Abstract
Fish oil dietary supplements have been linked with health benefits, due to high omega-3 concentration. The sources of these effects, polyunsaturated fatty acids such as eicosapentaenoic acid and docosahexaenoic acid, are almost exclusively found in seafood products. Our objectives were to characterize the composition of commercial omega-3 dietary supplements dietary supplements and to generate partial least square regression (PLSR) models using infrared spectroscopy and chemometrics. Fatty acid (FA) composition of oils was determined by FA methyl ester gas chromatography. The supplements encompassed a wide range of FA profiles and delivery methods. Infrared spectral data were collected by portable mid-infrared Fourier transform infrared (MID FT-IR) equipment. Principal components analysis (PCA) separated samples based on the type of ester present in the fish oil dietary supplements, showing a strong influence of the 1038 cm−1 band, which is typically associated with C=C and C–O stretching vibrations. In addition, PLSR was used to correlate the spectra data with GC-FAME results. PCA using the spectroscopy data allowed for tight clustering of fish oil into distinct classes, depending on the source and processing. PLSR using MID FT-IR spectra and FA composition generated multivariate models with high correlation coefficient (R ≥ 0.93), and SEP between 0.53 and 2.13 g of FA per 100 g of oil. Our results indicate that IR spectroscopy combined with chemometrics provides for robust screening of FA composition of fish oil supplements, and discriminate types of FAs esterification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish oil dietary supplements are usually made from mackerel, herring, tuna, halibut, salmon, cod liver, whale blubber, or seal blubber, and contain a large amount of polyunsaturated fatty acids (PUFA). Omega-3 fatty acids (FA) are long-chain n-3 FAs that are essential to the human diet and have been found to provide a wide array of health benefits, including anti-inflammatory, cardioprotective, and anti-arthritic effects [1]. The compounds primarily responsible for these benefits, PUFA such as eicosapentaenoic acid (EPA), 20:5, and docosahexaenoic acid (DHA), 22:6, are almost exclusively found in seafood products [2]. Some research has shown that Western diets are deficient in omega-3 FAs, leading to increased consumer awareness of dietary supplements that contain these compounds [3]. The composition of fish oils is highly variable, with fish type, catch location, season, and processing all playing a role in determining the ratios of the FAs found in the oils [4, 5]. As each PUFA has a different role in health promotion and disease prevention, it is important to understand the variability in composition within fish oil products, and whether they are supplied as triglycerides, esters, or free FAs. As a result, it is essential to analyze the properties of the oils that are not usually described on the label to ensure the delivery of claimed health benefits. The structure of these essential FAs plays a role in determining their health-promoting properties and efficacy [2]. Most fish oil supplements on the market are heavily processed and may be provided as ethyl esters, triacylglycerols, or less commonly in phospholipid form. Research has yielded mixed results on the bioavailability and bioactivity of different delivery forms. But at least two studies have demonstrated a small, yet significant, increase in bioavailability of triglycerides versus ethyl esters [6]. In addition, the effect of triglyceride substitution pattern on the bioavailability and metabolic fates of omega-3 FAs is not yet completely understood [7]. The high cost of fish oils and their myriad uses make them prime candidates for adulteration and mislabeling. The dietary supplement health and education act of 1994, which outlines regulations for dietary supplements, does not provide the same level of oversight for supplements that the FDA provides for food and drug manufacturers [8]. The delivery form, whether ethyl ester, triglyceride, or other, is not required to be stated on packaging. Many supplements are also processed and packaged outside the country of sale, making it difficult to consistently track quality and safety. Fish oils are susceptible to contamination with toxic and carcinogenic compounds, either from the fish itself or during processing [1]. Improper or incomplete purification can lead to high levels of polychlorinated biphenyls (PCB), dioxins, pesticides and other toxins [9, 10]. Modern, rapid techniques to determine composition, authenticity, and quality of fish oils are not yet readily available, but development of Fourier transform infrared (FT-IR) and attenuated total reflectance (ATR) spectrometry equipment has opened the door to novel methods. FT-IR systems provide a more robust alternative to older dispersive systems, and ATR systems can accommodate a wide variety of sample types, from gases to liquids and powders. Although the food and supplement industries have utilized near-infrared (NIR) spectroscopy for decades [11–14], the applications of mid-infrared (MID) spectroscopy have recently gained ground [15, 16]. MID FT-IR and NIR are non-destructive and rapid techniques that allow for the analysis of all the chemical bonds in a system. When combined with multivariate statistical analysis or chemometrics, the large amount of information provided by spectroscopy methods can be used to replace traditional techniques [17]. In oil analysis, these traditional techniques are both time consuming and expensive. Gas chromatography (GC) and high-performance liquid chromatography (HPLC) are used in the analysis of oils, but the extensive sample preparation, long run-times, and destructive nature of the analysis has piqued interest in spectroscopic alternatives. New methods, such as 1H and 13C NMR, have been used to classify fish oils by source, and provide a large amount of information regarding the connectivity of FAs [18, 19]. The high equipment costs and the inherent difficulty of quantification methods have prevented widespread adoption of these techniques. Several studies have demonstrated the potential for classification and detection of adulteration in edible oils, including fish oils, with infrared [20, 21]. Previous studies have looked at determining fish oil quality [22] and composition in different supplement forms of fish oils [23]. While several studies have undertaken analyses of retail fish oil supplements [24–26], more study is needed on the applications of IR spectroscopy to these products. The objective of this research was to evaluate a portable MID FT-IR equipment combined with chemometrics for characterization of dietary omega-3 oil supplements and for the rapid determination of their FA composition.
