Abstract
Background
In case of recurrence or persistent palmar hyperhidrosis, a sympathetic chain resection is suggested, however, many surgeons are still reluctant to offer further intervention because of the inability to predict the efficacy of such a procedure. We analyzed our large series of resympathectomy.
Methods
Substantive retrospective analysis of 39 patients underwent a resympathectomy (minimally invasive bilaterally sympathetic chain Th2-3 resection). Patients referred from other hospitals or primarily operated at our institution for recurrence or persistence palmar hyperhidrosis were included in the study group.
Results
No intraoperative complications were detected. Reoperation or chest tube positioning was necessary in 2 patients. Twenty-eight patients had a positive response (excellent or good results). Seven patients described a substantial, but not sufficient, reduction of the symptomatology. Four patients were very unsatisfied and regretted the operation.
Conclusions
Resympathectomy is highly effective procedure for patients who have persistent or recurrent symptoms. However, the indication of the operations should be more dissuasive as possible to avoid the risk of any undesirable psychologically side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary palmar hyperhidrosis (PH) affects approximately 2.8% of the population [1]. Video thoracoscopic sympathectomy/sympathicotomy (VATS-S.) is the first surgical choice in case of primary palmar hyperhidrosis refractory to medical therapy and has a high success rate [2]. Despite this high success rate, several patients are unresponsive and eventually require new surgery. In cases where a resympathectomy (RE-VATS) is needed, a sympathetic chain resection is suggested; however, many surgeons are still reluctant to offer further intervention to these patients because of a lack of experience with reoperative sympathectomy, the inability to predict the efficacy of such a procedure, or the lack of desire to treat a patient whose expectations have already not been met by the care of what is often another physician [3]. Several studies have previously analyzed RE-VATS [4,5,6,7,8,9,10] but none of the studies has objectively evaluated the degree of response to surgery or the improvement in quality of life. Moreover, in the literature, there are no standardized suggested procedure or therapeutic protocols in case of persistent or recurrence PH.
The aim of this study was to analyze our experience with RE-VATS in patients with persistent or recurrence palmar hyperhidrosis after various forms of sympathectomy/sympathicotomy. We assessed treatment response, quality-of-life improvement, technical aspects and rapprochement complications.
Materials and methods
Study design and population
A retrospective analysis of all patients undergoing bilateral RE-VATS for recurrence or persistent hyperhidrosis after VATS-S in our practice from 1 January 2005 to 31 December 2020 was performed. Patients referred from other hospitals or primary operated at our institution for recurrence or persistence PH were included in the study group.
Our study was performed according to the Vorarlberg Ethics Commission, Austria (only prospective studies need a IRB number) and, since it is a retrospective study, no written patients’ informed consent was required.
Educational preoperative management
All patients selected for this study underwent a standard multiphase education management to identify the patients with a real need of reoperation.
The three steps of the informed consent are as follow:
-
1.
The first phase of detailed description of operational techniques, minor and major complications and particularly the compensatory hyperhidrosis with the above-executed inconsistencies in the literature.
-
2.
The second patient’s reflection phase with a “free interval” of 10 days. Forensically traceable, this period of time can be used by the patient to exhaust all available sources, including the above-mentioned web sites and social media on the subject of undesirable side effects and complications, to compare the subjective preoperative distress with possible side effects. This is in analogy or alternative to the proposed drug-based sympathetic test blocks.
-
3.
The third final discussion and if the patient’s request persists, appointment for the operation.
Analyzed variables were
-
Demographics: age and sex, persistent or recurrence, method of previous VATS-S.
-
Clinical details: the degree of symptoms of each patient as well as evidence of significant compensatory sweating was assessed using the Hyperhidrosis Disease Severity Scale, respectively [1]. For the purposes of this investigation, significant compensatory sweating, requiring a RE-VATS was defined as level 3 or 4 of the Severity Scale.
-
Surgical data: extent of resection, duration (median min. –range-) and side of the operation, number and position of trocars (5 mm), require of the histology, number of pleura drainage.
-
Early postoperative complications: postoperative complications were defined as any complication occurring during hospitalization or within the first 30 postoperative days. Postoperative complications were divided into surgical and medical events and classified according to Clavien–Dindo criteria [11]. Surgical complications were further defined as: Pneumothorax requiring drainage and/or reoperation, bleeding requiring blood transfusion and/or reoperation and surgical site infection. Medical complications were defined as cardiovascular and pulmonary (pneumonia), events requiring reoperation, intensive care unit admission, or hospitalization longer than 15 days were defined as major complications (Clavien–Dindo > grade III).
