Abstract
[1-(2-isopropyl-5-methylphenoxy)-3-(yridine-3-ylmethylamino)propan-2-ol] is a novel oxipropanolamine derivative. It had been synthesized in a previous study with the idea that could be a prodrug and determined to show anti-α-glycosidase, antiacetylcholinesterase, anticarbonic anhydrase and antibacterial activities. However, to suggest it as a drug candidate, genotoxicity data related to it should be available. Thus, in this research, we aimed to evaluate its genotoxicity. We performed the chromosomal aberration and micronucleus tests in human peripheral lymphocytes. In all tests, lymphocyte cultures were treated with four concentrations (50, 25, 12.5, 6.25 μg/mL) of this derivative. According to our results, it significantly increased the chromosomal abnormalities at 25 and 50 μg/mL concentrations both 24 h and 48 h periods. Also, it significantly decreased the mitotic index at all concentrations. On the other hand, it significantly increased the micronucleus frequencies at 6.25, 12.5, and 25 μg/mL concentrations. In micronucleus test, no binucleate cells were detected at the highest concentration (50 μg/mL) and a total number of 4000 binucleate cells couldn’t be reached at the second-highest concentration (25 μg/mL). These results evaluated together, we can suggest that the test substance is cytotoxic and aneugenic at all used concentrations in human peripheral lymphocytes. Also, it’s clastogenic and genotoxic at high application concentrations.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Many therapeutic drugs discovered and frequently used may lose their effectiveness after a certain period time due to the ability of bacteria causing disease to become resistant to that drug or loss of sensitivity of the enzymes and receptors targeted in an organism to this drug. Therefore, the need for more effective drugs for disease or antibacterials has increased more than before. On the other hand, there is a need for variants of commercially available drugs with improved therapeutic effects and reduced side effects. In addition, effective drugs are also required for diseases that aren’t yet cured. For these reasons, efforts in the development of new pharmaceutical materials are increasing day by day. There are several approaches used to obtain new pharmaceuticals, such as synthesis of new compounds by combining different kinds of substances. When such an approach is used, it is important to know that components of the new compound are present in the structure of the pharmaceuticals in clinical use and have various biological activities. This enhances the probability of newly synthesized compounds exhibiting biological activity (Imming et al. 2006).
Thymol and oxypropanolamine are two compounds known to have a variety of biological activities and are found in the structure of various pharmaceuticals on the market. Thymol (2-isopropyl-5-methyl-phenol) is also a phenolic compound found in nature, especially in the essential oils of various plants of the Labiatae group. The best known activity of thymol is the antibacterial effect (Lambert et al. 2001; Pei et al. 2009; Sahoo et al. 2021). It also has antioxidant, antiviral, antifungal, and antitumor activity (Giweli et al. 2012). In recent years, thymol-containing compounds have also been investigated for activities such as obesity, anti-inflammatory, cicatrizant, and antileishmanial (Kordali et al. 2008; Jaafari et al. 2012; Kumar and Rawat 2013, Sahoo et al. 2021).
Oxypropanolamines have long been used as β-adrenoceptor antagonists (Crowther et al. 1972; Carre et al. 1984; Machin et al. 1984; Mauleon et al. 1988). They are used in the treatment of cardiovascular diseases, hypertension, thyrotoxicosis, angina pectoris, chronic lung diseases, skin infections, and diuretics (Ceccehetti et al. 1993; Bazylak and Nagels 2003; Wechsler et al. 2014). Also, there are some antibacterial properties of oxypropanolamines (Rokade and Sayyed 2009; Sabitha et al. 2010; Sahu et al. 2015).
Considering all aforementioned information, in a previous study Zengin et al. (2018) had synthesized compounds that contain both thymol and oxypropanolamine functionalities in the same structure. And also, they had investigated the anti-α-glycosidase, anti-acetylcholinesterase (anti-AChE), anti-carbonic anhydrase (anti-CA), and antibacterial activities of these compounds. Consequently, they reported that their novel thymol bearing oxypropanolamine derivatives can be acceptable prodrugs in the treatment of some diseases such as glaucoma, epilepsy, ulcer, osteoporosis, mountain sickness, diabetes mellitus, and neurological disorders. One of these [1-(2-isopropyl-5-methylphenoxy)-3-(yridine-3-ylmethylamino) propan-2-ol] was found to show an average antibacterial effect on S. aureus, A. baumannii and E. coli strains. Also, it was found to exhibit efficient anti-α-glycosidase, excellent anti-AChE, and impressively anti-CA activities. In recent years, novel pharmaceuticals that exhibit anti-carbonic anhydrase, anti-α-glycosidase, anti-acetylcholinesterase, and antibacterial activities attract great attention in drug development (Zengin et al. 2018).
