Abstract
The genotoxicity of copper oxychloride was investigated in human lymphocytes using chromosome aberration (CA) and micronucleus (MN) tests and the randomly amplified polymorphic DNA-polymerase chain reaction technique. The lymphocytes were treated with 3, 6, and 12 µg/mL of copper oxychloride for 24 and 48 h. Copper oxychloride increased CA and abnormal cells in a dose-dependent manner. The frequency of MN and micronucleated binuclear cells also increased at all concentrations and treatment periods. However, copper oxychloride cytotoxicity, observed through lower mitotic and nuclear division index, was significantly lower only at the higher concentrations (6 and 12 µg/mL). Copper oxychloride increased the polymorphic bands and decreased genomic template stability. In conclusion, in this study it was confirmed that copper oxychloride has genotoxic potential for human lymphocytes in vitro. Additionally, caution is advised for its use as a fungicide, because it may increase the risk of exposure through the food chain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
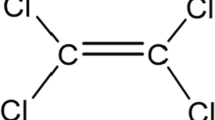
Copper oxychloride [3Cu(OH)2·CuCl2] is a common broad spectrum fungicide used to protect vegetables and fruits against diseases such as Venturia inaequalis, Taphrina deformans, Coryneum beijerinckii, Xanthomonas campestris pv, Vesicatoria, Phytophthora infestans, Alternaria solani, and Deuterophoma tracheiphila (Pérez-Rodríguez et al. 2013; Al-Assiuty et al. 2014).
Copper is a heavy metal that can be accumulated in various tissues of all living species (Snyman et al. 2004; Matache et al. 2013; Hurwitz et al. 2014). Its continuous application may lead to copper accumulation in crops, soil, and water (Chen et al. 1997; Masaka and Muunganirwa 2007) that consequently leads to human and animals to be exposed to copper through the food chain (La Pera et al. 2008), causing diseases such as kidney dysfunction, hepatocellular neoplasia, and hemolytic anemia (Waheed et al. 2013; Hurwitz et al. 2014; Ciji and Bijoy Nandan 2014).
Chronic copper poisoning can also cause weakness, abdominal pain, headache, dizziness, leg and back pain, anemia, and chronic kidney and liver damage in humans (Gunay et al. 2006; Hloch and Charvát 2012).
There are a few studies that have confirmed the genotoxic and cytotoxic effects of copper oxychloride in test animals using chromosome aberration and molecular toxicity tests (Pirtskhelani et al. 2008) or random amplified polymorphic DNA (RAPD)-polymerase chain reaction (PCR) (Atienzar et al. 2001; Gupta and Sarin 2009). To our best knowledge, so far none has examined its genotoxic effects on human lymphocytes as the most relevant markers of human exposure (Madle et al. 1993; Buyukleyla and Rencuzogullari 2009; Sevindik and Rencuzogullari 2014). The aim of this study was to fill this gap by investigating the genotoxic effects of copper oxychloride using widely adopted methods to detect DNA damage in humans, namely chromosome aberration (CA), micronucleus (MN), and RAPD-PCR tests (Albertini et al. 2000; Norppa and Falck 2003; Atienzar and Jha 2006; Kocaman and Topaktaş 2010).
Materials and methods
The test substance copper oxychloride was obtained in the commercial formulation that contains min 95 % copper oxychloride with min 51.4 % of the suspended active ingredient Cu (514 g Cu/L) (Tarem Inc., Delaware, DE, USA). The formula and other properties of the product are given in Table 1.
Chromosome aberration and micronucleus test
Chromosome aberration test was applied following the method described by Evans (1984) and the MN test was used as described by Fenech (2000) and Kirsch-Volders et al. (2003) with minor modifications. We also followed the IPCS guidelines (Albertini et al. 2000) and obtained the approval from the Ethics Committee of Adiyaman University (registered under no. 19.03.2013/03-01.1) in accordance with the Declaration of Helsinki.