-
Postoperative results: for treatment response analysis, we evaluated the degree of improvement after 1 day of surgery using a questionnaire recommended by the Society of Thoracic Surgery for the treatment of hyperhidrosis was applied [12, 13]. This questionnaire, distributed over 4 domains (functional, social, personal, emotional, and special circumstances), evaluates quality of results on a modified 4-point Likert scale ranging from 1 (excellent) to 4 (very bad). Postoperative pain was evaluated according to the VAS-Score and patients were classified in three postoperative pain levels: minor pain [1,2,3], moderate pain [4,5,6] and severe pain [7,8,9,10]
Statistical methods
Data are presented as median or average ± standard error for continuous variables and number (percentage) for categorical variables. Software used: data entered into Excel file, exported as .cvs. Survival and multivariate analyses were performed using SPSS version 24. All other analyses were performed using Python and Pandas software (Anaconda Inc., Berlin, Germany). Demographic information, procedural details and morbidities were extracted from inpatient and outpatient records.
Technique
General anesthesia was utilized for all subjects in this investigation. Neither central venous access nor intra-arterial blood pressure monitoring was performed. Throughout the procedure, patients were ventilated with 100% oxygen and were anesthetized with propofol (Diprivan). Peripheral arterial hemoglobin saturation (SaO2) was monitored with a pulse oximeter, with palmar temperature monitoring. A double-lumen endotracheal tube was utilized.
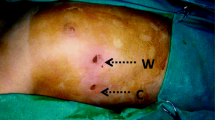
All patients were positioned in a semi-sitting position with the arms elevated above the head. The neck, chest, and abdomen were included in the operative field. First, a 0.5-cm trocar (camera-trocar) was placed in the third intercostal, along the anterior axillary line and just posterior to the pectoralis major muscle (or along the previous surgical incision). The ipsilateral endotracheal tube was clamped by the anesthesiologist to deflate the operative lung, then the pleural cavity was entered using mosquito forceps to avoid damaging the lung parenchyma. A 5-mm, 0° thoracoscope (Karl Storz Co, Tuttlingen, Germany) was introduced into the pleural cavity through an obtuse head trocar. Subsequently, a second 5-mm operative-trocar was placed in the fourth intercostal, along the anterior axillary line. Sometimes, creation of a third 5-mm incision in the third intercostal space along the median axillary line was helpful to facilitate lysis of adhesions (Fig. 1). When the temperature of both hands had elevated 0.5–1 °C, sympathectomy was considered adequate.
Figure shows where thoracoscopic trocars are place. A 5-mm, 0° thoracoscope is introduced into the pleural cavity through the lower trocar placed at the fourth intercostal space to permit a wider intrathoracic view during the procedure. Following operative trocars are placed at the third intercostal space
The sympathetic chain was identified at the level of the crossing of the second, third, and fourth costal heads. The parietal pleura was opened and midrib oriented the sympathetic chain was transected using a LigaSure™ Maryland Jaw Open and Laparoscopic Sealer/Divider with Nano-coating (Medtronic). The incision was extended laterally for approximately 2 cm on the second rib to include any accessory nerve fibers from the ganglion to the brachial plexus (the nerve of Kuntz). The procedure was performed bilaterally and we resected the sympathetic chain on the second, third, and fourth rib (Th2-4) (Fig. 2). All patients underwent a chest X-ray postoperatively, and in the absence of complications, they were discharged the day after surgery. The criteria for discharge from inpatient treatment were: freedom from pain and symptoms, irritation-free trocar incisions, postoperative residual pneumothorax that tended to regress radiologically, and eupnea when climbing stairs.