The newly synthesized compounds exhibit enzyme inhibitory and antibacterial activities aren’t enough to recommend them as pharmaceutical material. The safety of pharmaceuticals is more important than their effectiveness and treating people without harming their health is the basis of chemotherapy. Thus, toxicological investigations related to them should be carried out at the beginning of the drug investigation process. One stage of toxicological investigations is genotoxicity studies. In these studies, it is investigated whether the candidate pharmaceuticals will damage the genetic material. For this purpose, in vivo and in vitro short-term genotoxicity tests are performed. Since genetic material damages caused by genotoxic agents may cause important health problems in humans such as cancer, performing genotoxicity tests is very essential (Şen et al. 2018). The aim of this study; to investigate the genotoxic profile of the [1-(2-isopropyl-5-methylphenoxy)-3-(yridine-3-ylmethylamino) propan-2-ol] that had been synthesized with the idea that could be a prodrug. For this aim, we used the in vitro chromosomal aberrations (CA) and in vitro micronucleus (MN) tests in human peripheral blood lymphocytes.
Methods
This research was approved by the Non-Invasive Research Ethics Committee of Sakarya University, Faculty of Medicine (03/01/2019-E.87). In addition, this study is performed according to the Declaration of Helsinki and an informed written consent form was obtained from donors who donated blood.
Chemicals
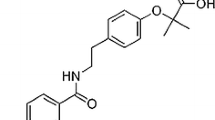
[1-(2-isopropyl-5-methylphenoxy)-3-(yridine-3-ylmethylamino) propan-2-ol] was synthesised by Zengin et al. (2018) in a previous study. In the synthesis step: 1 mol of thymol oxirane (4 g) was dissolved in methyl alcohol, 2 mol of 3-methylamino pyrine (3.94 mL) was added and then 1 mL of K2CO3 was added and stirred at room temperature for 24 h. At the end of the reaction, the methyl alcohol was extracted and extracted with ethyl acetate-brine. At the end of the extraction, the product was washed 4 times with hexane to give 4136 g of clean product (Zengin et al. 2018). The chemical structure and synthesis steps of the test substance are shown in Fig. 1.
Synthesis of [1-(2-isopropyl-5-methylphenoxy)-3-(yridine-3-ylmethylamino) propan-2-ol] (Zengin et al. 2018)
The other chemicals used for genotoxicity tests: Chromosome medium B (Cas no: F 5023) was obtained from Biochrome (Berlin, Germany). Mitomycin C (Cas no: 50-07-7), Colchicine (Cas no: 9754), Cytochalasin B (Cas no: 14930-96-2) were obtained from Sigma (St. Louis, MO, USA).
Collection of blood samples
Peripheral venous blood was obtained from 4 healthy donors (2 male, 2 female, non-smokers, aged 20–25 years) not exposed to any drug therapy or known mutagenic agent over the past 2 years, not exposed to ionizing radiation within the previous 6 months and with no history of chromosome fragility or recent viral infection.
Dose selection
The highest application concentration for the test compound was taken as 50 μg/mL. The other two concentrations of each compound were considered as 1/2, 1/4, and 1/8 of the highest concentrations. As a result, 25, 12.5, and 6.25 μg/mL concentrations were used for the test substance. Also, positive, solvent and negative controls were used. LC50 values were taken into account in the selection of the dose.
Chromosomal aberrations (CA) analysis in cultured human lymphocytes
0.2 mL heparinized peripheral blood samples of 4 healthy (2 male and 2 female) donors were cultured in 2.5 mL chromosome medium B and treated with 50, 25, 12.5, and 6.25 μg/mL concentrations of the test substance. Test substance was dissolved in dimethyl sulfoxide (DMSO). An untreated, a positive (Mitomycin C; 0.2 μg/mL) and solvent control (DMSO) were also maintained in all treatments. Cells in culture were exposed to the test substance for 24 and 48 h. Cultures were incubated for 72 h at 37 °C, and colchicine (final concentration: 0.06 μg/mL) was added to each culture 2 h before harvesting. Cells were then harvested by centrifugation (1200 rpm for 10 min), and the pellet was treated with 0.075 M of KCl for 30 min at 37 °C. Cells were centrifuged again and fixed in cold methanol/acetic acid (3:1). The fixation process was repeated three times. Slides were stained with 5% Giemsa (pH = 6.8) in Sorensen buffer for 20–25 min, washed in distilled water, dried at room temperature and mounted with entellan.
