Abstract
Biodiesel is conventionally produced by alkaline-catalyzed transesterification, which requires high-purity oils. However, low-quality oils can be used as feedstocks for the production of biodiesel by enzyme-catalyzed reactions. The use of enzymes has several advantages, such as the absence of saponification side reactions, production of high-purity glycerol co-product, and low-cost downstream processing. In this work, biodiesel was produced from lipase-catalyzed hydrolysis of waste cooking oil (WCO) followed by esterification of the hydrolyzed WCO (HWCO). The hydrolysis of acylglycerols was carried out at 30 °C in salt-free water (WCO/water ratio of 1:4, v/v) and the esterification of HWCO was carried out at 40 °C with ethanol in a solvent-free medium (HWCO/ethanol molar ratio of 1:7). The hydrolysis and esterification steps were carried out using immobilized Thermomyces lanuginosus lipase (TLL/WCO ratio of 1:5.6, w/w) and immobilized Candida antarctica lipase B (10 wt%, CALB/HWCO) as biocatalysts, respectively. The hydrolysis of acylglycerols was almost complete after 12 h (ca. 94 %), and in the esterification step, the conversion was around 90 % after 6 h. The purified biodiesel had 91.8 wt% of fatty acid ethyl esters, 0.53 wt% of acylglycerols, 0.003 wt% of free glycerol, viscosity of 4.59 cP, and acid value of 10.88 mg KOH/g. Reuse hydrolysis and esterification assays showed that the immobilized enzymes could be recycled five times in 10-h batches, under the conditions described above. TLL was greatly inactivated under the assay conditions, whereas CALB remained fully active. The results showed that WCO is a promising feedstock for use in the production of biodiesel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increase in the petroleum price, together with environmental concerns, has encouraged the use of biodiesel (a mixture of fatty acid monoalkyl esters) as a substitute for fossil fuels. Currently, biodiesel is produced by alkaline-catalyzed transesterification. This process has high conversion and reaction rates [1–3], but requires feedstocks that are free of fatty acids and water in order to avoid the formation of soaps that reduce the overall efficiency of the process and hinder purification of the biodiesel [1, 4–6].
The costs of refined oils account for 70–80 % of the total costs of biodiesel production. The use of non-edible or waste oils can be an attractive alternative to expensive edible oils (such as canola, soybean, and corn oils), making the biodiesel production process more cost-effective [2, 3, 7, 8]. However, these feedstocks have high contents of free fatty acids (FFAs) and their processing requires an additional step for alkaline-catalyzed transesterification, in order to lower the FFA content to below 0.5 %. If this value is exceeded, soap formation makes it difficult to separate the ester from the glycerol, and there is a decrease in the ester conversion rate [5, 6]. The FFAs therefore first need to be esterified using mineral acids, and this step requires resistant equipment and produces acid wastewater [5, 8].
Alternatively, the processing of these feedstocks can be performed using enzymes (lipases) as catalysts [1, 3]. Lipases are versatile enzymes that can catalyze hydrolysis, esterification, and transesterification reactions under mild conditions. Many studies have reported the use of lipases to catalyze the transesterification of edible and non-edible oils [3]. Lipases immobilized on solid supports are more attractive from an industrial point of view, because they can be reused over several cycles, increasing the cost-effectiveness of the process [1, 3, 6]. Several immobilized lipases are commercially available, but the most frequently used in industrial processes are Novozym® 435 and Lipozyme® TL IM, both marketed by Novozymes [3, 9].
Hydroesterification in two steps (defined herein as hydrolysis of oils followed by esterification of the hydrolyzed oils, both steps catalyzed by lipases and/or chemical catalysts) has recently attracted attention for biodiesel production as it produces high-purity glycerol and biodiesel in two consecutive steps [6, 8, 10–15]. Firstly, triglycerides, diglycerides, and monoglycerides are hydrolyzed in an aqueous medium, yielding FFAs in the oil phase and glycerol in the aqueous phase. The glycerol, a byproduct with high commercial value, is recovered at a high level of purity because of the absence of alcohol (such as methanol) and salts in the aqueous phase. Hence, the glycerol recovered in this step could be used for pharmaceutical and food purposes. In the second step, FFAs are converted into fatty acid alkyl esters (biodiesel) by esterification using short-chain alcohols (traditionally, methanol and ethanol). At the end of the reaction, the excess alcohol required to displace the esterification equilibrium towards the products is recovered by evaporation, yielding high-purity biodiesel with few or no downstream operations required. To date, only a handful of studies have explored the potential of low-cost acid feedstocks for production of biodiesel by hydroesterification in two steps (Table 1). Hybrid processes have been proposed in which the oil hydrolysis and FFA esterification steps are catalyzed by enzymes and/or chemical catalysts.