To prepare the lymphocytes for CA testing, we added 0.2 mL of heparinized whole blood from five healthy, young donors (three females and two males, all non-smokers, aged 21–22) to 2.5 mL chromosome medium B (Biochrom, Berlin, Germany, F5023) and the culture was incubated at 37 °C for 72 h. The non-cytotoxic copper oxychloride concentrations were established in preliminary study. Cultured cells were treated with 3, 6, and 12 μg/mL concentrations of copper oxychloride which was dissolved in sterile distilled water as described by the manufacturer, for 24 and 48 h. Untreated blood samples and samples treated with 0.22 μg/mL ethyl methanesulfonate (EMS, Sigma, M0880) were used as negative and positive control, respectively. After colchicine treatment, 2 h before harvesting, the cells were treated with a hypotonic solution of 0.4 % KCl for 5 min then fixed three times in a mixture of methanol and glacial acetic acid (3:1). Air dried slides were stained with 5 % Giemsa stain prepared with Sorensen’s buffer for CA test following the standard methods (Evans 1984).
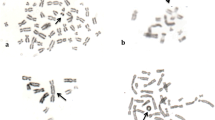
Chromosome aberration frequency and the percentage of abnormal cells (AC) were determined in 100 well-spread metaphases per donor (totaling 500 metaphases per concentration). Gaps were not counted as CA (Mace et al. 1978). Aberrations were classified according to the International System for Human Cytogenetic Nomenclature (ISCN) (Paz-y-Miño et al. 2002). The mitotic index (MI) was also determined by scoring 3000 cells from each donor. The MI detects delaying effects of chemicals on the G2 phase of the cell cycle (Evans 1984).
To score the number of micronuclei in binucleated cells, we used the cytochalasin B-induced cytokinesis block (Fenech 2000; Kirsch-Volders et al. 2003) by adding 0.2 mL of whole blood from each donor to the culture medium and treating the cultures with 3, 6, and 12 μg/mL of copper oxychloride solution for 24 and 48 h. Again, we used negative and positive control as described above. Six μg/mL of cytochalasin B (Sigma, St. Louis, MO, USA, C6762) was added to the cultures at hour of incubation in order to block cytokinesis. After 24-hour incubation with cytochalasin B at 37 °C, the lymphocytes were initially treated with 0.4 % KCl for 2 min then harvested by centrifugation (at 1200 rpm for 10 min). The harvested lymphocytes were fixed once with a mixture of methanol:glacial acetic acid (5:1) and an equal amount of 0.9 % NaCl, and then fixed twice with only methanol:glacial acetic acid (5:1). Air dried slides were then stained with 5 % Giemsa prepared in Sorensen’s buffer (Rencuzogullari et al. 2004). For each subject, 2000 binucleated cells were scored for MN frequency and a total of 1000 viable cells for the frequency of cells with 1, 2, 3, or 4 nuclei to calculate the nuclear division index (NDI) using the formula: NDI = (M1) + (2 × M2) + (3 × M3) + (4 × M4)/N (Fenech 2000), where M1–M4 represents the number of cells with one to four micronuclei and N the total number of viable cells scored.
Statistical analysis
To determine the statistical significance for CA, AC, and MN counts we used the one-way analysis of variance (ANOVA). Correlation and regression coefficients were used to determine dose–response relationships. For all statistical analyses we used MINITAB 14 version (Minitab Ltd., UK). The P value <0.05 was considered significant.
PCR/RAPD test
To determine molecular genotoxicity of copper oxychloride in human lymphocytes with the RAPD method we used 10 oligonucleotide primers (Table 2) with variable GC base pair proportions, as described earlier by Atienzar and Jha (2006) with minor modifications.
