Abstract
Leishmaniasis is a complex of parasitic protozoan diseases caused by more than 20 different species of parasites from Leishmania genus. Conventional treatments are high costly, and promote a sort of side effects. Besides, protozoan resistance to treatments has been reported. Natural products have been investigated as a source of new therapeutic alternatives, not only acting directly against the parasite but also being able to synergistically act on the host immune system in order to control parasitemia. Gallic acid (GA) and ellagic acid (EA) are plant-derived phenolic compounds which are able to induce antiinflammatory, gastroprotective, and anticarcinogenic activities. Therefore, the antileishmania, cytotoxic, and immunomodulatory activities of GA and EA were evaluated in this study. Both GA and EA were able to inhibit the growth of Leishmania major promastigotes (effective concentration (EC50) values 16.4 and 9.8 μg/mL, respectively). The cytotoxicity against BALB/c murine macrophages for GA and EA was also assessed (CC50 values 126.6 and 23.8 μg/mL, respectively). Interestingly, GA and EA also significantly reduced the infection and infectivity of macrophages infected by L. major (EC50 values 5.0 and 0.9 μg/mL, respectively), with selectivity index higher than 20. Furthermore, both GA and EA induced high immunomodulatory activity evidenced by the increase of phagocytic capability, lysosomal volume, nitrite release, and intracellular calcium [Ca2+ i] in macrophages. Further investigations are reinforced in order to evaluate the therapeutic effects of GA and EA in in vivo experimental infection model of leishmaniasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protozoal infections are considered a worldwide public health problem, especially in underdeveloped countries, where approximately 14% of the population are considered at risk of infection (Kondrashin et al. 2011; Waldron et al. 2011). In this context, leishmaniasis is considered by the World Health Organization (WHO) as one of the six major infectious diseases with a high incidence and capacity to produce deformities. This protozoan infection disease affects more than 12 million people, with up to 3 million new cases reported worldwide (Mitropoulos et al. 2010). Leishmaniasis is caused by more than 20 species from Leishmania genus. Among the clinical manifestations of leishmaniasis (cutaneous, mucocutaneous, and visceral), the cutaneous form is the most disseminated and responsible for causing physical deformities in patients. The species Leishmania major is responsible for the highest number of cases in the Old World, being endemic in African regions, such as the north of Sahara and Arabian Peninsula (Kaye and Scott 2011; Mitropoulos et al. 2010; Nozais 2003; Reithinger and Coleman 2007).
Leishmaniasis is widely distributed, and reaches the Middle East, Africa, and part of the Americas. The infection occurs by the blood meal of females of sand flies, which live in forest areas, caves, rodent dens, and also in the domiciliary area (Reithinger and Coleman 2007; Reithinger et al. 2007). The parasite is digenetic and presents two distinct morphologies: a flagellate form, the promastigote, infecting and present in the insect vector; and another non-flagellated form called amastigote, which is present in the vertebrate host (Madalosso et al. 2012; Montalvo et al. 2012). The parasite harbors and multiplies in the interior of mononuclear phagocytic cells of the vertebrate host, acting by immune evasive mechanisms, such as reducing iNOS (inducible nitric oxide synthase) activity, as well as increasing the Th2 response, which induces the cell signaling of antiinflammatory cytokines (e.g., IL4, IL10), leading to parasitemia. On the other hand, when a Th1 response occurs, the overexpression of IFN-γ and TNF-α promotes healing or protection (Islamuddin et al. 2015; Roy et al. 2014a).
Conventional treatments for leishmaniasis are limited, unsafe, and have a range of side effects, as well as contribute to the resistance of the parasite to the treatment. For example, the amphotericin B is widely used as the main drug in the treatment of leishmaniasis, but major side effects are reported, such as cardiotoxicity and nephrotoxicity, besides the high costs (Alizadeh et al. 2008; Ashford 2000). Therefore, studies focused on the development of safer, more effective, and affordable treatment for patients diagnosed with leishmaniasis are necessary.
A sort of natural products obtained from plants has been considered promising candidates due their antileishmania activity. They have cytotoxic potential against the parasite, as well as they are able to trigger immunomodulatory mechanisms involving the activation of iNOS and signaling the participation of cytokines responsible for the Th1 response, which increases the phagocytic capability and lysosomal volume in macrophages (Islamuddin et al. 2015; Rodrigues et al. 2015). In this context, tannins are classified as phenolic substances derived from the secondary metabolism of plants, where the most part is related to glucose metabolism by different biochemical reactions, such as shikimate or acetate pathway (de Jesus et al. 2012).
Among some important phenolic compounds, the gallic acid and their dimers, such as digallic acid, hexahydroxyidiphenic acid, and ellagic acid, are characterized as naturally occurring phenolic lactones in the form of hydrolysable tannins called ellagitannins, commonly found in Anacardium occidentale L., Myracrodruon urundeuva (Allemão), Anogeissus leiocarpus (DC.) Guill. & Perr., Quercus infectoria Olivier, and Stryphnodendron obovatum Benth. (Kheirandish et al. 2016; Murakami et al. 1991; Ribeiro et al. 2015; Shuaibu et al. 2008; Vattem et al. 2005).
Gallic acid (GA) has demonstrated several pharmacological properties such as antioxidant, antiinflammatory (Yang et al. 2015), antimicrobial (Sarjit et al. 2015), antimutagenic, and anticancer (Lee et al. 2003; Lu et al. 2010; Paolini et al. 2015). Besides, the most important biological activities induced by ellagic acid (EA) involve the anticancer, hepatoprotective, DNA topoisomerase inhibitor (Aggarwal and Shishodia 2006; Cortazar et al. 2007; Vattem et al. 2005), antioxidant (Devipriya et al. 2007a, b), antiinflammatory (Papoutsi et al. 2008; Yuce et al. 2007), and gastroprotective (Iino et al. 2001). The antileishmanial activity of GA and EA on promastigote forms has been already reported (Kayser et al. 2003; Kolodziej and Kiderlen 2005; Ribeiro et al. 2015). Interestingly, the increase of NO and cytokines in L. major-infected RAW 264.7 cells was previously reported for GA (Radtke et al. 2004). However, the antileishmanial effect of EA against macrophage-internalized amastigote forms of L. major as well as its immunomodulatory activity resulting in solving the parasited macrophages still remain unclear.