Production of biodiesel from feedstocks with high FFA content can be easily achieved by acid-catalyzed reactions, since esterification of FFAs and alcoholysis of triglycerides can be simultaneously catalyzed by acids. However, acid-catalyzed alcoholysis is slow, requires high temperatures, and when methanol is used the acid can also catalyze side reactions such as methanol etherification, which hampers the purification of biodiesel. Furthermore, the acid catalyst will preferentially bind to water produced during the reaction, leading to a reversible type of catalyst deactivation [11, 16]. If strong liquid acids are used, acid-resistant materials, efficient separation and product purification, and waste treatment are required to avoid corrosion of equipment and prevent contamination problems, hence increasing production costs [16]. Solid acid catalysts can overcome these drawbacks, but studies of the production of biodiesel by esterification of FFAs using solid acid catalysts are still very limited owing to expectations of low reaction rates and unfavorable side reactions [16]. Talukder et al. [12] reported the use of waste cooking oil (WCO) for biodiesel production by hydroesterification in two steps (enzymatic hydrolysis of WCO followed by chemical esterification of FFAs). The esterification step using Amberlyst-15 (an ion-exchange resin) was performed at low temperature to avoid catalyst degradation [16], requiring a large amount of catalyst (100 wt%, relative to the FFAs), which could make the process economically unviable.
Enzymes are promising catalysts for biodiesel production by hydroesterification in two steps, since enzymes work very well under mild conditions of temperature and pressure, and particularly because lipases exhibit high activity for both hydrolysis and esterification reactions. Furthermore, hydroesterification in two steps, both catalyzed by immobilized lipases, could reduce the energy demand of the plant as well as the costs of downstream processes (biodiesel and glycerol purification, and wastewater treatment). Although good results have been obtained for the hydrolysis of acid oils in buffered medium (Table 1), it would be more advantageous to carry out the reaction in salt-free water, because hydrolysis in buffered medium contaminates the glycerol, making its purification more costly.
This work investigates the production of biodiesel by enzymatic hydroesterification of WCO in two steps. The hydrolysis of WCO was carried out in salt-free water and the esterification of FFAs was carried out in solvent-free medium, using ethanol as acyl acceptor. The improper disposal of WCO can lead to environmental problems, but following suitable treatment, its use as a fuel substitute can be profitable [2]. Ethanol was chosen for the esterification, because its solubility in oils and esters is higher than that of methanol, reducing enzyme inactivation [9], and because it is obtained from renewable biomass, making the biodiesel sustainable. The hydrolysis step was carried out at 30 °C in a salt-free aqueous medium (WCO/water ratio of 1:4, v/v) using lipase from Thermomyces lanuginosus (TLL, enzyme/WCO ratio of 1:5.6, w/w), which presents high activity in oil/water emulsion [17]. The esterification step was carried out at 40 °C using ethanol as acyl acceptor (hydrolyzed WCO/ethanol molar ratio of 1:7) and lipase from Candida antarctica type B (CALB, 10 wt %, enzyme/hydrolyzed WCO), because it is known that loss of lipase activity induced by the acyl acceptor (methanol or ethanol) is greater for TLL than for CALB. Furthermore, the CALB biocatalyst promotes quantitative conversion during the biodiesel synthesis, while TLL requires a three-stage stepwise addition of ethanol [9]. The esterification step was carried out in the presence of molecular sieves (10 wt%, molecular sieves/hydrolyzed WCO) to adsorb water and displace the esterification equilibrium towards the products (fatty acid ethyl esters). Biocatalyst reuse was evaluated under the same conditions described above, except that the time of reaction was 10 h for each batch. After each batch, the biocatalysts (as well as molecular sieves in the esterification step) were recovered by filtration and washed with tert-butyl alcohol at around 30 °C before being reused in another cycle. Waste cooking oil could be enzymatically converted to biodiesel using hydroesterification in two steps, yielding a product with 91.8 wt% of FAEEs, 0.53 wt% of glycerides (MAGs, DAGs, and TAGs), 0.12 wt% of total glycerol, 0.003 wt% of free glycerol, viscosity of 4.59 cP at 40 °C, and acid value of 10.88 mg KOH/g.