We added peripheral blood from two healthy donors (one male and one female, both non-smokers, aged 21) to chromosome medium and incubated the culture at 37 °C for 72 h. The cultured cells were then treated with 3, 6 and 12 μg/mL concentrations of copper oxychloride solution 24 and 48 h. In addition to the negative and positive control cultures described above (untreated and EMS-treated, respectively), we used a second positive control culture treated with 2 μg/mL sodium azide (SA, Merck, Darmstadt, Germany, 106688). Ethyl methanesulfonate was used to cause frameshift mutation and SA base substitution (single-point mutation). At the end of the exposure period, the cultured cells were washed twice in sterile isotonic solution (0.9 % NaCl), and genomic DNA was isolated using the High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol (http://www.roche-applied-science.com).
Thirty-five RAPD-PCR reactions were performed with a Veriti 96-well thermal cycler (Applied Biosystems, Foster City, CA, USA) using the 10 oligonucleotide primers. PCR reaction mixtures (25 μL of total volume) consisted of the PCR buffer 1× [10 mM Tris–HCl, pH 8.8 and 50 mM KCl], 3 mM MgCl2, 2.5 U/μL of Taq DNA polymerase (Fermantas, Vilnius, Lithuania, SB38), 1.25 μM of each dNTPs (Fermantas, RO191), 4 pmol of each primer, and 50 ng of the DNA sample. PCR conditions were used as follows: an initial denaturation at 95 °C for 5 min, was followed by 35 cycles at 95 °C for 1 min, at 34 °C for 1 min and at 72 °C for 2 min, and a final extension at 72 °C for 10 min. PCR products were electrophoresed on 2 % agarose gel containing 0.5 μg/mL ethidium bromide and visualized under UV illumination. Fragment sizes were confirmed with DNA reference bands (DNA ladder, 100 bp) (Thermo Scientific, Waltham, MA, SM0321).
RAPD profiles and statistical analysis
All amplified bands in untreated control were scored as 0 and new and missing bands in treated groups were scored as 1. Bands in the treated groups that were the same as in control were scored as 0. By scoring the new and missing bands we were able to detect polymorphisms in RAPD profiles. The scores were then statistically analyzed using Student’s t test. The genomic template stability (GTS) rate was calculated using the following formula: GTS = (1 − y/z) × 100, where y is the total number of polymorphic bands in the treated groups (total number of new and missing bands) and z is the total number of bands in negative control, as described by Aras et al. (2011). Changes in RAPD profiles decreased the GTS rate, which indicated to genotoxic effects.
Results
Treatment with copper oxychloride significantly increased the percentage of CA and AC in human lymphocytes at all concentrations of all treatment periods when compared to negative control (Table 3). The effect was dose-dependent for CA in both 24 and 48-hour exposure (r = 0.998, P = 0.042 and r = 0.997, P = 0.048, respectively) and for AC in 24-hour exposure (r = 0.999, P = 0.020) (Fig. 1).
Copper oxychloride also significantly increased the MN frequency and the percentage of micronucleated binuclear cells (MNBN) at all concentrations of all treatment periods when compared to negative control (Table 4). However, the effect was not dose-dependent (Fig. 2).
Copper oxychloride decreased the MI at only the highest concentration (12 µg/mL) and decreased the NDI at the two highest concentrations (6 and 12 µg/mL) in both exposure periods (Table 5). Again, the effect was not dose-dependent for either index (Fig. 3).
The significant increase in total polymorphic bands, observed for all concentrations and treatment periods, was also not dose-dependent (Table 6). The GTS rate also significantly increased in all concentrations and treatment periods but not in a dose-dependent manner (Table 7; Fig. 4).
Discussion
As our study was the first to investigate the genotoxic and cytotoxic effects of copper oxychloride in human lymphocytes, we were not able to compare our findings with similar studies. However, Pirtskhelani et al. (2008) reported a strong genotoxic effect of copper oxychloride in mice and Shivanandappa et al. (1983) reported testicular atrophy in Gallus domesticus fed with acute doses of copper oxychloride. In addition, copper oxychloride caused infertility in some simple animals by decreasing the number of oocytes (Helling et al. 2000; Snyman et al. 2004, 2009). As for studies in humans, several have confirmed the genotoxic and mutagenic effects of copper oxide nanoparticles, which are used in a variety of industries (Ahamed et al. 2010; Wang et al. 2012; Alarifi et al. 2013; Di Bucchianico et al., 2013; Akhtar et al. 2013).