The lack of safe and effective conventional treatments against leishmaniasis has been arising the search for new therapeutic alternatives, focused not only in the cytotoxic effect against the parasite but also in the ability to synergistically modulate the immune response of the host (Islamuddin et al. 2015; Roatt et al. 2014). In this context, the antileishmania, cytotoxic, and immunomodulatory potential of gallic and ellagic acids were explored in this work.
Material and methods
Chemicals
Dimethyl sulfoxide (DMSO: 99%; PubChem CID: 679), Panoptic staining (PubChem CID: 13735) was purchased from Merck Chemical Company (Germany). The Schneider’s medium (PubChem CID: 2723893, RPMI medium (PubChem CID: 1640), fetal bovine serum (FBS; PubChem CID: 86289556), MTT (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide; PubChem CID: 64965), resazurin (PubChem CID: 11077), GA (PubChem CID: 370; Fig. 1a), EA (PubChem CID: 5281855; Fig. 1b), Fura-2/AM (PubChem CID: 24894734), and the antibiotics penicillin and streptomycin (PubChem CID 71311919) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The antibiotic amphotericin B (90% Anf B) was purchased from Cristália (São Paulo, SP, Brazil). The GA and EA were diluted in DMSO at a concentration of 80 mg/mL for the experiments.
Parasites and animals
The L. major strain (MHOM/IL/80/Friendlin) was obtained from the Medicinal Plants Research Center of Federal University of Piauí. Parasites were grown in supplemented Schneider’s medium (10% heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 26 °C) (Carneiro et al. 2012; Valadares et al. 2011).
Murine macrophages were collected from the peritoneal cavities of male and female BALB/c mice (4–5 weeks old; Medicinal Plants Research Center, UFPI, Brazil), and cultivated in RPMI 1640 medium (10% heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C and 5% CO2). Red blood cells were obtained by centrifugation of sheep blood (9 months old). All protocols were approved by the Animal Research Ethics Committee (CEEA-PI no. 053/2015).
Investigation of activity against promastigote forms of Leishmania major
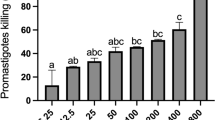
Promastigote forms of L. major in the late log phase (1 × 106 leishmania/100 μL of medium) were plated in 96-well culture plates containing supplemented Schneider’s medium. Then, GA and EA (6.25, 12.5, 25, 50, and 100 μg/mL) were added, and the plates were incubated during 48 h in a BOD (biochemical oxygen demand) incubator at temperature of 26 °C. Remaining 6 h to the end of this period, 20 μL of resazurin (1 × 10−3 mol/L) was added. Afterwards, the absorbances were read in a BioTek microplate reader (model ELx800) at a wavelength of 550 nm. The results were expressed as inhibition of parasite growth (%).
Amphotericin B (Amph B) was used as positive control at concentrations of 0.32, 0.75, 1.25, 2.5, and 5.0 μg/mL). The negative control was the Schneider’s medium with promastigotes (1 × 106 cells/well). The cell viability was considered as 100% for the parasite. The blank was read for each concentration and control in order to avoid interference of absorbance of medium of other compounds.
Determination of cytotoxicity for GA and EA
Cytotoxic effect on macrophages and the calculation of selective index
Cytotoxicity evaluation was carried out in 96-well plates using the MTT assay (de Medeiros et al. 2011; Gonçalves et al. 2016). Macrophages (2 × 105 per well) were incubated in 100 μL of supplemented RPMI 1640 medium at 37 °C and 5% CO2 for 4 h. Non-adherent cells were removed by washing with RPMI 1640 medium. Then, GA and EA were diluted in supplemented RPMI 1640 medium, and added at concentrations of 6.25, 12.5, 25, 50, and 100 μg/mL, followed by incubation at 37 °C with 5% CO2 for 2 days. The cytotoxicity of Amph B was assessed at concentration of 0.2 μg/mL. Afterwards, cytotoxicity was assessed by adding MTT (5 mg/mL). The supernatant was discarded, and the formazan crystals were dissolved by addition of 100 μL of DMSO. Finally, absorbance at 550 nm was measured using a BioTek (ELx800) plate reader. Selectivity index of each treatment was calculated by the ratio between the mean cytotoxic concentration (CC50) for BALB/c peritoneal macrophages and the mean effective concentration (EC50) for promastigote forms of L. major.
Red blood cell lysis assay
The hemolytic activity was evaluated by incubating 80 μL of 5.0% fresh sheep red blood cells suspension in PBS with 20 μL of different concentrations of GA, EA, or Amph B, as described previously. The samples were incubated at 37 °C for 1 h. The reaction was slowed by adding 200 μL of PBS, and then the suspension was centrifuged at 1000×g for 10 min). The supernatant was transferred to a 96-well plate, and cell lysis was measured at 540 nm, as previously described. The blank control and maximal lysis (positive control) were obtained by replacing the substance sample with in equal volume of PBS or distilled water, respectively (Lofgren et al. 2008).
Investigation of GA- and EA-induced activity on macrophages infected by Leishmania major
Macrophages (2 × 105 cells/mL) were harvested in 24-well plates containing sterile round coverslips at 13 mm and supplemented RPMI 1640 medium (10% inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin). Culture plates were incubated at 37 °C and 5% of CO2 for 3 h. Adhered macrophages were then incubated with a new medium containing axenic amastigotes at a ratio of 10 amastigotes per 1 macrophage at 5% CO2 and 37 °C for 4 h. The medium was subsequently aspirated in order to remove non-internalized parasites, and the wells were washed with 0.01 M phosphate buffered saline (PBS). The infected macrophages were then incubated with GA at 15, 30, and 60 μg/mL (non-toxic concentrations on host cells); EA at 1.75, 3.5, and 7 μg/mL (non-toxic concentrations on host cells); or Amph B at 0.2 μg/mL. After this period, the coverslips were removed and stained with Panoptic staining kit. For each treatment, the number of infected macrophages and the parasite load (survival index, obtained by counting the number of parasites in 100 macrophages) were counted using optical microscopy (Carneiro et al. 2012). Furthermore, the selectivity indexes of GA, EA, and Amph B were determined by the ratio of the mean CC50 against macrophages to the mean EC50 against macrophage-internalized amastigote forms of L. major.