Level of Th 2–4 sympathectomy: the sympathetic chain resection is performed from the lower limit of the stellate ganglion to the upper limit of the fourth ganglion. In the case of recurrence or persistence hyperhidrosis, the sympathetic chain resection with a consequent resection of lateral nerve branches is mandatory
Results
From 1 January 2005 to 31 December 2020, 39 patients underwent bilateral RE-VATS Th2-4 for recurrent or persistent primary palmar hyperhidrosis. Regarding gender, 25 (64.1%) patients were male. The median age was 34.6 (range 18–70) years and the mean body mass index (BMI) was 21.1 (range 19–32). Thirty-seven patients had major complaints about their hands (bilateral symptoms) or forearms before operation (intolerable and always interferes sweating: 79.4%). Two patients reported tolerable unilateral disturbs. In 20 cases, symptoms were bilateral. Twenty-nine patients were referred secondary to our institution due to hyperhidrosis after a first unsuccessfully operation or a long-term recurrence. Mean time from initial to RE-VATS was 21 months and did not differ significantly between patients undergoing an initial clipping or transection sympathectomy. Previous methods of sympathectomy/sympathicotomy and demographic data are displayed in Table 1. In all the patients, the bilateral resection of ganglions Th 2–4 was performed. Median operative time was 56 min (range 29–84) and only one patient required a third incision due to the presence of intrathoracic adhesions. In two patients, due to a parenchymal lesion occurring during the lysis of the adhesion, placed chest tube was necessary. A conversion to a thoracotomy was not necessary in any of the cases. Median duration of the hospitalization was 3 days (range 2–4).
Table 2 resumes postoperative results and complications. A high degree of response to RE-VATS occurred in 75% of patients. Twenty-eight patients having a positive response (excellent or good results). In 17 of these patients, the improvement in quality of life was considered excellent. Seventeen patients reported “not satisfied” results and complained about residual sweating also after the RE-VATS. However, six of these seven patients described a substantial, but not sufficient, reduction of the symptomatology. Four patients were very unsatisfied and regretted the operation. In terms of pain, 77.7% of the patients reported a minor or moderate pain level at the second postoperative day. No patient required intravenous pain relief therapy at the third postoperative day. The greatest number of complications were classified as Clavien–Dindo. A reoperation or a chest tube positioning was necessary in 2 patients due to a postoperative hemothorax, occurred 12 h after surgery, and a worsening pneumothorax at the second postoperative day.
Discussion
The extent of primary hyperhidrosis is exclusively defined by the patient’s subjective level of suffering. Sudorimetric tests provide information on the distribution and function of the sweat glands. Electro-neurophysiological tests detect the sympathetic nervous activity and the index formats are intended to objectify the quality of life before and after surgery.
Thus, there is no preoperative objective diagnostic tools to diagnose whether an operation is worth trying or even necessary. There are only arbitrarily fixed reference ranges, such as the S1 guidelines from the “Deutsche Dermatologische Gesellschaft” [12,13,14,15].
Neither is there an objective measurement option to identify particularly predestined risk patients for postoperative early nor later compensatory hyperhidrosis and, if necessary, to exclude them from the operation. Without a uniform topographical identification in the publications, a wide variety of technical and operative modifications to avoid this undesirable side effect with regard to height and extent are controversially discussed [13, 16,17,18,19,20,21,22,23,24]. Bilateral video-thoracoscopic sympathicotomy Th 2–4 seems to be the most used technique and it shows good results in term or recurrences rate and intraoperative/postoperative complications [13]. However, the actual coexistence of different surgical techniques shows that the ideal method, in case of primary hyperhidrosis does not seem to have been found yet. Therefore, also in case of recurrence or persistent hyperhidrosis, the identification of the standard technical approach and technique is still debated.
Although VATS-S is an easy, safe, and effective procedure, the immediate failure vary between 2 and 7%. Moreover, recurrences are more commonly reported in the literature and the incidence varies with the anatomic location of primary hyperhidrosis. Rates of recurrence vary from non-existing up to 5–8% [2, 4, 12, 18, 19].
Many explanations for these failures have been given. The definition of primary technical failure is quite obvious when it is unilateral [25].
In case of bilateral VATS-S, many factors are advocated as the causes or immediately or later recurrences of PH: inadequate interruption of the sympathetic chain (transection or resection) [7, 25], anatomic variations of the sympathetic chain [4, 7], and nerve regeneration [4, 26, 27]. Moreover, many authors reported a higher incidence of recurrence in case of inadequate primary surgery because of severe pleura adhesions [28]. Last but not least, psychological causes can trigger a relapse of hyperhidrosis [29, 30].