Micronucleus (MN) assay in cultured human lymphocytes
Heparinized 0.2 mL whole blood samples were added to 2.5 mL of Chromosome Medium B. Human lymphocytes were incubated at 37 °C for 72 h and treated with 50, 25, 12.5, and 6.25 μg/mL concentrations of the test substance. Test substance was dissolved in DMSO. An untreated, a solvent (DMSO) and a positive control (mitomycin C; 0.2 μg/mL) were also maintained in all treatments. Cytochalasin B (5.2 μg/mL) was added to arrest cytokinesis at 44 h after the start of culture. Then, cells were harvested by centrifugation (1000 rpm for 10 min), and the pellet was treated with hypotonic solution (0.075 M of KCl) for 5 min at 4 °C. Cells were recentrifuged and fixed three times in cold methanol/acetic acid (3:1). In the last fixative, 1% formaldehyde was added to preserve the cytoplasm. Slides were prepared and stained with 5% Giemsa (pH = 6.8) in Sorensen buffer for 13–15 min, washed in distilled water, dried at room temperature and mounted with entellan.
Slide evaluation
In human lymphocytes, 100 well-spread metaphases per donor (total, 400 metaphases per concentration) were analyzed for chromosome aberrations. The mitotic index (MI) was also determined by scoring 3000 cells from each donor (total, 12,000 cells per concentration). Micronuclei were scored from 1000 binucleated cells per donor (total, 4000 binucleated cells per concentration).
Statistical analyses
Statistical analysis was done by using the software program SPSS 20.0 (developed by SPSS, Chicago, IL, USA). For obtaining the percentage of abnormal cell, CA/cell, MI, MN, z-test was used and also, concentration-response relationships were determined from the regression coefficients for the percentage of abnormal cell, CA/cell, MN.
Results
To assess the genotoxic profile of [1-(2-isopropyl-5-methylphenoxy)-3-(yridine-3-ylmethylamino) propan-2-ol] in human lymphocytes, in vitro CA and in vitro MN tests were performed. To evaluate the cytotoxicity, mitotic index values were revealed. The results of the CA analysis and the mitotic index values were reported in Table 1.
Test compound induced four types of structural aberrations in 24 h treatment with the test compound. Chromatid breaks (Fig. 2a) were observed as the most common aberrations and followed by chromosome breaks (Fig. 2b), fragments (Fig. 2c) and sister chromatid union (Fig. 2d), respectively. One endoreduplication (Fig. 2e) was observed at the highest concentration of 24 h treatment. In 48 h treatment with test compound, five types of structural aberrations were observed: chromatid breaks, fragments, chromosome breaks, sister chromatid union and chromatid exchange (Fig. 2f), sort by most to least in 48 h treatment.
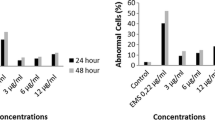
According to the CA test results, the test compound increased the abnormal cell percentage dose-dependent manner both in 24 h (r = 0,971 and r = 0,978, negative and solvent control, respectively) and in 48 h (r = 0,970 and r = 0,970, negative and solvent control, respectively) treatment periods. The abnormal cell percentage increases were statistically significant at two highest application concentrations (25, 50 μg/mL) compared with solvent control and at the highest concentration compared with negative control in 24 h treatment. In 48 h treatment period, the abnormal cell percentage was statistically significant at the highest application concentration (50 μg/mL) compared with both negative and solvent control (Table 1). Test compound increased the number of CA per cell (CAs/Cell) in a dose-dependent manner both 24 h (r = 0,967 and r = 0,967, negative and solvent control, respectively) and 48 h (r = 0,962 and r = 0,962, negative and solvent control, respectively) treatment periods. These increases were statistically significant at two highest application concentrations (25, 50 μg/mL) in 24 h treatment when compared with negative and solvent control. In 48 h treatment, CAs/Cell was statistically significant at highest application dose (50 μg/mL) compared with both negative and solvent control (Table 1). Furthermore, a cell with too many chromosomal abnormalities was found at the highest application concentration of 50 μg/mL during 24 h treatment period. These abnormalities were not considered. On the other hand, chromosomal abnormalities at 24 h were found to be slightly higher than in 48 h.
The effect of the test compound on the mitotic index was determined and statistically significant differences were observed between treatment and control cultures (Table 1). This compound decreased the mitotic index percentage in all application concentrations compared with both negative control and solvent control after 24 h and 48 h treatment periods. These declines were also dose-dependent manner in 24 h (r = −0,894 and r = −0,956, negative and solvent control, respectively) and in 48 h (r = −0,956 and r = −0,983, negative and solvent control, respectively) periods.
To determine the clastogenic and/or aneugenic effects of the test compound, the cytokinesis-block MN test was performed. The effects of the test compound on MN frequency are reported in Table 2.