The use of pesticides containing heavy metals such as copper may lead to their transfer to humans through the food chain (Chen et al. 1997; Masaka and Muunganirwa 2007; La Pera et al. 2008; Pose et al. 2009). Waheed et al. (2013) reported that high accumulation of Cu in various organs of herbivorous and carnivorous edible fish may have increased the risk of metal toxicity in humans. Our tested formulation contained other heavy metals such as cadmium, iron, and lead, but in such trace amounts (0.1, 0.01 and 10 ppm, respectively) that they could not affect our findings.
In our study, all copper oxychloride concentrations increased the frequency of chromatid and chromosome breaks and the number polymorphisms and decreased cell proliferation. The resulting DNA damage most probably inhibited DNA replication and cell proliferation (Vock et al. 1998; Kirkland and Muller 2000). On the other hand, the high frequency of chromosome aberrations may have triggered mitotic selection and pressured cell proliferation (Madle et al. 1993; Vock et al. 1998; Galloway et al. 1998; Hillard et al. 1998; Armstrong et al. 1992; Kirkland and Muller 2000; Kocaman et al. 2014). The cell death might have arisen from the oxidative stress and the high genotoxic effects that caused to apoptosis and necrosis which is the main mechanism of the cytotoxicity (Bakkali et al. 2008; Kocaman et al. 2011).
Copper (Cu) is essential for many biological systems as a cofactor of enzymes including cytochrome C oxidase, tyrosinase, dopamine-β-hydroxylase, superoxide dismutase (Suzuki et al. 2002; El-Gendy et al. 2009) and it has a very important role in hemoglobin synthesis involved in Fe metabolisms (WHO 1974). The estimated daily requirement of copper is 2 mg for adults because the copper intoxication may cause to hemolytic anemia (WHO 1974). On the contrary, the exceeding intake amount of Cu accumulated in liver and other organs and caused several diseases in humans such as chronic renal and liver disfunctions (Gunay et al. 2006; Hloch and Charvát 2012). The same results were reported for animals (Hurwitz et al. 2014; Ciji and Bijoy Nandan 2014). Woimant and Trocello (2014) divided the Cu disorders into two classes, in which one of them was genetically and related to inherited Cu transport disorders. The other ones are Cu disorders, which are associated with Cu deficiency or exceed. The mutant ATP7B gene caused Cu accumulation in tissue resulting in clinical disorders known as Wilson disease (Grazyna et al. 2014). The high concentrations of Cu led to oxidative stress and toxicity in all living beings (Gupta et al. 1996; Giller et al. 1998; Srivastava et al. 2006; Ciji and Bijoy Nandan 2014).
Even though our study on copper oxychloride and copper oxide nanoparticles demonstrate genotoxic and cytotoxic effects, copper oxychloride is still widely used as a fungicide. About twenty thousand metric tons of copper oxychloride were used in Turkey alone in 2013 (https://www.zauba.com). The most serious risk is that the copper oxychloride is still not classified as a mutagen or potential carcinogen for humans. Our short-term in vitro exposure has clearly shown the genotoxic effect of copper oxychloride, but for a better understanding of its genotoxicity long-term carcinogenic studies and in vivo animal genotoxicity studies are needed.
Conclusion
Our in vitro study has confirmed that copper oxychloride is genotoxic and cytotoxic to human lymphocytes. Therefore, it represents a certain risk for the environment and humans, and future studies should investigate that risk, especially via the food chain. In the meanwhile, caution is advised when using copper oxychloride as a fungicide for the sake of our and the next generations’ health.