Evaluation of parameters related to macrophage activation
Lysosomal activity
Macrophages (2 × 105/well) were incubated with GA (15, 30, and 60 μg/mL), EA (1.7, 3.5, and 7 μg/mL), or Amph B (0.2 μg/mL) in a 96-well plate at 37 °C and 5% de CO2. After 48 h, 10 μL of neutral red stock solution were added for 30 min. Then, the supernatant was discarded, the wells were washed with 0.9% saline at 37 °C, and 100 μL of extractive solution were added in order to solubilize the neutral red present within the lysosomal secretory vesicles. After 30 min on a Kline shaker, the absorbances were read in a BioTek (ELx800) plate reader at 550 nm (Bonatto et al. 2004).
Phagocytic capability
Macrophages (2 × 105/well) were incubated with GA (15, 30, and 60 μg/mL), EA (1.7, 3.5, and 7 μg/mL), or Amph B (0.2 μg/mL) in a 96-well plate for 48 h at 37 °C and 5% de CO2. After 48 h, 10 μL of zymosan-stained NR solution was added for 30 min. Next, the phagocytic process was interrupted adding 100 μL of Baker’s fixative solution during 30 min. Then, the wells were washed with 0.9% saline, and 100 μL of extractive solution were added. After solubilization in a Kline shaker, the absorbances were read in a BioTek (ELx800) plate reader at 550 nm (Grando et al. 2009).
Nitrite measurement
Non-infected macrophages or infected by L. major were obtained as described previously, and then incubated with GA (15, 30, and 60 μg/mL), EA (1.7, 3.5, and 7 μg/mL), or Amph B (0.2 μg/mL) at 37 °C and 5% de CO2 for 24 h. The lipopolysaccharide (LPS) from E. coli (2 μg/mL) was used as positive control. The standard curve was prepared with sodium nitrite in RPMI medium at varying concentrations of 1, 5, 10, 25, 50, 75, 100, and 150 μM diluted in RPMI 1640 medium. After 24 h, the supernatants were transferred, and then incubated with equal parts of Griess reagent. Thereafter, the absorbances were read in a BioTek (ELx800) plate reader at 550 nm (Soares et al. 2007).
Quantification of cytoplasmatic calcium levels (Ca2+ i) by confocal microscopy
Macrophages infected or non-infected by L. major as described previously were incubated with GA (30 μg/mL), EA (7 μg/mL) for 48 h at 5% CO2 at 37 °C. Amph B (0.2 μg/mL) and DMSO (60 μg/mL) were used as positive and negative controls, respectively. Then, the supernatant was removed, and macrophages were loaded with Fura-2/AM (10 μM) dye for 30 min (Goncalves et al. 2013). Right after, cells were washed twice with sterile PBS, and imaged in a DSU confocal fluorescence microscope (IX81, Olympus, Japan) coupled to a CCD camera (Hamamatsu, Japan), using 340/380-nm filters. ROIs were created around infected and non-infected macrophages, and the relative fluorescence emitted at 525 nm was quantified by proper software (Cell®, Olympus, Japan) and expressed as arbitrary units of absorbance (a.u.).
Statistical analyses
All assays were performed in triplicate in three independent experiments. The mean EC50 and mean CC50 with confidence limits of 95% were determined by regression of probits using the software SPSS 13.0. The selectivity index was calculated as the ratio between CC50 and EC50. One-way analysis of variance ANOVA followed by the Bonferroni test was performed using the GraphPad Prism version 5.0 program. The statistical significance was considered when p<0.05.
Results
Anti-Leishmania activity assay
GA and EA demonstrated concentration-dependent antileishmanial activity on promastigote forms of L. major. GA inhibited around 80% of the growth of L. major promastigote forms at the concentration of 100 μg/mL, whereas EA promoted maximal effect at 100 μg/mL. All tested concentrations were able to inhibit the promastigote growth. Amph B exhibited 90% of the growth inhibition of promastigote forms at 2.5 μg/mL. The EC50 values obtained against promastigote forms are listed in Table 1.
Citotoxicity assessment
The GA- and EA-induced cytotoxic effects against BALB/c murine peritoneal macrophages and sheep red blood erythrocytes are demonstrated in Fig. 2. The GA demonstrated significant cytotoxicity against macrophages by MTT test, starting from the 50 μg/mL, with CC50 value of 126.6 μg/mL, whereas EA reduced the viability of macrophages starting from 6.3 μg/mL, resulting in CC50 value of 23.8 μg/mL. The Amph B presents high cytotoxicity against murine macrophages, with CC50 of 8.750 μg/mL. GA, EA, and Amph B did not induced any toxicity on sheep erythrocytes (Table 1).
Cytotoxic effects of GA and EA against BALB/c murine peritoneal macrophages (a) and sheep red blood cells (b). Macrophages and red blood cells were incubated with GA, EA, or Amph B for 48 h. The macrophage viability was evaluated using tetrazolium salt (MTT) test. Data are presented as mean ± SEM of three experiments performed in triplicate *p < 0.05; **p < 0.01; ***p < 0.001 when compared with control (C) or Amph B
Effects of GA and EA against infection of macrophages by L. major
GA and EA were able to reduce the number of infected macrophages and survival index (Fig. 3). The concentration-dependent decrease of infected cells treated with GA reduced parasitism to approximately 44, 37, and 25% when incubated at concentrations of 15, 30, and 60 μg/mL, respectively. Likewise, the parasitism observed after treatment with EA at concentrations of 1.75, 3.5, and 7 μg/mL were 44, 38, and 30%, respectively (Fig. 4). By the way, Amph B at 0.2 μg/mL decreased the number of infected macrophages to approximately 39%, whereas the negative control was not able to affect the infection of macrophages by L. major.