Inadequate interruption may occur if simple transection of the sympathetic chain (sympathicotomy) is partial or incomplete. Higher recurrence rates are reported after clipping of the chain or ablation [12, 13, 23,24,25,26]. In theory, failure is less likely to occur after sympathectomy where the sympathetic chain is resected [10, 25]. Therefore, especially in case of recurrence, sympathectomy and histologic analysis is mandatory.
Anatomic variation of the sympathetic chain are reported in the literature. Many authors speculate on the existence of accessory nerve fibers or nerve of Kuntz. These accessory fibers could be responsible of a compensatory hyperhidrosis after the interruption of the main nerve way [25, 26].
A third explanation of failure exists in patients who were clipped if the clip was not tight enough to interfere with transmission of nerve impulses in the sympathetic chain and the nerves may recreate a normal conduction due to a nerve regeneration phenomenon [31].
In theory, it may also be argued that our higher failure rate could result from the use of single lumen intubation or the presence of severe pleura adhesion because of some detrimental effect on visualization [25].
Regarding the psychological risk factors, a recent controlled randomized study from 2018 shows a clear benefit for those patients who had to receive psychotropic medication due to psycho-social neurovegetative symptoms, with a substantial reduction in this after sympathectomy [30, 31]. Therefore, we could presume that psychologically risk factors may trigger or promote an early or later relapse of the symptoms. In addition, the World Wide Web is filled with descriptions of personal suffering, sometimes with highly emotional participation and choice of words. There are also appearances of more or less competent support groups as well as approaches drifting towards alternative medicine.
At the end, many reason are advocated as the causes of the persistent or recurrence PH after VATS-S. However, some authors suggest that the incidence of primary failure could have been reduced if we had measured changes in palmar temperature or blood flow believed to confirm complete interruption of the sympathetic chain [32, 33].
Despite these persistent or recurrence risks, the one-time bilateral clipping of the trunk below Th 3 using two trocars for hyperhidrosis of the upper extremity is generally considered to be the first surgical choice [13]. In the event of persistent or recurrent symptoms, following changing of the surgical indication and technical procedure are encouraged:
-
1.
Before the surgery, the surgeon should assess whether all conservative therapeutic approaches have been consistently exhausted, as well as the exclusion of any primary diagnoses or any organic contraindications.
-
2.
Suitable time interval between the first and the second surgeries is suggested to better select patients and to determinate the real severity of the persistent or relapse symptoms (multiphase education management).
-
3.
Sympathectomy Th 2–4 with consequent histologic analysis is mandatory. Histologic analysis confirms the definitive resection of the sympathetic chain and it allows the patient to be reassured that radical resection has taken place.
-
4.
Double lumen intubation and third trocar is suggested to avoid detrimental effect on the visualization with a consequent high risk of non-adequate surgery, especially in case of severe adhesions.
The result of this extensive preoperative assessment are that 2 out of 5 patients who came to our outpatient department for initial consultation chose to continue the conservative treatment to surgery for the moment.
Furthermore, we emphasize that the standard application of the concept, mentioned above, allowed the achievement of good postoperative results in terms of secondary compensatory hyperhidrosis. No data are available in the literature regarding secondary persistent or recurrence hyperhidrosis. During the first years of our experience, we scheduled everyone for telephonic follow-up after 1 month, and we lost just one patient during the follow-up. In our series, 27% of the patients reported not satisfactory results.
Our study reports that the effectiveness of RE-VATS remained high, while morbidity and length of stay were acceptable. Compensatory sweating, however, was significantly more common in our group as compared with the patients who underwent VATS-S for primary PH.
It remains unclear why patients undergoing RE-VATS have a higher risk of second relapse or persistence as compared with patients after VATS-S for primary PH. However, these patients also developed recurrent symptoms that were less severe than originally, and consequently, did not require a second re-intervention.
Although this investigation reports a large group of patients in the literature undergoing RE-VATS after various initial procedures, some weaknesses do exist in its design. As in any unusual condition, the absolute number of patients in this study remains small. Moreover, multicenter and randomized studies to determinate the gold-standard therapy are required.
Conclusion
In conclusion, despite widely disparate techniques of initial sympathectomy/sympathicotomy for primary palmar hyperhidrosis, RE-VATS is highly effective for patients who have persistent or recurrent symptoms. In the case of neurovegetative indications, information should be as dissuasive as possible for personal protection. This, in conjunction with its documented decision interval, does not necessarily protect against expressed dissatisfaction with the surgical result or any undesirable psychologically side effects, but represents a hard forensic fact in the case of expert or even legal steps on the part of the operating person.