References
Ahamed M, Siddiqui MA, Akhtar MJ, Ahmad I, Pant AB, Alhadlaq HA (2010) Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem Biophys Res Commun 396:578–583
Akhtar MJ, Kumar S, Alhadlaq SA, Alrokayan SA, Abu-Salah KM, Ahamed M (2013) Dose-dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells. Toxicol Ind Health. doi:10.1177/0748233713511512
Alarifi S, Ali D, Verma A, Alakhtani S, Ali BA (2013) Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int J Toxicol 32:296–307
Al-Assiuty AN, Khalil MA, Ismail AW, van Straalen NM, Ageba MF (2014) Effects of fungicides and biofungicides on population density and community structure of soil oribatid mites. Sci Total Environ 466–467:412–420
Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DEG, Tice R, Waters MD, Aitio A (2000) IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res 463:111–172
Aras S, Beyaztas T, Cansaran-Duman D, Gokce-Gunduzer E (2011) Evaluation of genotoxicity of Pseudevernia furfuracea (L.) Zopf by RAPD analysis. Genet Mol Res 10:3760–3770
Armstrong MJ, Bean CL, Galloway SM (1992) A quantitative assessment of cytotoxicity associated with chromosomal aberration detection in Chinese hamster ovary cells. Mutat Res 265:45–60
Atienzar FA, Jha AN (2006) The random amplified polymorphic DNA (RAPD) assay and related techniques applied to genotoxicity and carcinogenesis studies: a critical review. Mutat Res 613:76–102
Atienzar FA, Cheung VV, Jha AN, Depledge MH (2001) Fitness parameters and DNA effects are sensitive indicators of copper-induced toxicity in Daphnia magna. Toxicol Sci 59:241–250
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475
Buyukleyla M, Rencuzogullari E (2009) The effects of thymol on sister chromatid exchange, chromosome aberration and micronucleus in human lymphocytes. Ecotoxicol Environ Saf 72:943–947
Chen TB, Wong JWC, Zhou HY, Wong MH (1997) Assessment of trace metal distribution and contamination in surface soils of Hong Kong. J Environ Pollut 96:61–68
Ciji PP, Bijoy Nandan S (2014) Toxicity of copper and zinc to Puntius parrah (Day, 1865). Mar Environ Res 93:38–46
Di Bucchianico S, Fabbrizi MR, Misra SK, Valsami-Jones E, Berhanu D, Reip P, Bergamaschi E, Migliore L (2013) Multiple cytotoxic and genotoxic effects induced in vitro by differently shaped copper oxide nanomaterials. Mutagenesis 28:287–299
El-Gendy KS, Radwan MA, Gad AF (2009) In vivo evaluation of oxidative stress biomarkers in the land snail, Theba pisana exposed to copper-based pesticides. Chemosphere 77:339–344
Evans HJ (1984) Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. In: Kilbey BJ, Legator M, Nichols W, Ramel C (eds) Handbook of mutagenicity test procedures, 2nd edn. Elsevier Science Publishers BV, Amsterdam, pp 405–427
Fenech M (2000) The in vitro micronucleus technique. Mutat Res 455:81–95
Galloway SM, Miller JE, Armstrong MJ, Bean CL, Skopek TR, Nichols WW (1998) DNA synthesis inhibition as an indirect mechanism of chromosome aberrations: comparison of DNA reactive and non-DNA-reactive clastogens. Mutat Res 400:169–186
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30:1389–1414
Grażyna G, Agata K, Adam P, Tomasz L, Agata WC, Karolina D, Grzegorz C, Anna C (2014) Treatment with D-penicillamine or zinc sulphate affects copper metabolism and improves but not normalizes antioxidant capacity parameters in Wilson disease. Biometals 27:207–215
Gunay N, Yildirim C, Karcioglu O, Gunay NE, Yilmaz M, Usalan C, Kose A, Togun I (2006) A series of patients in the emergency department diagnosed with copper poisoning: recognition equals treatment. Tohoku J Exp Med 209:243–248