Macrophages experimentally infected by Leishmania major (a). Amph B was used as a positive control at the concentration of 0.2 μg/mL (b). For GA treatment, concentrations of 15 (c.1), 30 (c.2), and 60 μg/mL (c.3) were used. The EA was evaluated at concentrations of 1.75 (d.1), 3.5 (d.2), and 7.0 μg/mL (d.3). The arrows indicate macrophage-internalized amastigote forms of L. major
Effects of GA, EA and amphotericin B on infected macrophages and survival index of BALB/c murine macrophages infected with Leishmania major. Cells were treated with GA , EA or Amph B for 48 h. Data are presented as mean ± SEM of three experiments performed in triplicate. *p < 0.05; **p < 0.01; ***p < 0.001 when compared with control (C)
The assessment of survival index showed the negative control group (vehicle) with around seven amastigotes/macrophage, and the positive control group (0.2 μg/mL Amph B) with two amastigotes/macrophage. In this study, after treatment with GA at concentrations of 15, 30, and 60 μg/mL, a concentration-dependent reduction of the amount of amastigotes per macrophages by 2.0, 1.1, and 0.5, respectively, was observed. Besides, EA at concentrations of 1.8, 3.0, and 7.0 μg/mL reduced survival index to 1.8, 1.6, and 1.4 amastigotes/macrophages, respectively (Fig. 4). The EC50 values for survival index of amastigotes on macrophages by L. major were calculated for GA and EA, and the values of 5.0 and 0.9 μg/mL were determined, respectively. Interestingly, GA and EA demonstrated higher selectivity to parasites than mammalian cells, resulting in the values of 25 and 26, respectively (Table 1).
Determination of lysossomal activity and phagocytic capability
The GA- or and EA-induced macrophage activation were assessed based on the retention of neutral red and zymosan particles by macrophages, suggesting the possible immunomodulatory effects on the lysosomal activity and phagocytic capability, respectively. In this work, GA was able to increase the lysosomal activity in macrophages at 15 μg/mL (Fig. 5a). Otherwise, EA was not able to induce any alteration (Fig. 5b). Besides, GA and EA were able to increase the phagocytic capability in all tested concentrations.
Effects of GA, EA, and Amph B on lysosomal activity (a) and phagocytic capability (b). Murine peritoneal macrophages were treated at ranging concentrations for 48 h. Lysosomal activity and phagocytic capacity were assessed by quantification of neutral red (NR). Phagocytic capability was assessed by the incorporation of zymosan to NR, solubilized by the extraction solution. Data are presented as mean ± SEM of three experiments performed in triplicate. *p < 0.05; **p < 0.01; ***p < 0.001 when compared with control (C)
Measurement of nitrite production
The nitrite production was measured after incubation of GA or EA with non-infected or infected by L. major. The GA increased significantly the production of nitrite in non-infected macrophages only at concentration of 60 μg/mL, whereas in the macrophages infected by L. major, the nitrite production synthesis occurred in all tested concentrations (Fig. 6a). Besides, non-infected macrophages treated with EA did not show any increase in nitrite levels. Interestingly, a significant increase of nitrite production was observed in macrophages infected by L. major at all tested concentrations (Fig. 6b). The LPS was used as a positive control, demonstrating significant nitrite production when compared with the control group.
Nitrite measurement in infected or non-infected BALB/c murine peritoneal macrophages treated with GA (a) or EA (b) and Amph B for 24 h. The culture supernatant was mixed in equal parts with the Griess reagent. LPS (lipopolysaccharide from Escherichia coli; 2 μg/mL) was used as positive control. Data are presented as mean ± SEM of three experiments performed in triplicate. p < 0.05 when compared with non-infected (−) or infected (+) macrophages from control group; p < 0.05 when compared with non-infected (−) or infected (+) macrophages from the LPS group HNS71387
Cytoplasmic calcium level (Ca2+ i) in murine macrophages
The cytoplasmic levels of Ca2+ (Ca2+ i) were determined in the presence of GA, EA, or Amph B in non-infected and infected macrophages by L. major (Fig. 7). A significant increase of 130 a.u. in the relative fluorescence was observed for GA (30 μg/mL) in both infected and non-infected macrophages. A similar increase was also observed in infected macrophages treated with 7 μg/mL of EA, which increased the relative fluorescence to 120 and 150 (a.u.) for non-infected and infected macrophages, respectively. The negative control (DMSO 60 μg/mL) and Amph B (0.2 μg/mL) did not cause any alteration on [Ca2+ i].
Quantification of cytoplasmic calcium in BALB/c murine macrophages in the absence (−) or presence (+) of internalized Leishmania major amastigote forms treated with vehicle (DMSO 60 μg/mL), Amph B, GA, or EA. The culture supernatant was removed, and RPMI containing Fura-2/AM (10 μM) was added. The coverslips were evaluated by confocal microscopy using the 340/380-nm filters. Data are presented as mean ± SEM of three images of each coverslip captured by microscopy. The experiment was performed in triplicate *p < 0.05, **p < 0.01, and ***p < 0.001 when compared with non-infected (−) or infected (+) macrophages
Discussion
Studies focused on natural products and their potential application for new treatments for leishmaniasis have been quite promising. The search for new therapeutic alternatives is based on the discovery of the bioactive molecules commonly derived from plants. In this sense, the plant-derived polyphenols have demonstrated marked antileishmania activity against different species and forms of the parasite in both in vitro and in vivo studies. Among these compounds, stilbenoids, phenylpropanoids, flavonoids, and quinones have been reported (de Jesus et al. 2012). Ogungbe et al. (2014) have described around 352 phenolic compounds with in silico antileishmanial activity towards protein targets, including 10 aurones, 6 cannabinoids, 34 chalcones, 20 chromenes, 52 coumarins, 92 flavonoids, 41 isoflavonoids, 52 lignans, 25 quinones, 8 stilbenoids, 9 xanthones, and 3 different phenolic compounds. Thus, there is a wide range of substances probably acting sole or sinergistically, and then studies regarding the antileishmanial potential of this new molecules are reinforced.