Materials and Methods
Material
Waste cooking oil was collected from a local restaurant in São Carlos (São Paulo, Brazil). Immobilized T. lanuginosus lipase (TLL IM-T2-150, 10,000 tributyrin hydrolysis units (TBU)/g at 40 °C and pH 7.5) and C. antarctica type B lipase (CALB IM-T2-350, 2500 TBU/g at 40 °C and pH 7.5) were purchased from Chiral Vision (Leiden, the Netherlands). Anhydrous ethanol (purity ≥99.7 %) was acquired from J. T. Baker (New Jersey, USA). UOP 3 Å molecular sieves (rod-shaped, size 1/16 in) were acquired from Sigma-Aldrich Co. (St. Louis, USA). tert-Butyl alcohol was acquired from Vetec (Rio de Janeiro, Brazil). All other chemicals used were analytical grade.
Physical and Chemical Characterization of the Waste Cooking Oil
The WCO was filtered to remove suspended solids and then characterized in terms of saponification value [18], acid value [19], and water content [20]. Viscosity at 40 °C was measured using a Brookfield viscometer. The fatty acid profile analysis was kindly performed in the laboratory of IMCOPA S.A. (Araucaria, Brazil), using standard methods [21, 22].
Hydrolysis Reactions
The hydrolysis reactions of the WCO were carried out for 24 h at 30 °C in a closed 0.3-L thermostatically controlled reactor, with mechanical stirring at 300 rpm. The reaction medium consisted of 60 mL of WCO, 240 mL of distilled water (oil/water ratio of 1:4, v/v), and 3 g of immobilized TLL (enzyme/reaction medium ratio of 1:100, w/v). The reaction was followed by measurement of FFAs in the reaction medium. Briefly, 2 g of the reaction medium was mixed with 25 mL of ethyl ether/95 % ethyl alcohol (2:1, v/v) solution. The mixture was titrated with 0.021 M KOH solution, using phenolphthalein as indicator. At the end of the reaction, the biocatalyst was recovered by filtration and the two phases (oil and water) were separated by centrifugation. The oil phase was washed with distilled water at around 60 °C and dried using molecular sieves. The contents of free glycerol, monoacylglycerols (MAGs), diacylglycerols (DAGs), and triacylglycerols (TAGs) in the oil phase were determined by gas chromatography, enabling calculation of the mass percentage of FFAs in the oil phase. The assay was performed in duplicate and the results (conversion vs. time) were expressed as mean values ± average deviations. Conversions as a function of the time were calculated using Eq. (1).
where FFA is the free fatty acid content (in moles) determined by titration, TFA is the total fatty acid content in the WCO, calculated as (oil mass × 3/MM), and MM is the molecular mass of the WCO.
Esterification Reactions
The esterification reactions were carried out at 40 °C in closed flasks, with agitation in a shaker. The reaction medium was composed of 15 g of hydrolyzed WCO, henceforth named HWCO (wt% composition: 97.7 % FFAs, 0.017 % glycerol, 0.36 % MAGs, 0.25 % DAGs, and 1.67 % TAGs; acid value of 197.92 ± 1.89 mg KOH/g), 21.06 g of anhydrous ethanol (acid/alcohol molar ratio of 1:7), 1.5 g of immobilized CALB (10 wt%, enzyme/HWCO), and 1.5 g of molecular sieves. The reaction was followed by measurement of FFAs, as described above. At the end of the reaction, the biocatalyst was recovered by filtration and the oil phase (biodiesel) was recovered by centrifugation. The biodiesel was washed with hot distilled water, dried in an oven for 2 h at 130 °C, and the mass contents of fatty acid ethyl esters (FAEEs), MAGs, DAGs, and TAGs were determined by gas chromatography. The viscosity of the biodiesel was measured at 40 °C. The assay was performed in quadruplicate and the results (conversion vs. time) were expressed as means ± standard deviations. Conversions as a function of time were calculated using Eq. (2).
where the FFA contents (in moles) at t = 0 and t = t were determined by titration.
Reuse Assays
For the reuse assays of the immobilized lipases (TLL and CALB), batches of hydrolysis and esterification were repeated for five cycles, each lasting 10 h. For the hydrolysis assays, each batch consisted of 6 mL of WCO, 24 mL of distilled water (oil/water ratio of 1:4, v/v), and 0.3 g of immobilized TLL (enzyme/reaction medium ratio of 1:100, w/v). After 10 h of stirring at 300 rpm and 30 °C, the biocatalyst was recovered by filtration, washed with tert-butyl alcohol at room temperature (30–32 °C) to remove adsorbed products, and reused in another batch. The two phases (oil and water) were separated by centrifugation and the oil phase was dried in an oven for 2 h at 130 °C, followed by measurement of FFAs using 0.016 M KOH as titrant, as described above. For the esterification assays, each batch consisted of 1.5 g of HWCO, 2.106 g of anhydrous ethanol (acid/alcohol molar ratio of 1:7), 0.15 g of immobilized CALB (10 wt%, enzyme/HWCO), and 0.15 g of molecular sieves. After 10 h of stirring at 300 rpm and 40 °C, the biocatalyst was recovered by filtration, washed with tert-butyl alcohol at room temperature (30–32 °C), and reused in another cycle. The oil phase (biodiesel) was recovered by centrifugation, dried in an oven for 2 h at 130 °C, and the mass contents of the FAEEs were determined by gas chromatography. The reuse assays were performed in duplicate and the results were expressed as mean values ± standard deviations.