Gupta M, Sarin NB (2009) Heavy metal induced DNA changes in aquatic macrophytes: random amplified polymorphic DNA analysis and identification of sequence characterized amplified region marker. J Environ Sci 21:686–690
Gupta M, Sinha S, Chandra P (1996) Copper-induced toxicity in aquatic macrophyte, Hydrilla verticillata: effect of pH. Ecotoxicology 5:23–33
Helling B, Reinecke SA, Reinecke AJ (2000) Effects of the fungicide copper oxychloride on the growth and reproduction of Eisenia fetida (Oligochaeta). Ecotoxicol Environ Saf 46:108–116
Hillard CA, Armstrong MJ, Bradt CI, Hill RB, Greenwood SK, Galloway SM (1998) Chromosome aberrations in vitro related to cytotoxicity of non-mutagenic chemicals and metabolic poisons. Environ Mol Mutagen 31:316–326
Hloch O, Charvát J (2012) Acute copper poisoning by suicidal attempt. Vnitr Lek 58:25–328
Hurwitz BM, Center SA, Randolph JF, McDonough SP, Warner KL, Hazelwood KS, Chiapella AM, Mazzei MJ, Leavey K, Acquaviva AE, Lindsay MM, Sanders L, Pintar J (2014) Presumed primary and secondary hepatic copper accumulation in cats. J Am Vet Med Assoc 244:68–77
Kirkland DJ, Muller L (2000) Interpretation of the biological relevance of genotoxicity test results: the importance of thresholds. Mutat Res 464:137–147
Kirsch-Volders M, Sofuni T, Aardema M, Albertini S, Eastmond D, Fenech M, Ishidate MJ, Kirchner S, Lorge E, Morita T, Norppa H, Surrallés J, Vanhauwaert A, Wakata A (2003) Report from the in vitro micronucleus assay working group. Mutat Res 540:153–163
Kocaman AY, Topaktaş M (2010) Genotoxic effects of a particular mixture of acetamiprid and alpha-cypermethrin on chromosome aberration, sister chromatid exchange, and micronucleus formation in human peripheral blood lymphocytes. Environ Toxicol 25:157–168
Kocaman AY, Rencüzoğulları E, Topaktaş M, Istifli ES, Büyükleyla M (2011) The effects of 4-thujanol on chromosome aberrations, sister chromatid exchanges and micronucleus in human peripheral blood lymphocytes. Cytotechnology 63:493–502
Kocaman AY, Rencuzogullari E, Topaktas M (2014) In vitro investigation of the genotoxic and cytotoxic effects of thiacloprid in cultured human peripheral blood lymphocytes. Environ Toxicol 29:631–641
La Pera L, Dugo G, Rando R, Di Bella G, Maisano R, Salvo F (2008) Statistical study of the influence of fungicide treatments (mancozeb, zoxamide and copper oxychloride) on heavy metal concentrations in Sicilian red wine. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25:302–313
Mace MLJ, Daskal Y, Wray W (1978) Scanning-electron microscopy of chromosome aberrations. Mutat Res 52:199–206
Madle S, Beek B, Nowak C (1993) Zum Verständnis von chromosomenmutationstests an somazellen. [The understanding of chromosome and somatic cell mutation test]. In: Fahrig R (ed) Mutationsforschung und genetische toxikologie [Mutation research and genetic toxicology]. Wissenschaftliche Buchgesellschaft, Darmstadt, pp 224–242
Masaka J, Muunganirwa M (2007) The effects of copper oxy chloride waste contamination on selected soil biochemical properties at disposal site. Sci Total Environ 387:228–236
Matache ML, Marin C, Rozylowicz L, Tudorache A (2013) Plants accumulating heavy metals in the Danube river wetlands. J Environ Health Sci Eng 11:39
Norppa H, Falck GC (2003) What do human micronuclei contain? Mutagen 18:221–233
Paz-y-Miño C, Bustamante G, Sánchez ME, Leone PE (2002) Cytogenetic monitoring in a population occupationally exposed to pesticides in Ecuador. Environ Health Perspect 110:1077–1080