In this study, the antileishmania and cytotoxic potential of gallic and ellagic acids, as well as the immunomodulatory mechanisms related to macrophage activation, were investigated. Both GA and EA demonstrated a high potential as growth inhibitors of promastigote forms of L. major, corroborating with studies of antileishmania activity induced by phenolic compounds (Ogungbe et al. 2014; Rizk et al. 2014). Similar EC50 values to GA and EA were previously reported to Stryphnodendron obovatum leiocarpus (DC.) Guill. & Perr. and Anogeissus leiocarpus Benth (Ribeiro et al. 2015; Shuaibu et al. 2008).
The urge to obtain novel molecules with antileishmania activity with higher selectivity towards the parasite, and thus less toxic to the host cells, is urgent. Thus, toxicological investigation of potential therapeutic alternatives against leishmaniasis is markedly important, since the parasite is an obligate intracellular organism (de Medeiros et al. 2011; Islamuddin et al. 2015). The selectivity index (SI) represents how much a treatment is more toxic to the parasite rather than the mammalian host cells. For macrophage-internalized amastigotes, the SI of drug candidates is recommended to be close or greater than 20 (Nwaka and Hudson 2006). In this study, both GA and EA showed significant cytotoxicity against these cells, resulting in a CC50 value of 126.6 μg/mL by GA and 23.8 μg/mL by EA. Interestingly, the ratios of CC50 on macrophage cells to EC50 against macrophage-internalized amastigotes for both compounds indicate selectivity indexes higher than 20 (Table 1). Besides, GA and EA did not promote hemolytic effect on sheep erythrocytes. Sheep, human (O+-type blood), and rabbit erythrocytes have been widely used to assess damage of cell membranes, since they lack high expression levels of proteins or glycoproteins, that could be activated by the new drugs, which might result false positive hemolytic activity (Carneiro et al. 2012).
The experimental model based on macrophage-internalized amastigotes forms of Leishmania spp. is considered the in vitro model that adequately resembles the infection that occurred in the host cells (Carneiro et al. 2012). Therefore, compounds able to reduce the survival index of parasitized cells by Leishmania spp. are quite promising and relevant to be investigated using in vivo experimental models. In this study, GA and EA were highly efficient in reducing the survival index of murine macrophages by L. major in a concentration-dependent manner. Microscopy data show a large agglomeration of amastigotes around the parasitophorous vacuoles in the control group, due to the structural integrity and accumulation of F-actin and myosin Va in the peripheral region, a mechanism established by the parasite to counter cellular defenses, being essential for the establishment of leishmanial infection (Azevedo et al. 2012; Lodge and Descoteaux 2005; Roy et al. 2014a, b). In the groups treated with GA or EA, the parasitophorous vacuoles present a “smog” aspect due to the activation of phagolysosomes, probably due to the depolymerization of F-actin against the evasive mechanisms of the parasite, and then solving the infection (Lodge and Descoteaux 2005). In our study, no alterations were observed in cells treated with Amph B, probably due to its leishmanicidal activity is related to the inhibition of the ergosterol synthesis in the parasite membrane (Lachaud et al. 2009; Sundar et al. 2007). According to Kolodziej and Kiderlen (2005) and de Macedo-Silva et al. (2011), this aspect observed after treatment with GA or EA is because these cells were activated and capable of solving the infection, not only because of the ability of the compounds to act against the parasite but also other signaling pathways which make the intracellular environment more hostile to the parasite are triggered. Natural products derived from plants are characteristic in reducing the infection and infectivity of macrophages affected by Leishmania, which corroborates to this study (Carneiro et al. 2012; de Medeiros et al. 2011; Dias et al. 2013; dos Santos et al. 2012; Rodrigues et al. 2013, 2015).
In this study, parameters of macrophage activation able to induce microbicidal activity, such as phagocytic capacity, lysosomal activity, induction of NO synthesis, and quantification of cytoplasmic calcium, were evaluated. They acting synergistically in order to control Leishmania infections. The dichotomy between the Th1- and the Th2-type cellular immune response in hosts affected by the disease leads to the investigation of novel therapeutic alternatives which possess activity not only targeted to the parasite but also induce immunomodulatory activity in order to prevail immune response of the Th1-type host (Islamuddin et al. 2015). The substances evaluated in this study (GA and EA) induced immunomodulatory effects (Kolodziej and Kiderlen 2005). Phagocytosis and lysosomal activity are functions activated in the innate immune response, important for the control of infections, leading to parasitic degradation and presentation of antigens by MHC class II to CD4+ T cells (Niedergang and Chavrier 2004). After the endocytosis of the parasite, the formation of phagosomes from the endosomes occurs, followed by the fusion with lysosomes, then producing the phagolysosomes (Niedergang and Chavrier 2004).
The phagolysosome is a compartment filled with acid hydrolases and reactive oxygen species, and where degradation of pathogens occurs. Thus, pathogens are destroyed within the phagolysosome (Lee et al. 2003; Lopes et al. 2006). One important and highly pathway underlying the mechanism of antileishmania activity is the induction of NO production in macrophages, the most effective mechanism being involved in the defense against Leishmania (Gantt et al. 2001). The NO is synthesized after activation of macrophages by cytokines acting in synergism, such as interferon-γ (IFN-γ) and tumor necrosis factor alpha (TNF-α), which increases the expression of inducible nitric oxide synthase (iNOS), an enzyme which catalyzes l-arginine to generate NO and citrulline (Liew et al. 1990). Once inside the phagolysosome, NO combines with superoxide to produce peroxide nitrite, which is highly reactive and acts as microbicidal agent (Bogdan and Rollinghoff 1998; Ueda-Nakamura et al. 2006). Interestingly, the NO pathway induced by EA in macrophages was probably co-stimulated after infection with L. major, thus being a high immunomodulatory capacity only in the presence of the pathogen, without altering the cell physiology. These observations corroborate with previous reports for natural products and polyphenols (Islamuddin et al. 2015; Kheirandish et al. 2016; Ogungbe et al. 2014; Rodrigues et al. 2015).