Gas Chromatography Analyses
Free Fatty Acid Ethyl Esters
Fatty acid ethyl esters were analyzed according to the EN 14103 method [23]. Samples (250 mg) were diluted in 5 mL of a 10 mg/mL solution of ethyl heptadecanoate (Sigma, St. Louis, MO, USA) as internal standard. Aliquots (1 µL) were injected into an Agilent 7890 GC equipped with a Restek 12423 column (30 m × 0.25 mm × 0.25 µm) and a flame ionization detector operated at 250 °C. The analysis was performed for 25 min using nitrogen as carrier gas (split ratio of 1:50), with the following heating program: 150 °C for 2 min, ramp to 180 °C at 10 °C/min, with a hold for 3 min, and ramp to 230 °C at 10 °C/min, with a hold for 7 min.
Acylglycerols and Glycerol
Free glycerol and mono-, di-, and triglycerides were analyzed according to the ASTM D6584 method [24], using an Agilent 7890 GC with a DB-5HT capillary column (15 m × 0.320 mm × 0.1 µm) and nitrogen as carrier gas (3.0 mL/min). The compounds were detected using an FID detector heated at 380 °C. The oven temperature heating program was as follows: 50 °C for 1 min, ramp to 180 °C at 15 °C/min, ramp to 230 °C at 7 °C/min, ramp to 380 °C at 30 °C/min, and a final hold at 380 °C for 10 min. The internal standards (IS) used were 1,2,4-butanetriol (1 mg/mL in pyridine, IS1) and tricaprin (8 mg/mL in pyridine, IS2). IS1 was used for identification of the glycerin peak, while IS2 was used to identify the mono-, di-, and triglycerides. Dried samples (100 mg) were weighed out into a 10-mL vial, followed by addition of 100 µL of each internal standard and 100 μL of MSTFA (derivatization reagent). The samples were maintained at room temperature for 20 min to complete the derivatization. Subsequently, heptane (8 mL) was added and an aliquot of 1 µL was injected into the GC. The compounds were quantified using calibration curves for glycerol and the mono-, di-, and triglycerides.
Results and Discussion
Characterization of Feedstock
The acid value (1.43 ± 0.02 mg KOH/g) and viscosity at 40 °C (36.6 ± 1.3 cP) were higher than for most vegetable oils [4, 5], suggesting some degradation of the oil during the frying process due to oxidative, hydrolytic, and polymerization reactions, which produce complex molecules, volatile compounds, and FFAs, glycerol, MAGs, and DAGs [25]. The acid value was higher than the value (0.5 wt%, or 1.0 mg KOH/g) required for an effective alkaline transesterification employing KOH [5, 8].
The WCO presented the following fatty acid composition (wt%): 43.8 % of polyunsaturated fatty acids (mainly linoleic acid, at 38.2 %, and alpha-linolenic acid, at 3.7 %), 32.8 % of monounsaturated fatty acids (mainly oleic acid, at 30.2 %, and cis-vaccenic acid, at 1.7 %), 21.8 % of saturated fatty acids (mainly palmitic acid, at 13.7 %, and stearic acid, at 6.0 %), and 1.6 % of other unidentified compounds. This composition, as well as the saponification value (193.4 ± 0.5 mg KOH/g), was close to that of soybean oil, which is widely used for cooking in Brazil. The average molecular weights of the WCO and the WCO fatty acids were 870 and 277 g/mol, respectively.
