB, Moh Qrimida A, Allzrag AMM, Ming LC, Pagano E, Capasso R (2021) Andrographis paniculata (Burm. f.) Wall. ex Nees: An updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life (Basel) 11:348–360. https://doi.org/10.3390/life11040348
Iwu MM, Jackson JE, Schuster BG (1994) Medicinal plants in the fight against leishmaniasis. Parasitol Today 10(94):65–68. https://doi.org/10.1016/0169-4758
Jeong WS, Lachance PA (2001) Phytosterols and fatty acids in Fig (Ficus carica, var. Mission) fruit and tree components. J Food Sci 66:278–281. https://doi.org/10.1111/j.1365-2621.2001.tb11332.x
Keskin D, Ceyhan N, Uğur A, Dbeys AD (2012) Antimicrobial activity and chemical constitutions of West Anatolian olive (Olea europaea L.) leaves. J Food Agric Environ 10:99–102. https://doi.org/10.1234/4.2012.2896
Khadabadi SS, Gond NY, Ghiware NB, Shendarkar GR (2007) Hepatoprotective effect of Ficus carica leaf in chronic hepatitis. Indian Drugs 44:54–59
Kheirandish F, Mosaffa N, Tarahi MJ, Fallahi S (2018) Olive (Olea europaea) leaf extract alters the cytokine profile of Leishmania major-infected macrophages: New insight into the underlying mechanism. Parasite Immunol 40:e12520. https://doi.org/10.1111/pim.12520
Khoshzaban F, Ghaffarifar F, Jamshidi Koohsari HR (2014) Peganum harmala aqueous and ethanol extracts effects on lesions caused by Leishmania major (MRHO/IR/75/ER) in BALB/c mice. Jundishapur J Microbiol 7:e10992. https://doi.org/10.5812/jjm.10992
Liu F, Yang Z, Zheng X, Luo S, Zhang K, Li G (2011) Nematicidal coumarin from Ficus carica L. J Asia Pac Entomol 14:79–81. https://doi.org/10.1016/j.aspen.2010.10.006
Maleki F, Zarebavani M, Mohebali M, Dayer MS, Hajialiani F, Tabatabaie F (2017) In vitro and in vivo susceptibility of Leishmania major to some medicinal plants. Asian Pac J Trop Biomed 7:37–42. https://doi.org/10.1016/j.apjtb.2016.11.008
Mawa S, Husain K, Jantan I (2013) Ficus carica L. (Moraceae): Phytochemistry, traditional uses and biological activities. Evid Based Complement Alternat Med 2013:974256. https://doi.org/10.1155/2013/974256
McGwire BS, Satoskar AR (2014) Leishmaniasis: clinical syndromes and treatment. QJM 107:7–14. https://doi.org/10.1093/qjmed/hct116
Mishra J, Saxena A, Singh S (2007) Chemotherapy of leishmaniasis: Past, present and future. Curr Med Chem 14:1153–1169. https://doi.org/10.2174/092986707780362862
Mohamad S, Zin NM, Wahab HA, Ibrahim P, Sulaiman SF, Zahariluddin ASM, Noor SSM (2011) Antituberculosis potential of some ethnobotanically selected Malaysian plants. J Ethnopharmacol 133:1021–1026. https://doi.org/10.1016/j.jep.2010.11.037
Msomi NZ, Simelane MB (2017) Olea europaea subsp. africana (Oleaceae). Active ingredients from aromatic and medicinal plants. InTechOpen, London, UK. https://doi.org/10.5772/65725
Niazi M, Saki M, Sepahvand M, Jahanbakhsh S, Khatami M, Beyranvand M (2019) In vitro and ex vivo scolicidal effects of Olea europaea L. to inactivate the protoscolecs during hydatid cyst surgery. Ann Med Surg (Lond) 42:7–10. https://doi.org/10.1016/j.amsu.2019.04.006
Oliveira AP, Silva LR, Andrade PB, Valentão P, Silva BM, Gonçalves RF (2010) Further insight into the latex metabolite profile of Ficus carica. J Agric Food Chem 58:10855–10863. https://doi.org/10.1021/jf1031185
Oliveira AP, Valentão P, Pereira JA (2009a) Ficus carica L., metabolic and biological screening. Food Chem Toxicol 47:2841–2846. https://doi.org/10.1016/j.fct.2009.09.004
Oliveira AP, Valentão P, Pereira JA, Silva BM, Tavares F, Andrade PB (2009b) Ficus carica L.: Metabolic and biological screening. Food Chem Toxicol 47:2841–2846. https://doi.org/10.1016/j.fct.2009.09.004
Palumbo E (2009) Current treatment for cutaneous leishmaniasis: A review. Am J Ther 16:178–182. https://doi.org/10.1097/MJT.0b013e3181822e90
Patil Vikas V, Bhangale SC, Patil VR (2010) Evaluation of anti-pyretic potential of Ficus carica leaves. Int J Pharma Sci Rev Res 2:48–50
Pereira AP, Ferreira ICFR, Marcelino F, Valentão P, Andrade PB, Seabra R, Estevinho L, Bento A, Pereira JA (2007) Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 12:1153–1162. https://doi.org/10.3390/12051153
Pérez-Bonilla M, Salido S, van Beek TA, Linares-Palomino PJ, Altarejos J, Nogueras M, Sánchez A (2006) Isolation and identification of radical scavengers in olive tree (Olea europaea) wood. J Chromatogr A 1112:311–318. https://doi.org/10.1016/j.chroma.2005.12.055
Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, López-Vélez R, García-Hernández R, Pountain AW, Mwenechanya R, Papadopoulou B (2017) Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis 11:e0006052. https://doi.org/10.1371/journal.pntd.0006052
Saberi R, Moin-Vaziri V, Hajjaran H, Niyyati M, Taghipour N, Kheirandish F, Abadi A (2018) Identification of Leishmania species using N-acetylglucosamine-1-phosphate transferase gene in a zoonotic cutaneous leishmaniasis focus of Iran. J Vector Borne Dis 55:14–19. https://doi.org/10.4103/0972-9062.234621
Sahranavard S, Kamalinejad M, Faizi M (2014) Evaluation of anti-inflammatory and anti-nociceptive effects of defatted fruit extract of Olea europaea. Iran J Pharm Res 13:119–123
Saija A, Uccella N (2000) Olive biophenols: functional effects on human wellbeing. Trends Food Sci Technol 11:357–363. https://doi.org/10.1016/S0924-2244(00)00068–6
Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R (2007) Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun 362:793–798. https://doi.org/10.1016/j.bbrc.2007.06.130
Sgariberi VC, Gupte SM, Karmer DE, Whitakar (1964) Ficus enzymes, I. Separation of the proteolytic enzymes of Ficus carica and Ficus glabrata lattices. J Biol Chem 239:2170–2177
Shiraishi SI, Kawakami K, Widodo SE, Shiraishi M, Kitazaki M (1996) Organic acid profiles in the juice of fig fruits. J Fac Agric Kyushu Univ 41:29–33. https://doi.org/10.5109/24126
Solomon A, Golubowicz S, Yablowicz Z, Grossman S, Bergman M, Gottlieb HE, Altman A, Kerem Z, Flaishman MA (2006) Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J Agric Food Chem 54:7717–7723. https://doi.org/10.1021/jf060497h
Soosaraei M, Fakhar M, Hosseini Teshnizi S, Ziaei Hezarjaribi H, Banimostafavi ES (2017) Medicinal plants with promising antileishmanial activity in Iran: a systematic review and meta-analysis. Ann Med Surg (Lond) 21:63–80. https://doi.org/10.1016/j.amsu.2017.07.057
Stiti N, Hartmann MA (2012) Nonsterol triterpenoids as major constituents of Olea europaea. J Lipids 2012: 476595. https://doi.org/10.1155/2012/476595
Sudjana AN, D’Orazio C, Ryan V, Rasool N, Justin Ng, Islam N, Riley TV, Hammer KA (2009) Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int J Antimicrob Agents 33:461–463. https://doi.org/10.1016/j.ijantimicag.2008.10.026
Taran M, Mohebali M, Esmaeli J (2010) In vivo efficacy of gum obtained Pistacia atlantica in experimental treatment of cutaneous leishmaniasis. Iran J Public Health 39:36–41
Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R (2017) Leishmaniasis: a review. F1000Res 6:750–759. https://doi.org/10.12688/f1000research.11120.1
Vaya J, Mahmood S (2006) Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.). BioFactors 28:169–175. https://doi.org/10.1002/biof.5520280303
Wang G, Wang H, Song Y, Jia C, Wang Z, Xu H (2004) Studies on anti-HSV effect of Ficus carica leaves. Zhong Yao Cai 27:754–756
Yancheva SD, Golubowicz S, Yablowicz Z, Perl A, Flaishman MA (2005) Efficient Agrobacterium-mediated transformation and recovery of transgenic fig (Ficus carica L.) plants. Plant Sci 168:1433–1441. https://doi.org/10.1016/j.plantsci.2004.12.007
Yigit A, Sahan Y, Korukluoglu M (2001) Antimicrobial substances found in olive leaves and olive. 2nd International AltInoluk “Antandros” Olive Busines Symposium. Ankara, Turkey
Acknowledgements
The authors thank Shahid Sadoughi University of Medical Sciences, Yazd, Iran. And International Centre for Empirical Research and development, Dhaka, Bangladesh.
Funding
This work was supported by a grant (no. 5436) from the Deputy of Research, Birjand University of Medical Sciences, Birjand, Iran. The funders had no role in study design, data collection, analysis and interpretation, the decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The current research was approved by the Ethical Committee of the Faculty of Medicine, Birjand University of Medical Sciences, Birjand, Iran (IR.BUMS.REC.1399.152).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siyadatpanah, A., Mirzaei, F., Hossain, R. et al. Anti-parasitic activity of the Olea europaea and Ficus carica on Leishmania major: new insight into the anti-leishmanial agents. Biologia 77, 1795–1803 (2022). https://doi.org/10.1007/s11756-022-01066-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01066-y