Availability of data and material
All data are freely available for review.
Code availability
Not applicable.
References
Strutton DR, Kowalski JW, Glaser DA, Stang PEJ. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. Am Acad Dermatol. 2004;51:241–8.
Ibrahim M, Menna C, Andreetti C, Ciccone AM, D’Andrilli A, Maurizi G. Bilateral single-port sympathectomy: long-term results and quality of life. Biomed Res Int. 2013;2013:348017.
Freeman RK, Van Woerkom JM, Vyverberg A, Ascioti AJ. Reoperative endoscopic sympathectomy for persistent or recurrent palmar hyperhidrosis. Ann Thorac Surg. 2009;88(2):412–6 (discussion 416–7).
Lin TS. Video-assisted thoracoscopic ‘resympathicotomy’ for palmar hyperhidrosis: analysis of 42 cases. Ann Thorac Surg. 2001;72:895–8.
Lin TS, Fang HY, Wu CY. Repeat transthoracic endoscopic sympathectomy for palmar and axillary hyperhidrosis. Surg Endosc. 2014;14:134–6.
Kim DH, Paik HC, Lee DY. Video assisted thoracoscopic re-sympathetic surgery in the treatment of re-sweating hyperhidrosis. Eur J Cardiothorac Surg. 2005;27:741–4.
Hsu CP, Chen CY, Hsia JY, Shai SE. Resympathectomy for palmar and axillary hyperhidrosis. Br J Surg. 1998;85:1504–5.
Amir M, Arish A, Weinstein Y, Pfeffer M, Levy Y. Impairment in quality of life among patients seeking surgery for hyperhidrosis (excessive sweating): preliminary results. Isr J Psychiatry Relat Sci. 2000;37:25–31.
Lin T-S. Video-Assisted Thoracoscopic “Resympathicotomy” for Palmar Hyperhidrosis: Analysis of 42 Cases. Ann Thorac Surg. 2001;72(3):895–8.
de Campos JRM, Lembrança L, Fukuda JM, Kauffman P, Teivelis MP, Puech-Leão P, Wolosker N. Evaluation of patients who underwent resympathectomy for treatment of primary hyperhidrosis. Interact Cardiovasc Thorac Surg. 2017;25(5):716–9.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
de Campos JR, Kauffman P, Werebe EC, Kusniek S, Wolosker N, Biscegli JF. Quality of life, before and after thoracic sympathectomy: report on 378 operated patients. Ann Thorac Surg. 2003;76:886e91.
Cerfolio RJ, De Campos JRM, Bryant AS, Connery CP, Miller DL, DeCamp MM, McKenna RJ, Krasna MJ. The society of thoracic surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg. 2011;91:1642–8.
Finlay AY, Khan GK. The Dermatology Life Quality Index: A simple practical measure for routine clinical use. British Association of Dermatologists Annual Meeting, Oxford, July 1993. Br J Dermatol. 1993;129(Suppl 42):27.
Rzany B., Bechara F.G., Feise K., Heckmann M., Rapprich S., Wörle B.. Aktualisierung der S1 Leitlinie zur Definition und Therapie der primären Hyperhidrose AWMF-Register Nr. 013/059 Klasse: S1 Aktualisierung Stand: 01.11.2017 Freigabe durch die Deutsche Dermatologische Gesellschaft (DDG): 27.11.2017.
Fibla JJ, Molins L. Sympathetic disorders of the upper limbs. In: ESTS Textbook of Thoracic Surgery, Vol. 2, 2015, pp. 35–43. Kuzdzal J. (editor.) Asamura H, Detterbeck F., Godstraw P., Lerut A., Thomas P., Treasure T. (associate editors)
Soares TJ, Dias PG, Sampaio SM. Impact of Video-Assisted Thoracoscopic Sympathectomy and Related Complications on Quality of Life According to the Level of Sympathectomy. Ann Vasc Surg. 2020;63:63–7.
Cai S, Huang S, An J, Li Y, Weng Y, Liao H, Chen H, Liu L, He J, Zhang J. Effect of lowering or restricting sympathectomy levels on compensatory sweating. Clin Auton Res. 2014;24(3):143–9.