Hydrolysis of Waste Cooking Oil
Hydrolysis of the WCO was carried out in salt-free water (WCO/water ratio of 1:4, v/v) at 30 °C and with mechanical stirring at 300 rpm, using immobilized TLL as catalyst (enzyme/reaction medium ratio of 1:100, w/v). Excess water was used to displace the hydrolysis equilibrium towards the products, ensuring higher FFA yield. The use of excess water also resulted in the formation of oil droplets in water, which could be easily stirred. At the end of the reaction, the phases (oil and water/glycerol) could be simply separated by gravitational settling (recovery of the oil phase was above 90 wt%, relative to the WCO), avoiding the use of costly and toxic solvent [8, 11–13] or heating [10] to separate the oil and water layers. The temperature of 30 °C was chosen on the basis of the literature, since the temperature for maximum TLL activity is reported to be in the range 20–50 °C [26, 27]. Although it has been reported previously that TLL is more active at higher temperature [13], use of a lower temperature saves energy and reduces enzyme inactivation.
The hydrolysis profile of the WCO in an aqueous medium is shown in Fig. 1. It can be observed that the hydrolysis was very fast, achieving nearly 90 % conversion after 9 h of reaction, after which the conversion increased slowly, reaching around 100 % after 24 h. Gas chromatography analysis showed that the oil phase obtained after 24 h contained (wt%) glycerol (0.017), MAGs (0.36), DAGs (0.25), and TAGs (1.67). As FFAs are not measured directly by gas chromatography, their content was calculated from a mass balance (100 % minus the MAGs, DAGs, and TAGs present), resulting in a value of 97.7 %. The acid value measured for the HWCO was 197.92 ± 1.89 mg KOH/g, corroborating the assumed amount of FFAs. As TLL is a 1,3-specific lipase that hydrolyzes ester bonds at the sn-1 and sn-3 positions in the glycerol backbone, the theoretical conversion should be only 66 %. However, as around 100 % conversion was obtained, acyl migration from position 2 to position 1 must have occurred during the hydrolysis [28, 29].
Time course of waste cooking oil (WCO) hydrolysis catalyzed by immobilized Thermomyces lanuginosus lipase (TLL-IM-T2-150). The reaction was carried out at 30 °C for 24 h using an oil/water ratio of 1:4 (v/v), enzyme/reaction medium ratio of 1:100 (w/v), and uncontrolled pH. The values are expressed as averages ± standard deviations for duplicates
The findings of this study were similar to results reported previously (Table 1). In hydrolysis of acid oils catalyzed by soluble Candida rugosa lipase, Talukder et al. [12] and Watanabe et al. [10] achieved high conversions (100 and 92 %, respectively) under conditions similar to those used in this work (oil/water medium, 30 °C, and 10 or 24 h). In hydrolysis of soybean oil catalyzed by soluble T. lanuginosus lipase, Cavalcanti-Oliveira et al. [13] obtained 89 % conversion after 48 h at 60 °C. Although these conversions were similar to the yields achieved here, it is more difficult to recycle soluble enzymes than immobilized enzymes. As shown in Table 1, some studies carried out the hydrolysis of refined or acid oils catalyzed by microbial or plant lipases in buffered medium. Although comparisons of conversion according to time are difficult, because of the use of different operational conditions, it is important to emphasize that the hydrolysis carried out in salt-free water is advantageous from an economic perspective, because glycerol can be recovered after gravitational settling or centrifugation in an aqueous medium free from salts and emulsifiers. Therefore, after distillation, high purity (>99 %) glycerol could be used in the food, cosmetics, and pharmaceutical industrial sectors [13], contributing to the economic feasibility of enzymatic biodiesel produced on an industrial scale.
Esterification of HWCO with Ethanol
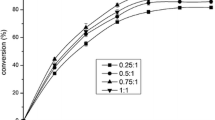
Esterification of HWCO was carried out in solvent-free medium using ethanol as acyl acceptor (HWCO/ethanol molar ratio of 1:7), at 40 °C and with agitation in a shaker, using immobilized CALB (10 wt%, enzyme/HWCO) and molecular sieves (10 wt%, based on the HWCO weight). A temperature of 40 °C was chosen because the temperature for maximum activity of CALB has been reported to be in the range 30–60 °C [27]. Talukder et al. [11] reported that in the methanolysis of free fatty acids catalyzed by Novozym 435 (CALB immobilized on acrylic resin), the highest initial reaction rate was achieved at 60 °C, but that the maximum biodiesel yield at this temperature was slightly lower than at 40 °C. A 1:7 HWCO/ethanol molar ratio was chosen on the basis of the work by Lima et al. [30], who studied the enzymatic transesterification of vegetable oils using ethanol as acyl acceptor. Although the stoichiometric molar ratio for the esterification of FFAs is 1:1, excess ethanol was used to displace the esterification equilibrium towards the products. Furthermore, it was reported previously by Watanabe et al. [10] that the esterification yield of FFAs and the residual activity of immobilized Candida antarctica were highest using methanol/FFAs molar ratios in the range from 1:5 to 1:7, when comparison was made with the presence of an equimolar amount of methanol. Although ethanol was used as acyl acceptor in the present work, similar behavior would be expected. Watanabe et al. [10] also described the use of glycerol (10 wt%) in the esterification of FFAs with methanol, with the aim of achieving a complete esterification yield, since water in the oil layer was attracted to the glycerol layer, avoiding shifting of the reaction equilibrium to the left. However, the presence of glycerol in the esterification step requires its separation at the end of the reaction, increasing the costs of purification of the biodiesel. Therefore, in this work molecular sieves were evaluated as a dehydrating agent, because they could be easily recovered and recycled together with the biocatalyst.