Test substance produced statistically significant increases in the frequency of micronucleus compared with controls. These differences were slightly dose-dependent manner when compared to negative control (r = 0,987) and solvent control (r = 0,995). No binucleate cells were found at 50 μg/mL concentration and 2356 binucleate cells were found at 25 μg/mL concentration. The rise in MN frequency nearly 5, 7 and 12 fold higher compared with NC for the 3 doses. Most cells showed just one MN (Fig. 3a), four cells with two MN (Fig. 3b), two cells with three MN (Fig. 3c) and one cells with five MN (Fig. 3d).
Discussion
[1-(2-isopropyl-5-methylphenoxy)-3-(yridine-3-ylmethylamino) propan-2-ol] is a novel compound that synthesised as a prodrug. It is recognized several biological activities such as anti-α-glycosidase, anti acetylcholinesterase, anti-carbonic anhydrase and antibacterial. Therefore, in the future, this compound might be used in the treatment of some diseases such as glaucoma, epilepsy, ulcer, osteoporosis, mountain sickness, diabetes mellitus, and neurological disorders. CA and MN are the most commonly used and reliable genotoxicity test systems. The human peripheral blood lymphocytes are mostly used cells in these tests. It has been reported in many studies in the scientific literature that the use of lymphocytes is appropriate for the evaluation of genotoxicity. Therefore, we also used CA and MN tests in human peripheral lymphocytes for genotoxic evaluation of our test compound.
Chromosomal aberration is one of the endpoints pointing to the genotoxicity and mutagenicity of chemicals (Fei et al. 2015). It has been reported that high CA levels in lymphocytes significantly increase the risk of developing cancer (Hagmar et al. 1998; Chandirasekar et al. 2014). Our test substance significantly increased the chromosomal abnormalities especially at high concentrations (25 μg/mL and 50 μg/mL) of 24 h and 48 h treatment periods. However, the test substance didn’t produce significant differences at low concentrations of both treatment periods. Since we have obtained similar results both in 24 h and 48 h application periods, we can say that chromosomal abnormality formation is affected by treatment concentrations rather than the exposure time. These results indicate that test substance is clastogenic and genotoxic at high concentrations. The dose-effect relationship observed at both treatment periods confirms this opinion. Furthermore, in our study, chromosomal abnormalities were found slightly higher at 24 h than in 48 h. From this result, it can be thought that the repair system starts to work in long-term exposure and corrected chromosomal abnormalities.
The basic skeletal structure of our test compound contains thymol and oxypropanolamine (Zengin et al. 2018). We came across the studies reporting that thymol induces chromosomal aberrations and DNA damage at high concentrations. Aydın et al. (2005) tested genotoxic effects of thymol by comet assay and observed that thymol had no effect to increase DNA strand breakage at concentrations below 0.1 mM (0.005, 0.01, 0.025, 0.05 and 0.1 mM). However, at the higher concentrations (0.2, 0.5, 1, and 2 mM) thymol caused significant increases in DNA damages. Azirak and Rencuzogullari (2008) examined the genotoxic effects of thymol on bone marrow cells in rats in vivo and reported that thymol (40, 60, 80, and 100 mg/kg b.w.) significantly induced the structural and total chromosome abnormalities for all treatment periods (6, 12, and 24 h) and also induced the numerical chromosomal abnormalities especially at the highest concentration (100 mg/kg b.w.) for all treatment periods. Buyukleyla and Rencuzogullari (2009) reported that 25, 50, 75 and 100 μg/mL concentrations of thymol increased the frequency of structural chromosomal abnormalities in human peripheral lymphocytes, and also chromosomal abnormalities were higher at 24 h than at 48 h treatment period. These results are consistent with our results. However, different from these results, LLana-Ruiz-Cabello et al. (2014) suggested that thymol didn’t show any mutagenic and genotoxic activity at any concentration assayed (0–250 μM) using the Ames Salmonella test and the alkaline, Endo III and formamidopyrimidine glycosylase (FPG)-modified comet assays. Shettigar et al. (2015) investigated the anticytotoxic and antigenotoxic ability of thymol against mercury chloride (HgCl2) induced cytotoxicity and genotoxicity in human hepatocarcinoma cell line. They reported that thymol pretreatment reduced HgCl2 induced cytotoxicity and inhibited apoptotic and necrotic cell death induced by HgCl2.
We encountered studies in the literature reporting that oxypropanolamine derivatives induce chromosomal abnormalities and DNA damage. The cytotoxic and genotoxic studies revealed that propranolol (oxypropanolamine derivative) increased polyploid, chromosome and chromatid breaks (Sedigh-Ardekani et al. 2013), beta-blocker pharmceuticals containing propanolamine have a genotoxic effect (Brambilla and Martelli 2006) and beta blocker drugs caused DNA fragmentation (Robbiano et al. 1991).