Cheng A, Johnsen H, Chang MY. Patient satisfaction after thoracoscopic sympathectomy for palmar hyperhidrosis: do method and level matter? Perm J. 2015;19(4):29–31.
Inan K, Goksel OS, Uçak A, Temizkan V, Karaca K, Ugur M, Arslan G, Us M, Yılmaz AT. Thoracic endoscopic surgery for hyperhidrosis: comparison of different techniques. Thorac cardiovasc Surg. 2008;56(4):210–3.
Wolosker N, Leiderman DB, de Campos JR, Kauffman P, Tedde ML, Yazbek G, Puech-Leão P. Number of preoperative hyperhidrosis sites does not affect the sympathectomy postoperative results and compensatory hyperhidrosis occurrence. Thorac Cardiovasc Surg. 2019;67(5):407–14.
Loscertales J, Congregado M, Jimenez-Merchan R, Gallardo G, Trivino A, Moreno S, Loscertales B, Galera-Ruiz H. Sympathetic chain clipping for hyperhidrosis is not a reversible procedure. Surg Endosc. 2012;26(5):1258–63.
Thomsen LL, Mikkelsen RT, Derejko M, Schrøder HD, Licht PB. Sympathetic block by metal clips may be a reversible operation. Interact Cardiovasc Thorac Surg. 2014;19(6):908–13.
Hynes CF, Yamaguchi S, Bond CD, Marshall MB. Reversal of sympathetic interruption by removal of clips. Ann Thorac Surg. 2015;99(3):1020–3.
Licht PB, Clausen A, Ladegaard L. Resympathicotomy. Ann Thorac Surg. 2010;89(4):1087–90.
Singh B, Moodley J, Ramdial PK, Ramsaroop L, Satyapal KS. Pitfalls in thoracoscopic sympathectomy: mechanisms for failure. Surg Laparosc Endosc Percutan Tech. 2001;11(6):364–7.
Singh B, Moodley J, Haffejee AA, Ramdial PK, Robbs JV, Rajaruthnam P. Resympathectomy for sympathetic regeneration. Surg Laparosc Endosc. 1998;8(4):257–60.
Lin CC, Mo LR. Experience in thoracoscopic sympathectomy for hyperhidrosis with concomitant pleura adhesion. Surg Laparosc. 1996;6:258–61.
Qian K, Feng YG, Zhou JH, Wang RW, Tan QY, Deng B. Anxiety after Sympathectomy in patients with primary palmar hyperhidrosis may prolong the duration of compensatory hyperhidrosis. J Cardiothorac Surg. 2018;13(1):54.
Wang HY, Zhu YJ, Liu J, Li LW, Liu YH. The relationship between preoperative psychological evaluation and compensatory sweating. J Cardiothorac Surg. 2018;13(1):42.
Lee DY, Paik HC, Kim DH, Kim HW. Comparative analysis of T3 selective division of rami communicantes (ramicotomy) to T3 sympathetic clipping in treatment of palmar hyperhidrosis. Clin Auton Res. 2003;13(Suppl 1):I45–7.
Crandall CG, Meyer DM, Davis SL, Dellaria SM. Palmar skin blood flow and temperature responses throughout endoscopic sympathectomy. Anesth Analg. 2005;100(1):277–83.
Sáiz-Sapena N, Vanaclocha V, Panta F, Kadri C, Torres W. Operative monitoring of hand and axillary temperature during endoscopic superior thoracic sympathectomy for the treatment of palmar hyperhidrosis. Eur J Surg. 2000;166(1):65–9.
Funding
We did not require any type of funding.
Author information
Authors and Affiliations
Contributions
PG, MH, VH, DL, and IK: data collection and writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Paolo N.C. Girotti MD, Martin Hufschmidt MD, Peter Tschann MD, Vebi Hodja MD, Daniel Lechner MD, and Ingmar Königsrainer MD have no conflicts of interest.
Ethics approval
Approved by Vorarlberg Ethics Commission, Austria.
Consent to participate
Because of retrospective study, no written informed consent was obtained according to the regulations of the local ethics commission.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Girotti, P.N.C., Hufschmidt, M., Tschann, P. et al. Thoracoscopic resympathectomy for persistent or recurrent palmar hyperhidrosis: single-center experience. Gen Thorac Cardiovasc Surg 70, 651–658 (2022). https://doi.org/10.1007/s11748-022-01788-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-022-01788-5