Figure 2 shows the profile of FFAs esterification according to time. It can be observed that the esterification was very fast, with around 90 % conversion after 6 h of reaction, after which there was no further significant increase up to 12 h of reaction. Application of Tukey’s test (at the 95 % confidence level) showed that the conversions at 3, 6, 9, and 12 h were not significantly different. The gas chromatography results for the biodiesel composition are shown in Table 2, together with several physical and chemical properties. The acid value of the biodiesel was 10.88 ± 1.54 mg KOH/g, representing 5.37 ± 0.76 % (w/w) of the FFAs. From the mass balance, it could be estimated that unsaponifiable compounds probably accounted for 1.5–3.0 % (w/w) of the biodiesel. Conversions of around 90–93 % have been reported previously for enzymatic transesterification of WCO [31, 32]. However, in these studies methanol was used as acyl acceptor, and its addition was carried out in three stages to avoid enzyme inactivation. As a result, batch processes required long reaction times of around 25 h at 40 °C, using immobilized Candida sp. 99–125 lipase [32], and around 50 h at 30 °C, using C. antarctica lipase [31].
Time course of esterification of hydrolyzed waste cooking oil (HWCO) with anhydrous ethanol catalyzed by immobilized Candida antarctica lipase type B (CALB IM-T2-350). The reaction was carried out at 40 °C for 12 h using an oil/ethanol molar ratio of 1:7, 10 wt% enzyme/HWCO, and 10 % wt% molecular sieves/HWCO. The values are expressed as averages ± standard deviations for quadruplicates
Although the production of biodiesel by hydroesterification in two steps has been reported previously (Table 1), few studies have performed the esterification step enzymatically. Similar conversions of FFAs have been reported using ethanol as acyl acceptor. Soares et al. [15] achieved 93 % conversion after 31 h at 50 °C, using fermented solid from Burkholderia cepacia as biocatalyst, while Aguieiras et al. [8] obtained 91 % conversion after 8 h at 40 °C, using fermented solid from R. miehei as biocatalyst. Using methanol as acyl acceptor, conversions of up to 96–98 % have been obtained using immobilized C. antarctica lipase as biocatalyst [10, 11]. However, stepwise addition of methanol or organic solvent was required.
Some parameters of the enzymatically produced biodiesel, such as FAEE content and acid value (Table 2), did not comply with the standards established by the Brazilian National Petroleum Agency [33] for sale of biodiesel in Brazil. Process optimization would be able to adjust these parameters to the required specifications. According to Aguieiras et al. [8] and Watanabe et al. [10], esterification of FFAs in two steps could enable the minimum required FAEEs value to be achieved.
Reuse Assays
The reuse of immobilized lipases is desirable in the production of biodiesel, because the ability to recycle the biocatalyst could reduce the costs of production, contributing to making enzymatic biodiesel competitive with its chemical counterpart [6, 8, 11].
Figure 3 shows that the activity of the immobilized TLL decreased during recycling, with 42 % FFA conversion after five cycles. The low pH of the reaction medium (around pH 4.6 after 10 h hydrolysis) probably caused inactivation of the enzyme, because the pH for maximum activity of TLL is reported to lie in the range pH 7–9 [27, 29]. Talukder et al. [11] recycled the heavy phase of the hydrolysis of oil (water, glycerol, and soluble C. rugosa lipase), obtaining a hydrolysis yield of around 92 % after 10 cycles. The presence of glycerol at concentrations of 10–40 wt% during hydrolyses of oils or fats can stabilize the lipase [11]. This strategy seems to be of potential interest in further studies, because the use of glycerol could assist recycling of the immobilized TLL, as well as lead to savings of water and energy in the plant.