Materials and Methods
A total of 37 unique omega-3 oil dietary supplements included 21 fish oil (FO) supplements, 4 cod liver oil (CLO) supplements and 4 flaxseed oil supplements (Table 1), from 16 different manufacturers, all purchased at retail. New duplicate samples of 7 FO and 1 flaxseed oil were purchased at a later date, ensuring separate lots from the original supplements. All FA standards were purchased from Nu-Chek Prep (Elysian, MN, USA). All solvents and chemicals of analytical grade used in this study were purchased from Fisher Scientific (Waltham, MA, USA).
Sample Separation
Oils supplied in gel capsule form were cleanly perforated and the oil transferred to sealed, 1.5-mL flip-cap tubes for cold storage (4 °C). For each encapsulated supplement, three capsules were selected from the bottle at random for spectral collection and subsequent FA composition analysis. In the case of bulk oils, the containers were agitated and an aliquot was removed and stored in the same manner and location as the encapsulated oils.
Fatty Acid Profile
Determination of FA content in fresh oils was achieved with a FA methyl ester (FAME) derivatization [27]. Briefly, esterification was achieved by adding methanolic-sulfuric acid (10 mL, 4 % sulfuric acid) to the oil sample (~0.05 g) with benzene (1 mL) in a glass test tube with a Teflon screwtop cap. The mixture was heated to 90 °C for 120 min in an oven. Methyl esters were extracted using a partition of hexane and distilled water. An aliquot of the hexane portion (1 mL) was collected in a 1.5 mL GC vial with crimp top and was evaporated under nitrogen flush. The dried samples were re-diluted using iso-octane (0.5 mL). Samples were analyzed, in duplicate, on an HP-6890 GC (Agilent, Santa Clara, CA, USA) equipped with a flame ionization detector (FID) and HP G1513A autosampler (Agilent). Separation of the components was done using an HP-88 60 m × 0.25 mm × 0.2 µm column (Agilent) using helium as the carrier gas. The injection volume was 0.2 μL in the splitless mode. The oven conditions were 125–220 °C (5 °C/min), then hold for 15 min. Injector temperature was 220 °C and the detector temperature was 250 °C. The identification of FAs was carried out by comparing the retention times with EPA and DHA reference standards (NuChek Prep U-99-A and U-84-A) and 18 standards (NuChek Prep A-68C). An internal standard (Heptadecanoic acid, NuChek Prep, N-17-A) was added at 0.005 g per methylation and the ratio of FA area to internal standard (IS) peak area was used for quantification via a standard curve.
Free Fatty Acids
Determination of free fatty acids (FFA) was carried out using the EasyPlus™ Titration (Mettler Toledo, Columbus, OH, USA) coupled with a 10-mL burette. The sample was weighed (~5 g) in a glass beaker and 25 mL of pre-neutralized 1:1 xylene/ethanol were added to dissolve the oil. Potassium hydroxide (0.1 N) in methanol was used to neutralize the FFAs [28]. Results were expressed in grams of oleic acid per 100 g of oil [29].