In this study, the test substance significantly decreased the mitotic index values at all concentrations both 24 h and 48 h treatment periods. These results show that the test substance is a cytotoxic agent. One reason for mitotic index reduction maybe that cell cycle-specific proteins are the targets of the test substance. Another reason may be that the test substance blocks the G2 phase of the cell cycle or suppresses the ATP production in the cell (Epel 1963, Tunca et al. 2017). Several studies emphasized that the thymol is cytotoxic or reduces mitotic index. Aydın and Türkez (2014) reported that thymol induced cytotoxicity on cultured human blood cells in a time and dose-dependent manner in lactate dehydrogenase (LDH) release and [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) assay. Buyukleyla and Rencuzogullari (2009) found that thymol decreased the mitotic index at the higher concentration (100 μg/mL) in peripheral lymphocytes without dose-dependent effect. Azirak and Rencuzogullari (2008) reported decreased mitotic index in bone marrow cells of rats maintained intraperitoneally with 40, 60, 80, and 100 mg/kg b.w. thymol.
The MN assay has the ability to detect both aneugens (chromosome lagging due to dysfunction of mitotic apparatus) and clastogens (chromosome breakage) (Kirsch et al. 2011). Clastogenic and/or aneugenic agents produce chromosomal fragments or chromosomal losses that do not integrate into the nucleus of daughter cells during cell division, thus causing MN formation (Arealdi et al. 2015). The MN test is an early diagnostic test that has proven very useful for detecting precancerous lesions (Jyoti et al. 2015). It was also reported that increased MN frequency is related to cytotoxicity (Kirkland 2010). In this study, MN analysis showed that test substance significantly increased the micronucleus frequencies at 6.25, 12.5, 25 μg/mL application concentrations. Significant increases in micronucleus frequencies at these three application concentrations indicate that the test compound is clastogenic/aneugenic. On the other hand, in the micronucleus test, no detection of binucleated cells at the highest concentration (50 μg/mL) and failure to achieve 4000 binucleate cell at the second highest concentration (25 μg/mL) also support the test compound is cytotoxic. Buyukleyla and Rencuzogullari (2009) reported 25, 50, 75 and 100 μg/mL concentrations of thymol increased the micronucleus frequency and exhibited clastogenic/aneugenic activity. This result is compatible with ours. Some studies have suggest results that are contrary to ours. Maisanaba et al. (2015) investigated the in vitro genotoxicity of thymol (0–250 μM) with MN test. As a result of this study, they reported negative results for thymol with the MN with and without the S9 fraction. This result reinforces the view that thymol is not genotoxic in mammalian cells. Aydın and Türkez (2014) were treated human blood cells with thymol (0–200 mg/L) for 24 and 48 h and analyzed the DNA damage by micronucleus (MN) test, sister chromatid exchanges (SCE) assay and 8-oxo-2-deoxyguanosine (8-OH-dG) level. They were found that thymol had no mutagenic effects on human lymphocytes. Belato et al. (2018) reported that MN frequency in murine macrophages treated with thymol was similar to the control group and thymol had no genotoxic effect. There are a few studies that were found the effect of the propanolamine derivatives on micronucleus formation. Okine et al. (1983) researched the mutagenic potential of nine β-adrenergic blocking agents containing the propanolamine group by micronucleus test. Among the examined drugs, only the highest doses of oxprenolol and propranolol showed a weak but not statistically significant response. Aruna and Krishnamurthy (1986) evaluated the mutagenic effects of propranolol in somatic and germ cells of mice. Propranolol induced a significant increase in the frequency of micronuclei in erythrocytes of Swiss albino mice was at higher dose levels. Martelli et al. (1994) studied the genotoxic effect of metoprolol and N-nitroso derivative NO-metoprolol in vivo MN on rats. They gave 772 mg/kg metoprolol and 1000 mg/kg NO-metoprolol to rats by gavage and performed MN analysis in liver, spleen and bone marrow tissues. As a result of the application of both substances, no significant difference was observed in terms of MN formation compared to the control group.
In conclusion, the data presented here reveal that the test substance has cytotoxic effect at all application concentrations and genotoxic effect at high concentrations. It can also be said that the test substance has an aneugenic effect in all application groups. In the light of this information, it can be stated that the test substance exhibit an important risk at the genetic level in vitro in selected sample size, concentrations and experimental conditions. And also, it can exhibit genotoxic effects with clastogenic effects at high concentrations. In order to say that this substance is safe in terms of cytotoxic and genotoxicity, it should be evaluated with other test methods, especially in vivo methods. As a result of this study, the test substance is both genotoxic and cytotoxic effect at high concentrations in vitro. On the other hand, especially, while no genotoxic effect at low doses, it has a cytotoxic effect, so; this substance could be considered as an agent of cell division inhibitor, evaluated with results obtained in future studies.