Reuse assays. Hydrolysis conditions: WCO/water ratio of 1:4 (v/v), immobilized TLL/reaction medium ratio of 1:100 (w/v), 10 h at 30 °C and 300 rpm. Esterification conditions: HWCO/ethanol molar ratio of 1:7, immobilized CALB/HWCO and molecular sieves/HWCO ratios of 10 wt%, 10 h at 40 °C and 300 rpm. The values are expressed as averages ± standard deviations for duplicates
Figure 3 also shows the results for recycling of CALB in the esterification step, with FAEEs mass content of around 86 % achieved after five cycles. The high stability of CALB has been reported previously by Talukder et al. [11], who found that when Novozym 435 (CALB immobilized on acrylic resin) was washed with tert-butyl alcohol and freeze-dried after each cycle, it could be repeatedly used during 50 cycles without losing its activity.
Conclusions
Biodiesel was produced by hydroesterification using waste cooking oil as feedstock, ethanol as acyl acceptor, and enzymes as biocatalysts. The enzyme-catalyzed steps were energy efficient and attractive from an industrial point of view. The hydrolysis step in salt-free water provided around 90 % conversion after 9 h at a low temperature (30 °C), and the esterification step in solvent-free medium using ethanol as acyl acceptor provided around 90 % FAEEs mass content after 6 h at 40 °C. The biodiesel parameters evaluated showed that the process evaluated in this work requires an optimization study in order to adjust the biodiesel to the specifications required for its sale.
References
Ranganathan SV, Narasimhan SL, Muthukumar K (2008) An overview of enzymatic production of biodiesel. Bioresour Technol 99:3975–3981
Patil PD, Gude VG, Reddy HK, Muppaneni T, Deng S (2012) Biodiesel production from waste cooking oil using sulfuric acid and microwave irradiation process. J Environ Protec 3:107–113
Tan T, Lu J, Nie K, Deng L, Wang F (2010) Biodiesel production with immobilized lipase: a review. Biotechnol Adv 28:628–634
Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70:1–15
Canakci M, Gerpen JV (2001) Biodiesel production from oils and fats with high free fatty acids. Am Soc Agric Eng 44:1429–1436
Ting W-J, Huang C-M, Nair GR, Wu W-T (2008) An enzymatic/acid-catalyzed hybrid process for biodiesel production from soybean oil. J Chin Inst Chem Eng 39:203–210
Kaieda M, Samukawa T, Matsumoto T, Ban K, Kondo A, Shimada Y, Noda H, Nomoto F, Ohtsuka K, Izumoto E, Fukuda H (1999) Biodiesel fuel production from plant oil catalyzed by Rhizopus oryzae lipase in a water-containing system without an organic solvent. J Biosci Bioeng 88:627–631
Aguieiras ECG, Cavalcanti-Oliveira ED, Castro AM, Langone MAP, Freire DMG (2014) Biodiesel production from Acrocomia aculeata acid oil by (enzyme/enzyme) hydroesterification process: use of vegetable lipase and fermented solid as low-cost biocatalysts. Fuel 135:315–321
Hernández-Martín E, Otero C (2008) Different enzyme requirements for the synthesis of biodiesel: Novozym® 435 and Lipozyme® TL IM. Bioresour Technol 99:277–286
Watanabe Y, Nagao T, Nishida Y, Takagi Y, Shimada Y (2007) Enzymatic production of fatty acid methyl esters by hydrolysis of acid oil followed by esterification. J Am Oil Chem Soc 84:1015–1021
Talukder MdMR, Wu JC, NgM Fen, Melissa YLS (2010) Two-step lipase catalysis for production of biodiesel. Biochem Eng J 49:207–212
Talukder MdMR, Wu JC, Chua LP-L (2010) Conversion of waste cooking oil to biodiesel via enzymatic hydrolysis followed by chemical esterification. Energy Fuel 24:2016–2019
Cavalcanti-Oliveira ED, Silva PR, Ramos AP, Aranda DAG, Freire DMG (2011) Study of soybean oil hydrolysis catalyzed by Thermomyces lanuginosus lipase and its application to biodiesel production via hydroesterification. Enzym Res. doi:10.4061/2011/618692
de Sousa JS, Cavalcanti-Oliveira ED, Aranda DAG, Freire DMG (2010) Application of lipase from the physic nut (Jatropha curcas L.) to a new hydrid (enzyme/chemical) hydroesterification process for biodiesel production. J Mol Catal B Enzym 65:133–137
Soares D, Pinto AF, Gonçalves AG, Mitchell DA, Krieger N (2013) Biodiesel production from soybean soapstock acid oil by hydrolysis in subcritical water followed by lipase-catalyzed esterification using a fermented solid in a packed-bed reactor. Biochem Eng J 81:15–23
Lotero E, Liu Y, Lopez DE, Suwannakarn K, Bruce DA, Goodwin JG (2005) Synthesis of biodiesel via acid catalysis. Ind Eng Chem Res 44:5353–5363
Salis A, Svensson I, Monduzzi M, Solinas V, Adlercreutz P (2003) The atypical lipase B from Candida antarctica is better adapted for organic media than typical lipase from Thermomyces lanuginosa. Biochim Biophys Acta 1646:145–151
AOCS Official Method Cd 3-25: Saponification value - revised 2013 (2013) In: Firestone D (ed) Official methods and recommended pratices of the AOCS, 6th edn. (3rd Printing). AOCS Press, Urbana.