Another interesting factor is the cytoplasmatic calcium levels in the host cell. As an important microbicidal agent, intracellular calcium promotes the activation of the classical protein kinase C (PKC) pathway, which activates pro-inflammatory cytokines, such as TNF-α and IFN-γ, in order to activate the cellular Th1 immune response, and then promoting the recruitment of cells from mononuclear phagocytic system in order to control the infection (Islamuddin et al. 2015; Roy et al. 2014a). The action of new drugs on the increase of intracellular calcium is directly related to this signaling pathway. In this study, GA and EA were able to increase cytoplasmic calcium levels, possibly promoting this activation. Furthermore, the depolymerization of F-actin from the peripheral region is another calcium-dependent mechanism. Phagocytosis by neutrophils, macrophages, and other professional phagocytes requires rapid remodeling of actin. The disaggregation of periphagosomal F-actin, phagocytosis, and phagocytosome maturation are calcium-dependent processes in macrophages when they interact with pathogens in order to solve infections (Tejle et al. 2002).
Radtke et al. (2004), Kolodziej and Kiderlen (2005), and Yadav et al. (2012) have described the immunomodulatory activity of phenolic compounds, including GA, capable of promoting increased NO synthesis, as well as increasing the expression of genes, resulting from the production of pro-inflammatory cytokines, such as TNF-α and IFN-γ. Besides, miltefosine is one of the conventional drugs widely used in Africa and Europe as the only one to be administered orally to combat leishmaniasis. Its mechanism of action is described by Verma and Dey (2004) as immunomodulatory activity through the IP3/PLC/PKC pathway. In this sense, evidences that GA and EA might act by similar mechanisms can be hypothesized.
In conclusion, GA and EA induced marked activity against promastigote forms of L. major, reducing the number of infected macrophages and survival index of L. major internalized amastigotes in parasitized macrophages, as well as induced macrophage activation by increase of phagocytic capability, lysosomal volume, NO synthesis, and cytoplasmic calcium release. Therefore, GA and EA are promising molecules for studies regarding the treatment of leishmaniasis. Further investigations are reinforced in order to evaluate the therapeutic effects of GA and EA in in vivo experimental infection model of leishmaniasis.
References
Aggarwal BB, Shishodia S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71:1397–1421. doi:10.1016/j.bcp.2006.02.009
Alizadeh BH, Foroumadi A, Ardestani SK, Poorrajab F, Shafiee A (2008) Leishmanicidal evaluation of novel synthetic chromenes. Arch Pharm 341:787–793. doi:10.1002/ardp.200800128
Ashford RW (2000) The leishmaniases as emerging and reemerging zoonoses. Int J Parasitol 30:1269–1281
Azevedo E, Oliveira LT, Castro Lima AK, Terra R, Dutra PM, Salerno VP (2012) Interactions between Leishmania braziliensis and macrophages are dependent on the cytoskeleton and myosin Va. J Parasitol Res 2012:275436. doi:10.1155/2012/275436
Bogdan C, Rollinghoff M (1998) The immune response to Leishmania: mechanisms of parasite control and evasion. Int J Parasitol 28:121–134
Bonatto SJ et al (2004) Lifelong exposure to dietary fish oil alters macrophage responses in Walker 256 tumor-bearing rats. Cell Immunol 231:56–62. doi:10.1016/j.cellimm.2004.12.001
Carneiro SM, Carvalho FA, Santana LC, Sousa AP, Neto JM, Chaves MH (2012) The cytotoxic and antileishmanial activity of extracts and fractions of leaves and fruits of Azadirachta indica (A Juss.) Biol res 45:111–116. doi:10.4067/S0716-97602012000200002
Cortazar TM, Coombs GH, Walker J (2007) Leishmania panamensis: comparative inhibition of nuclear DNA topoisomerase II enzymes from promastigotes and human macrophages reveals anti-parasite selectivity of fluoroquinolones, flavonoids and pentamidine. Exp Parasitol 116:475–482. doi:10.1016/j.exppara.2007.02.018
Devipriya N, Srinivasan M, Sudheer AR, Menon VP (2007a) Effect of ellagic acid, a natural polyphenol, on alcohol-induced prooxidant and antioxidant imbalance: a drug dose dependent study. Singap med J 48:311–318
Devipriya N, Sudheer AR, Menon VP (2007b) Dose-response effect of ellagic acid on circulatory antioxidants and lipids during alcohol-induced toxicity in experimental rats. Fundam Clin Pharmacol 21:621–630. doi:10.1111/j.1472-8206.2007.00551.x
Dias CN, Rodrigues KA, Carvalho FA, Carneiro SM, Maia JG, Andrade EH, Moraes DF (2013) Molluscicidal and leishmanicidal activity of the leaf essential oil of Syzygium cumini (L.) SKEELS from Brazil. Chem Biodivers 10:1133–1141. doi:10.1002/cbdv.201200292
Gantt KR et al (2001) Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J Immunol 167:893–901
Goncalves JC et al (2013) The monoterpene (−)-carvone: a novel agonist of TRPV1 channels. Cytometry Part A 83:212–219. doi:10.1002/cyto.a.22236
Gonçalves JCR et al (2016) Antitumoral activity of novel 1,4-naphthoquinone derivative involves L-type calcium channel activation in human colorectal cancer cell line. J Appl Biomed 14:229–234. doi:10.1016/j.jab.2016.03.002