Abbreviations
- anti-AChE:
-
Anti-acetylcholinesterase
- anti-CA:
-
Anti-carbonic anhydrase
- BN:
-
Binucleated
- CA:
-
Chromosomal aberrations
- csb:
-
Chromosome breaks
- ctb:
-
Chromatid break
- cte:
-
Chromatid exchanges
- dc:
-
Dicentric chromosomes
- DMSO:
-
Dimethyl sulfoxide
- er:
-
Endoredublication
- f:
-
Fragment
- LC50:
-
50% lethal concentration
- μg/mL:
-
Microgram/ml
- MI:
-
Mitotic index
- MN:
-
Micronucleus
- NC:
-
Negative control
- PC:
-
Positive control
- SC:
-
Solvent control
- SCE:
-
Sister chromatid exchange
- scu:
-
Sister chromatid union
- SE:
-
Standard error
- TS:
-
Test substance
References
Araldi RP, Melo TCD, Mendes TB, Sa’Junior PL, Nozima BHN, Ito ET, Carvalho RF, Souza EB, Stocco R (2015) Using the comet and micronucleus assays genotoxicity studies: a review. Biomed Pharmacother 72:74–82. https://doi.org/10.1016/j.biopha.2015.04.004
Aruna N, Krishnamurthy NB (1986) Mutagenic evaluation of propranolol in somatic and germ cells of mice. Mutat Res Lett 173:207–210. https://doi.org/10.1016/0165-7992(86)90037-0
Aydın E, Türkez H (2014) In vitro cytotoxicity, genotoxicity and antioxidant potentials of thymol on human blood cells. J Essent Oil Res 26(2):133–140. https://doi.org/10.1080/10412905.2013.860411
Aydın S, Başaran AA, Başaran N (2005) The effects of thyme volatiles on the induction of DNA damage by the heterocyclic amine IQ and mitomycin C. Mutat Res/Genet Toxicol Environ Mutagen 581(1–2):43–53. https://doi.org/10.1016/j.mrgentox.2004.10.017
Azirak S, Rencuzogullari E (2008) The in vivo genotoxic effects of carvacrol and thymol in rat bone marrow cells. Environ Toxicol: An Int J 23(6):728–735. https://doi.org/10.1002/tox.20380
Bazylak G, Nagels LJ (2003) A novel potentiometric approach for detection of beta-adrenergics and beta-adrenolytics in high-performance liquid chromatography. II Farmaco 58(8):591–603. https://doi.org/10.1016/S0014-827X(03)00096-X
Belato KK, de Oliveira JR, de Oliveira FS, de Oliveira LD, Camargo SEA (2018) Cytotoxicity and genotoxicity of thymol verified in murine macrophages (RAW 264.7) after antimicrobial analysis in Candida albicans, Staphylococcus aureus and Streptococcus mutans. J Funct Foods 40:455–460. https://doi.org/10.1016/j.jff.2017.11.035
Brambilla G, Martelli A (2006) Genotoxicity and carcinogenicity studies of antihypertensive agents. Mutat Res/Rev Mutat Res 612(2):115–149. https://doi.org/10.1016/j.mrrev.2005.12.002
Buyukleyla M, Rencuzogullari E (2009) The effects of thymol on sister chromatid exchange chromosome aberration and micronucleus in human lymphocytes. Ecotoxicol Environ Saf 72(3):943–947. https://doi.org/10.1016/j.ecoenv.2008.10.005
Carre MC, Youlassani A, Caubere P (1984) Synthesis of a novel series of (aryloxy) propanolamines: new selective. Beta 2-blocking agents. J Med Chem 27(6):792–799. https://doi.org/10.1021/jm00372a016
Cecchetti V, Fravolini A, Schiaffella F, Tabarrini O, Bruni G, Segre G (1993) o-chlorobenzenesulfonamidic derivatives of (aryloxy) propanolamines as. beta.-blocking/diuretic agents. J Med Chem 36(1):157–161. https://doi.org/10.1021/jm00053a020
Chandirasekar R, Kumar BL, Sasikala K, Jayakumar R, Suresh K, Venkatesan R, Rachel J, Kavitha H, Ganesh GH (2014) Assessment of genotoxic and molecular mechanisms of cancer risk in smoking and smokeless tobacco users. Mutat Rese/Gene Toxicol Environ Mutagen 767:21–27. https://doi.org/10.1016/j.mrgentox.2014.04.007
Crowther AF, Howe R, McLoughlin BJ, Mallion KB, Rao BS, Smith LH, Turner RW (1972) Beta.-Adrenergic blocking agents. 12. Heterocyclic compounds related to propranolol. J Med Chem 15(3):260–266. https://doi.org/10.1021/jm00273a013
Epel D (1963) The effects of carbon monoxide inhibition on ATP level and the rate of mitosis in the sea urchin egg. J Cell Biol 17(2):315–319. https://doi.org/10.1083/jcb.17.2.315