AOCS Official Method Ca 5a-40: free fatty acids - revised 2012 (2013) In: Firestone D (ed) Official methods and recommended pratices of the AOCS, 6th edn. (3rd Printing). AOCS Press, Urbana.
AOCS Official Method Ca 2c-25: moisture and volatile matter air oven method -reapproved 2009 (2013) In: Firestone D (ed) Official methods and recommended pratices of the AOCS, 6th edn. (3rd Printing). AOCS Press, Urbana.
AOCS Official Method Ce 2-66: preparation of methyl esters of fatty acids - reapproved 2009 (2013) In: Firestone D (ed) Official methods and recommended pratices of the AOCS, 6th edn. (3rd Printing). AOCS Press, Urbana.
ISO 5508 (1990) Animal and vegetable fats and oils—analysis by gas chromatography of methyl esters of fatty acid. International Standard, Switzerland
EN 14103 (2011) Fat and oil derivatives—fatty acid methyl esters (FAME)—determination of ester and linolenic acid methyl ester contents
ASTM D6584 (2013) Standard test method for determination of total monoglycerides, total diglycerides, total triglycerides, and free and total glycerol in B-100 biodiesel methyl esters by gas chromatography. ASTM International, 2013
Freire PCM, Mancini-Filho J, Ferreira TAPC (2013) Major physical and chemical changes in oils and fats used for deep frying: regulation and effects on health. Rev Nutr 26:353–368
Rodrigues RC, Volpato G, Wada K, Ayub MAS (2008) Enzymatic synthesis of biodiesel from transesterification reaction of vegetable oils and short chain alcohols. J Am Oil Chem Soc 85:925–930
Lipase enzymes (2016) Novozymes, Denmark. http://www.novozymes.com/en/solutions/pharmaceuticals/biocatalysis/lipase-enzymes. Acessed 21 Sep 2016.
Du W, Xu Y-Y, Liu D-H, Li Z-B (2005) Study of acyl migration in immobilized lipozyme TL-catalyzed transesterification of soybean oil for biodiesel production. J Mol Catal B-Enzym 37:68–71
Fernandez-Lafuente R (2010) Lipase from Thermomyces lanuginosus: uses and prospects as an industrial biocatalyst. J Mol Catal B Enzym 62:197–212
Lima LN, Oliveira GC, Rojas MJ, Castro HF, Da Rós PCM, Mendes AA, Giordano RLC, Tardioli PW (2015) Immobilization of Pseudomonas fluorescens lipase on hydrophobic supports and application in biodiesel synthesis by transesterification of vegetable oils in solvent-free systems. J Ind Microbiol Biotechnol 42:523–535
Shimada Y, Watanabe Y, Sugihara A, Tominaga Y (2002) Enzymatic alcoholysis for biodiesel fuel production and application of the reaction oil processing. J Mol Catal B Enzym 17:133–142
Nie K, Xie F, Wang F, Tan T (2006) Lipase catalyzed methanolysis to produce biodiesel: optimization of the biodiesel production. J Mol Catal B Enzym 43:142–147
ANP Resolution No. 14 of 11/05/2012. National agency of petroleum, natural gas and biofuels (ANP), Brazil. http://nxt.anp.gov.br/nxt/gateway.dll/leg/resolucoes_anp/2012/maio/ranp%2014%20-%202012.xml. Accessed 24 Feb 2015
Acknowledgments
The authors are grateful for the financial support provided by the São Paulo Research Foundation (FAPESP, grant #2011/23194-0), National Council for Scientific and Technological Development (CNPq), and Improvement Commission of Higher Level Personnel (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Vescovi, V., Rojas, M.J., Baraldo, A. et al. Lipase-Catalyzed Production of Biodiesel by Hydrolysis of Waste Cooking Oil Followed by Esterification of Free Fatty Acids. J Am Oil Chem Soc 93, 1615–1624 (2016). https://doi.org/10.1007/s11746-016-2901-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-016-2901-y