Grando FC et al (2009) Modulation of peritoneal macrophage activity by the saturation state of the fatty acid moiety of phosphatidylcholine. Braz J Med Biol Res = Rev Bras Pesqui Med Biol 42:599–605
Iino T, Nakahara K, Miki W, Kiso Y, Ogawa Y, Kato S, Takeuchi K (2001) Less damaging effect of whisky in rat stomachs in comparison with pure ethanol. Role of ellagic acid, the nonalcoholic component. Digestion 64:214–221
Islamuddin M, Chouhan G, Farooque A, Dwarakanath BS, Sahal D, Afrin F (2015) Th1-biased immunomodulation and therapeutic potential of Artemisia annua in murine visceral leishmaniasis. PLoS Negl Trop dis 9:e3321. doi:10.1371/journal.pntd.0003321
de Jesus NZ et al (2012) Tannins, peptic ulcers and related mechanisms. Int J Mol Sci 13:3203–3228. doi:10.3390/ijms13033203
Kaye P, Scott P (2011) Leishmaniasis: complexity at the host-pathogen interface. Nat rev Microbiol 9:604–615. doi:10.1038/nrmicro2608
Kayser O, Kiderlen AF, Croft SL (2003) Natural products as antiparasitic drugs. Parasitol res 90(Suppl 2):S55–S62. doi:10.1007/s00436-002-0768-3
Kheirandish F, Delfan B, Mahmoudvand H, Moradi N, Ezatpour B, Ebrahimzadeh F, Rashidipour M (2016) Antileishmanial, antioxidant, and cytotoxic activities of Quercus infectoria Olivier extract. Biomed Pharmacother 82:208–215. doi:10.1016/j.biopha.2016.04.040
Kolodziej H, Kiderlen AF (2005) Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells. Phytochemistry 66:2056–2071. doi:10.1016/j.phytochem.2005.01.011
Kondrashin AV, Baranova AM, Morozova LF, Stepanova EV (2011) Global trends in malaria control. Progress and topical tasks in malaria control programs Med Parazitol 3–8
Lachaud L, Bourgeois N, Plourde M, Leprohon P, Bastien P, Ouellette M (2009) Parasite susceptibility to amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clin Infect Dis Off Publ Infect Dis Soc Am 48:e16–e22. doi:10.1086/595710
Lee WL, Harrison RE, Grinstein S (2003) Phagocytosis by neutrophils. Microbes Infect 5:1299–1306
Liew FY, Millott S, Parkinson C, Palmer RM, Moncada S (1990) Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J Immunol 144:4794–4797
Lodge R, Descoteaux A (2005) Leishmania donovani promastigotes induce periphagosomal F-actin accumulation through retention of the GTPase Cdc42. Cell Microbiol 7:1647–1658. doi:10.1111/j.1462-5822.2005.00582.x
Lofgren SE, Miletti LC, Steindel M, Bachere E, Barracco MA (2008) Trypanocidal and leishmanicidal activities of different antimicrobial peptides (AMPs) isolated from aquatic animals. Exp Parasitol 118:197–202. doi:10.1016/j.exppara.2007.07.011
Lopes L, Godoy LM, de Oliveira CC, Gabardo J, Schadeck RJ, de Freitas BD (2006) Phagocytosis, endosomal/lysosomal system and other cellularaspects of macrophage activation by Canova medication. Micron 37:277–287. doi:10.1016/j.micron.2005.08.005
Lu Y et al (2010) Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur J Pharmacol 641:102–107. doi:10.1016/j.ejphar.2010.05.043
de Macedo-Silva ST, de Oliveira Silva TL, Urbina JA, de Souza W, Rodrigues JC (2011) Antiproliferative, ultrastructural, and physiological effects of amiodarone on promastigote and amastigote forms of Leishmania amazonensis. Mol Biol Int 2011:876021. doi:10.4061/2011/876021
Madalosso G, Fortaleza CM, Ribeiro AF, Cruz LL, Nogueira PA, Lindoso JA (2012) American visceral leishmaniasis: factors associated with lethality in the state of sao paulo. Braz J Trop Med 2012:281572. doi:10.1155/2012/281572
de Medeiros M, da Silva AC, Cito AM, Borges AR, de Lima SG, Lopes JA, Figueiredo RC (2011) In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitol Int 60:237–241. doi:10.1016/j.parint.2011.03.004
Mitropoulos P, Konidas P, Durkin-Konidas M (2010) New World cutaneous leishmaniasis: updated review of current and future diagnosis and treatment. J am Acad Dermatol 63:309–322. doi:10.1016/j.jaad.2009.06.088
Montalvo AM, Fraga J, Monzote L, Garcia M, Fonseca L (2012) Leishmaniasis diagnosis: going from microscopic observation of parasite to DNA detection. Rev Cubana med Trop 64:108–131
Murakami S, Isobe Y, Kijima H, Nagai H, Muramatu M, Otomo S (1991) Inhibition of gastric H+, K(+)-ATPase and acid secretion by ellagic acid. Planta med 57:305–308. doi:10.1055/s-2006-960103
Niedergang F, Chavrier P (2004) Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome. Curr Opin Cell Biol 16:422–428. doi:10.1016/j.ceb.2004.06.006
Nozais JP (2003) The origin and dispersion of human parasitic diseases in the old world (Africa, Europe and Madagascar). Mem Inst Oswaldo Cruz 98(Suppl 1):13–19
Nwaka S, Hudson A (2006) Innovative lead discovery strategies for tropical diseases. Nat Rev Drug Discov 5:941–955. doi:10.1038/nrd2144
Ogungbe IV, Erwin WR, Setzer WN (2014) Antileishmanial phytochemical phenolics: molecular docking to potential protein targets. J Mol Graph Model 48:105–117. doi:10.1016/j.jmgm.2013.12.010
Paolini A, Curti V, Pasi F, Mazzini G, Nano R, Capelli E (2015) Gallic acid exerts a protective or an anti-proliferative effect on glioma T98G cells via dose-dependent epigenetic regulation mediated by miRNAs. Int J Oncol 46:1491–1497. doi:10.3892/ijo.2015.2864
Papoutsi Z, Kassi E, Chinou I, Halabalaki M, Skaltsounis LA, Moutsatsou P (2008) Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br J Nutr 99:715–722. doi:10.1017/S0007114507837421