Pérez-Rodríguez P, Paradelo M, Rodríguez-Salgado I, Fernández-Calviño D, López-Periago JE (2013) Modeling the influence of raindrop size on the wash-off losses of copper-based fungicides sprayed on potato (Solanum tuberosum L.) leaves. J Environ Sci Health B 48:737–746
Pirtskhelani AG, Pirtskhelani NA, Gakhokidze RA, Bichikashvili NV, Kalandiia EA (2008) The influence of polyvitamin complex polijen on mutagenic and cytotoxic effect of copper oxychloride in white mice. Georgian Med News 159:44–47
Pose E, Rial-Otero R, Paradelo M, López-Periago JE (2009) Influence of soil characteristics on copper sorption from a copper oxychloride fungicide. J Agric Food Chem 57:2843–2848
Rencuzogullari E, Ila HB, Kayraldiz A, Arslan M, Diler SB, Topaktas M (2004) The genotoxic effect of the new acaricide etoxazole. Genetika 40:1571–1575
Sevindik N, Rencuzogullari E (2014) The genotoxic and antigenotoxic effects of Salvia fruticosa leaf extract in human blood lymphocytes. Drug Chem Toxicol 37:295–302
Shivanandappa T, Krishnakumari MK, Majumder SK (1983) Testicular atrophy in Gallus domesticus fed acute doses of copper fungicides. Poult Sci 62:405–408
Snyman RG, Reinecke AJ, Reinecke SA (2004) Changes in oocyte numbers in the ovotestis of Helix aspersa, after experimental exposure to the fungicide copper oxychloride. Bull Environ Contam Toxicol 73:398–403
Snyman RG, Reinecke AJ, Reinecke SA (2009) Quantitative changes in digestive gland cells and oocytes of Helix aspersa, as biomarkers of copper oxychloride exposure under field conditions. Bull Environ Contam Toxicol 83:19–22
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Gupta DK (2006) Copper-induced oxidative stress and responses of antioxidants and phytochelatins in Hydrilla verticillata (L.f.) Royle. Aquat Toxicol 80:405–415
Suzuki KT, Someya A, Komada Y, Ogra Y (2002) Roles of metallothionein in copper homeostasis: responses to Cu-deficient diets in mice. J Inorg Biochem 88:173–182
Vock EH, Lutz WK, Hormes P, Hoffmann HD, Vamvakas S (1998) Discrimination between genotoxicity and cytotoxicity in the induction of DNA double-strand breaks in cells treated with etoposide, melphalan, cisplatin, potassium cyanide, Triton X-100 and c-irradiation. Mutat Res 413:83–94
Waheed S, Kamal A, Malik RN (2013) Human health risk from organ-specific accumulation of toxic metals and response of antioxidants in edible fish species from Chenab River, Pakistan. Environ Sci Pollut Res Int 21:4409–4417. doi:10.1007/s11356-013-2385-311
Wang Z, Li N, Zhao J, White JC, Qu P, Xing B (2012) CuO nanoparticle interaction with human epithelial cells: cellular uptake, location, export, and genotoxicity. Chem Res Toxicol 25:1512–1521
WHO (1974) Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. Seventeenth report of the Joint FAO/WHO Expert Committee on Food Additives, World Health Organization technical rep ser no. 539; FAO Nutrition Meetings Report Series, 53
Woimant F, Trocello JM (2014) Disorders of heavy metals. Handb Clin Neurol 120:851–864
Acknowledgments
This study was supported by the Adiyaman University Research Fund (Grant No. FEFBAP/2013-0001). The authors wish to thank Dr. Muhsin Aydin and Mr. Dado Čakalo for language advice and editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declaration of interest.
Rights and permissions
About this article
Cite this article
Bayram, S., Genc, A., Buyukleyla, M. et al. Genotoxicity and cytotoxicity of copper oxychloride in cultured human lymphocytes using cytogenetic and molecular tests. Cytotechnology 68, 2027–2036 (2016). https://doi.org/10.1007/s10616-016-9943-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-016-9943-8