Spectra Collection
The collection of mid-infrared spectral (4000–700 cm−1) data was performed on a FT-IR portable spectrometer (Cary 630; Agilent), equipped with a temperature-controlled, 5-bounce ZnSe crystal ATR. Sealed sample containers of oil taken from the refrigerator were allowed to equilibrate to room temperature prior to data collection and measured in duplicate. Then, 80 μL of oil was deposited by micropipette on the ATR (which was maintained at 65 °C), and spectra were collected over a range of 4000–700 cm−1 at 4 cm−1 resolution. Spectra were collected in terms of absorbance using MicroLab software (Agilent).
Multivariate Analysis
Principal components analysis (PCA) was used to analyze spectra of the supplements. SNV and 2nd derivative (Savitzky-Golay second-order polynomial filter with a 35-point window) transforms were applied to all samples to reduce peak overlap and eliminate baseline shift of the spectra [30]. Diagrams of scores and loadings were used to analyze the relationship between the original variable and sample grouping. Partial least squares regression (PLSR) was used to correlate GC-FAME FA composition information with the spectra. Construction of the PLSR models was done by cross-validation (CV), using the leave-five-out method. The optimal number of PLS factors were selected by plotting the SECV versus number of factors and selecting the number of factors that did not significantly decrease the SECV when this number were included in the model. The IR regions included in the model were selected based on the functional groups assigned previously by other authors. The goodness of fit for each model was evaluated using an independent validation set. Random sample selection was used to split the calibration [27] and validation [10] sets. The performance of the PLSR models were evaluated by means of the standard error of prediction (SEP), coefficient of correlation of prediction (r Pred) and ratio of performance deviation (RPD), which is the ratio between the validation set and the standard deviation of the reference set divided by SEP. Chemometric analysis was carried out using Pirouette 4.0 rev. 2 (Infometrix, Bothell, WA, USA).
Results and Discussion
Characterization of Fish Oils
Analysis of FA composition by gas chromatography showed a wide range of EPA (0–61 %) and DHA (0–57 %) concentrations, as well as large variation in other majority components of the FA profile (Table 2). These values were comparable to the label claims, with most of the samples having a higher concentration of EPA and DHA than claimed on the label. Studies on whether commercially available fish oil products meet their label claims have produced mixed results [24, 26, 31–34]. In summary, the FA profile showed that the fish oil supplements are a rich source of polyunsaturated fats, contributed primarily by eicosapentaenoic (EPA), docosahexaenoic (DHA), oleic, linoleic, palmitoleic and eicosenoic acids. The profiles exhibited moderate concentrations of saturated FA (~18 %), with C16:0 being the most abundant (Table 2). Flax oil supplements contained no EPA or DHA, but were rich in linoleic (~25 %) and linolenic acids (~40 %). Low concentrations of FFA (<0.4 %) were found in all dietary oil supplements, with the exception of a conjugated linoleic acid (CLA) dietary oil supplement, which was 100 % pure CLA (a free fatty acid). Table 3 shows that the fish body oil samples were divided into two major groups based on their EPA/DHA levels: samples containing 65–91 % total EPA/DHA and samples containing 32–37 % total EPA/DHA. Cod liver oils contained levels of EPA/DHA ranging from 23 to 28 %, exhibiting a narrower window of concentrations from the fish body oils. The fish body oil with the lowest EPA/DHA levels in our study was found to contain only 8 % total EPA/DHA. This supplement was advertised and labeled as “minimally processed and not molecularly distilled”, which may explain the distinction from the rest of the oils. Natural, non-concentrated fish oils contain approximately 18 % EPA and 12 % DHA, although the levels are highly variable, and dependent on factors previously described [6]. As a result, oils with higher EPA/DHA concentrations will have undergone a range of processing, most commonly trans-esterification and molecular distillation.
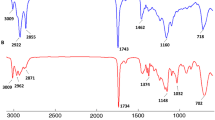
Spectral comparison of four samples, representing low and high concentrations of EPA/DHA in fish body oils, CLO, and flaxseed oil, revealed strong similarities between spectra (Fig. 1). Differences were evident primarily in the 3006–3012 cm−1 region associated with the =C–H stretching vibration band and the C=O carbonyl band at 1720–1746 cm−1 [19, 35]. The fingerprint region (800–1500 cm−1) showed a large amount of variation, especially for the high EPA/DHA fish oils in the 1050–950 cm−1 region, which has been ascribed to =C–H out-of-plane bending (Fig. 1).