Fei C, Zhang J, Lin Y, Wang X, Zhang K, Zhang L, Zheng W, Wang M, Li T, Xiao Z, Xue F, Wang C (2015) Safety evaluation of a triazine compound nitromezuril by assessing bacterial reverse mutation, sperm abnormalities, micronucleus and chromosomal aberration. Regul Toxicol Pharmacol 71:585–589. https://doi.org/10.1016/j.yrtph.2015.01.011
Giweli A, Džamić AM, Soković M, Ristić MS, Marin PD (2012) Antimicrobial and antioxidant activities of essential oils of Satureja thymbra growing wild in Libya. Molecules 17(5):4836–4850. https://doi.org/10.3390/molecules17054836
Hagmar L, Bonassi S, Stromberg U, Brøgger A, Knudsen LE, Norppa H, Reuterwall C (1998) Chromosomal aberrations in lymphocytes predict human cancer: a report from the European Study Group on Cytogenetic Biomarkers and Health (ESCH). Cancer Res 58:4117–4121
Imming P, Sinning C, Meyer A (2006) Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov 5:821–834. https://doi.org/10.1038/nrd2132
Jaafari A, Tilaoui M, Mouse HA, M’bark LA, Aboufatima R, Chait A, Lepoivre M, Zyad A (2012) Comparative study of the antitumor effect of natural monoterpenes: relationship to cell cycle analysis. Rev Brasi Farmaco 22(3):534–540. https://doi.org/10.1590/S0102-695X2012005000021
Jyoti S, Siddique YH, Khan S, Naz F, Ali F (2015) Effect on micronucleus frequency and DNA damage in buccal epithelial cells of various factors among pan masala and gutkha chewers. Oral Sci Int 12:9–14. https://doi.org/10.1016/S1348-8643(14)00030-5
Kirkland D (2010) Evaluation of different cytotoxic and cytostatic measures for the in vitro micronucleus test (MNVit): summary of results in the collaborative trial. Mutat Res 702:139–147. https://doi.org/10.1016/j.mrgentox.2010.02.001
Kirsch-Volders M, Plas G, Elhajouji A, Lukamowicz M, Gonzalez L, Loock KV, Decordier I (2011) The in vitro MN assay in 2011: origin and fate, biological significance, protocols, high throughput methodologies and toxicological relevance. Arch Toxicol 85:873–899. https://doi.org/10.1007/s00204-011-0691-4
Kordali S, Cakir A, Ozer H, Cakmakci R, Kesdek M, Mete E (2008) Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresource Technol 99(18):8788–8795. https://doi.org/10.1016/j.biortech.2008.04.048
Kumar D, Rawat DS (2013) Synthesis and antioxidant activity of thymol and carvacrol based Schiff bases. Bioorgan Med Chem Lett 23(3):641–645. https://doi.org/10.1016/j.bmcl.2012.12.001
Lambert RJW, Skandamis PN, Coote PJ, Nyshas GJ (2001) A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91(3):453–462. https://doi.org/10.1046/j.1365-2672.2001.01428.x
LLana-Ruiz-Cabello M, Maisanaba S, Puerto M, Prieto AI, Pichardo S, Jos Á, Cameán AM (2014) Evaluation of the mutagenicity and genotoxic potential of carvacrol and thymol using the Ames Salmonella test and alkaline, Endo III-and FPG-modified comet assays with the human cell line Caco-2. Food Chem Toxicol 72:122–128. https://doi.org/10.1016/j.fct.2014.07.013
Machin PJ, Hurst DN, Bradshaw RM, Blaber LC, Burden DT, Melarange RA (1984) Beta 1 selective adrenoceptor antagonists. 3. 4-Azolyl linked phenoxypropanolamines. J Med Chem 27(4):503–509. https://doi.org/10.1021/jm00370a012
Maisanaba S, Prieto AI, Puerto M, Gutiérrez-Praena D, Demir E, Marcos R, Cameán AM (2015) In vitro genotoxicity testing of carvacrol and thymol using the micronucleus and mouse lymphoma assays, Mutat Res/Gene Toxicol Environ Mutagene. 784:37–44. https://doi.org/10.1016/j.mrgentox.2015.05.005
Martelli A, Allavena A, Sottofattori E, Brambilla G (1994) Low clastogenic activity in vivo of the N-nitroso derivatives of 5 β-adrenergic-blocking drugs proved to be potent genotoxins in vitro. Toxicol Lett 73(3):185–191. https://doi.org/10.1016/0378-4274(94)90057-4
Mauleón D, Pujol MD, Rosell G (1988) Beta-adrenergic antagonists: N-alkyl and N-amidoethyl (arylalkoxy) propanolamines related to propranolol. Eur J Med Chem 23(5):421–426. https://doi.org/10.1016/0223-5234(88)90138-9