Radtke OA, Kiderlen AF, Kayser O, Kolodziej H (2004) Gene expression profiles of inducible nitric oxide synthase and cytokines in Leishmania major-infected macrophage-like RAW 264.7 cells treated with gallic acid. Planta med 70:924–928. doi:10.1055/s-2004-832618
Reithinger R, Coleman PG (2007) Treating cutaneous leishmaniasis patients in Kabul, Afghanistan: cost-effectiveness of an operational program in a complex emergency setting. BMC Infect dis 7:3. doi:10.1186/1471-2334-7-3
Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S (2007) Cutaneous leishmaniasis. Lancet Infect dis 7:581–596. doi:10.1016/S1473-3099(07)70209-8
Ribeiro TG et al (2015) Antileishmanial activity of standardized fractions of Stryphnodendron obovatum (Barbatimao) extract and constituent compounds. J Ethnopharmacol 165:238–242. doi:10.1016/j.jep.2015.02.047
Rizk YS et al (2014) In vitro activity of the hydroethanolic extract and biflavonoids isolated from Selaginella sellowii on Leishmania (Leishmania) amazonensis. Mem Inst Oswaldo Cruz 109:1050–1056. doi:10.1590/0074-0276140312
Roatt BM et al (2014) Immunotherapy and Immunochemotherapy in visceral Leishmaniasis: promising treatments for this neglected disease. Front Immunol 5:272. doi:10.3389/fimmu.2014.00272
Rodrigues KA et al (2013) Eugenia uniflora L. essential oil as a potential anti-Leishmania agent: effects on Leishmania amazonensis and possible mechanisms of action. Evid Based Complement Alternat Med eCAM 2013:279726. doi:10.1155/2013/279726
Rodrigues KA, Amorim LV, Dias CN, Moraes DF, Carneiro SM, Carvalho FA (2015) Syzygium cumini (L.) Skeels essential oil and its major constituent alpha-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J Ethnopharmacol 160:32–40. doi:10.1016/j.jep.2014.11.024
Roy N et al (2014a) Regulation of PKC mediated signaling by calcium during visceral leishmaniasis. PLoS One 9:e110843. doi:10.1371/journal.pone.0110843
Roy S, Kumar GA, Jafurulla M, Mandal C, Chattopadhyay A (2014b) Integrity of the actin cytoskeleton of host macrophages is essential for Leishmania donovani infection. Biochim Biophys Acta 1838:2011–2018. doi:10.1016/j.bbamem.2014.04.017
dos Santos KK et al (2012) Cytotoxic, trypanocidal, and antifungal activities of Eugenia jambolana L. J med Food 15:66–70. doi:10.1089/jmf.2010.0298
Sarjit A, Wang Y, Dykes GA (2015) Antimicrobial activity of gallic acid against thermophilic Campylobacter is strain specific and associated with a loss of calcium ions. Food Microbiol 46:227–233. doi:10.1016/j.fm.2014.08.002
Shuaibu MN et al (2008) Castalagin from Anogeissus leiocarpus mediates the killing of Leishmania in vitro. Parasitol res 103:1333–1338. doi:10.1007/s00436-008-1137-7
Soares DC, Pereira CG, Meireles MA, Saraiva EM (2007) Leishmanicidal activity of a supercritical fluid fraction obtained from Tabernaemontana catharinensis. Parasitol Int 56:135–139. doi:10.1016/j.parint.2007.01.004
Sundar S, Chakravarty J, Rai VK, Agrawal N, Singh SP, Chauhan V, Murray HW (2007) Amphotericin B treatment for Indian visceral leishmaniasis: response to 15 daily versus alternate-day infusions. Clin Infect Dis Off Publ Infect Dis Soc Am 45:556–561. doi:10.1086/520665
Tejle K, Magnusson KE, Rasmusson B (2002) Phagocytosis and phagosome maturation are regulated by calcium in J774 macrophages interacting with unopsonized prey. Biosci rep 22:529–540
Ueda-Nakamura T et al (2006) Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum. Parasitol Int 55:99–105. doi:10.1016/j.parint.2005.10.006
Valadares DG et al (2011) Leishmanicidal activity of the Agaricus blazei Murill in different Leishmania species. Parasitol Int 60:357–363. doi:10.1016/j.parint.2011.06.001
Vattem DA, Ghaedian R, Shetty K (2005) Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry. Asia Pac J Clin Nutr 14:120–130
Verma NK, Dey CS (2004) Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob Agents Chemother 48:3010–3015. doi:10.1128/AAC.48.8.3010-3015.2004
Waldron LS, Ferrari BC, Cheung-Kwok-Sang C, Beggs PJ, Stephens N, Power ML (2011) Molecular epidemiology and spatial distribution of a waterborne cryptosporidiosis outbreak in Australia. Appl Environ Microbiol 77:7766–7771. doi:10.1128/AEM.00616-11
Yadav DK, Khan F, Negi AS (2012) Pharmacophore modeling, molecular docking, QSAR, and in silico ADMET studies of gallic acid derivatives for immunomodulatory activity. J Mol Model 18:2513–2525. doi:10.1007/s00894-011-1265-3
Yang YH, Wang Z, Zheng J, Wang R (2015) Protective effects of gallic acid against spinal cord injury-induced oxidative stress. Mol med rep 12:3017–3024. doi:10.3892/mmr.2015.3738
Yuce A, Atessahin A, Ceribasi AO, Aksakal M (2007) Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin Pharmacol Toxicol 101:345–349. doi:10.1111/j.1742-7843.2007.00129.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All protocols were approved by the Animal Research Ethics Committee from Federal University of Piaui, Brazil (CEEA-PI no. 053/2015).
Rights and permissions
About this article
Cite this article
Alves, M.M.d.M., Brito, L.M., Souza, A.C. et al. Gallic and ellagic acids: two natural immunomodulator compounds solve infection of macrophages by Leishmania major . Naunyn-Schmiedeberg's Arch Pharmacol 390, 893–903 (2017). https://doi.org/10.1007/s00210-017-1387-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-017-1387-y