Okine LKN, Ioannides C, Parke DV (1983) Studies on the possible mutagenicity of β-adrenergic blocker drugs. Toxicol Lett 16(3–4):167–174. https://doi.org/10.1016/0378-4274(83)90175-3
Pei RS, Zhou F, Ji BP, Xu J (2009) Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J Food Sci 74(7):379–383. https://doi.org/10.1111/j.1750-3841.2009.01287.x
Robbiano L, Martelli A, Allavena A, Mazzei M, Gazzaniga GM, Brambilla G (1991) Formation of the N-nitroso derivatives of six β-adrenergic-blocking agents and their genotoxic effects in rat and human hepatocytes. Cancer Res 51(9):2273–2279
Rokade YB, Sayyed RZ (2009) Naphthalene derivatives: a new range of antimicrobials with high therapeutic value. Rasayan J Chem 2(4): 972–980. RJC-499
Sabitha G, Arundhathi K, Sudhakar K, Sastry BS, Yadav JS (2010) A novel three-component one-pot reaction involving β-naphthol, aldehydes, and urea promoted by TMSCl/NaI. J Heterocycl Chem 47(2): 272–275. http://doi.org/123456789/7866
Sahoo CR, Paidesetty SK, Padhy RN (2021) The recent development of thymol derivative as a promising pharmacological scaffold. Drug Develop Res. https://doi.org/10.1002/ddr.21848
Sahu PK, Sahu PK, Thavaselvam D, Alafeefy AM, Agarwal DD (2015) Synthesis and evaluation of antimicrobial activity of 2-aminobenzothiazolomethyl naphthol derivatives. Med Chem Res 24(2):725–736. https://doi.org/10.1007/s00044-014-1150-6
Sedigh-Ardekani M, Saadat I, Saadat M (2013) Propranolol induced chromosomal aberrations in Chinese hamster ovary cell line. Mol Biol Res Commun 2(1–2):11–18. https://doi.org/10.22099/mbrc.2013.1460
Şen S, Beceren A, Aksoy H (2018) The importance of genotoxicity tests in new drug development process. J Hum Sci 15(3):1634–1649. https://doi.org/10.14687/jhs.v15i3.5400
Shettigar NB, Das S, Rao NB, Rao SB (2015) Thymol, a monoterpene phenolic derivative of cymene, abrogates mercury-induced oxidative stress resultant cytotoxicity and genotoxicity in hepatocarcinoma cells. EnvironToxicol 30(8):968–980. https://doi.org/10.1002/tox.21971
Tunca H, Berber AA, Çanakçi K, Tuna M, Yildiz SZ, Aksoy H (2017) Synthesis, characterization, and determination of genotoxic effect of a novel dimeric 8-hydroxyquinoline Cd (II) SCN complex. Drug Chem Toxicol 40(3):300–308. https://doi.org/10.1080/01480545.2016.1223094
Wechsler JB, Hsu CL, Bryce PJ (2014) IgE-mediated mast cell responses are inhibited by thymol-mediated, activation-induced cell death in skin inflammation. J Allergy Clin Immun 133(6):1735–1743. https://doi.org/10.1016/j.jaci.2013.12.024
Zengin M, Genc H, Taslimi P, Kestane A, Guclu E, Ogutlu A, Karabay O, Gulçin İ (2018) Novel thymol bearing oxypropanolamine derivatives as potent some metabolic enzyme inhibitors–their antidiabetic, anticholinergic and antibacterial potentials. Bioorg Chem 81:119–126. https://doi.org/10.1016/j.bioorg.2018.08.003
Acknowledgments
This research was approved by the Non-Invasive Research Ethics Committee of Sakarya University, Faculty of Medicine (03/01/2019-E.87). In addition, this study is performed according to the Declaration of Helsinki and an informed written consent form was obtained from donors who donated blood.
Funding
This study was supported by Sakarya University Scientific Research Projects Commission Presidency, Sakarya, Turkey (Project No: 2019-7-24-40).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declared no potential conflicts of interest concerning for to the research, authorship, and/or publication of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meredova, G., Yıldız, E., Şen, S. et al. Genotoxicity of a novel thymol bearing oxipropanolamine derivative in human peripheral lymphocytes. Biologia 77, 559–567 (2022). https://doi.org/10.1007/s11756-021-00965-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00965-w