Spectral comparisons of the 4 major supplement groups in this study. Peak assignment adapted from Guillen and Cabo [31]
PCA of Oil Supplements
Principal components analysis (PCA) using MID FT-IR spectra revealed the clustering of 4 major classes among the oil supplements (Fig. 2A–D). Further examination revealed that these clusters were highly associated with oil source and processing characteristics. Cluster A was associated to pure CLA, B corresponded to samples containing negligible (Flax), low (11 %, extra virgin marine oil) and moderate (34 %) EPA/DHA levels in fish body oils and CLO (25 % EPA/DHA), while C was related to concentrated marine oils (65–91 % EPA/DHA). Cluster D corresponded to a single supplement (denoted in Table 1 as sample C) with EPA and DHA concentrations of 24 and 14 %, respectively, similar to samples containing moderate EPA/DHA levels in class B but clustered farthest away as a different group (D) (Fig. 2). This fish oil supplement (sample C) was manufactured using a molecular distillation process, involving purification of FA esters in a vacuum system based on the variations in boiling points of different FAs. Sub-groupings were evident in samples included in Class B with flaxseed and CLO samples being differentiated from the marine oil samples. The slightly higher ratio of DHA to EPA found in CLO versus fish body oils may play a role in differentiating these groups [5]. Also, the nature of CLO processing and the fact that CLO concentrates are not readily available [24] may explain the low variability within this group.
Loading plots show the spectral weights (4000–700 cm−1) responsible for sample clustering (Fig. 3). PC1 and PC2 showed high correlations with the 1750–1745 cm−1 region, attributed to the C–O stretching in esters [35], and describing the shift in the carbonyl group (1745–1710 cm−1) of the oils depending on the type of FAs esterification. PC3 exhibited a high correlation with bands in the 3070–2800 cm−1 and 1250–1150 cm−1 regions attributed to the C–H groups of the FAs and C=C and C–O stretching, respectively [35]. Examination of raw spectra reveals a distinct carbonyl band shift (from 1704 to 1745 cm−1) in the samples (Fig. 4) that may reflect the delivery of these supplements as primarily FA ethyl esters instead of triglycerides. Figure 4 showed that the conjugated linoleic acid sample (Class A) showed strong absorptions at 1704 cm−1 attributed to the free FA; Class B samples showed a shift of the absorption band to 1745 cm−1 associated with the carbonyl signal of triacylglycerides, while samples in Class C and D showed a major band at 1737 cm−1 assigned to the absorption of the carbonyl methyl ester band [36]. High levels of EPA/DHA in many fish oil supplements are obtained through esterification, fractionation and concentration of omega-3 fatty alkyl esters. As a result, clustering of fish oils may be due to the presence of these ethyl esters in the supplements, either intentionally as a cheap way to increase levels of EPA and DHA, or as a result of incomplete re-esterification after molecular distillation [24]. Alkyl esters of omega-3 fatty acids are more prone to oxidation than their triglyceride counterparts, and some evidence suggests that the bioavailability of alkyl esters is lower than that of intact triglycerides [6]. The lack of distinction between products containing triglycerides and FA alkyl esters reveals a weakness of the dietary supplements health and education act. Only one of the concentrated fish oils claimed to contain ethyl ester FAs, and consumers that wish to choose one form of supplement over the other have very little reliable information on which to base their decision.
Partial Least Squares Regression Analysis
Random sample selection was used to select the samples for the calibration and validation sets. All FA ranges were comparable, but the standard deviations were always smaller for the validation set (Table 4). PLSR models for estimating FA levels were constructed using the 3200–2800 cm−1 region related to the olefinic (=CH–) and aliphatic (–CH2–) stretching vibration bands in their infrared spectra [19, 37]. The C=O carbonyl MID region (1800–1600 cm−1) was used to develop the FFA model.
The correlation coefficients of validation (>0.93) for all models can be considered as excellent [38]. The standard error of prediction (SEP) ranged from 0.6 to 2.3 % (Table 5). Overall, the low SEP and very high goodness of fit (Table 5) showed that this method is capable of quantifying levels of major FAs in dietary supplements across a broad compositional range. PLSR models for estimating EPA and DHA showed high correlation coefficients (r val = 0.99) and low SEPs of 2.2 and 1.3 %, respectively. RPDs were used as an indicator of predictive ability of the models. RPDs can be broken down into the following ranges: 0–2.3 indicates poor model or predictions, and using this model is not recommended; RPD values between 2.4 and 3 shows that models can only distinguish between high and low values; values between 3.1 and 4.9 are good for screening applications, values between 5.0 and 6.4 are acceptable for quality control applications and above 6.5 for process control purposes [39]. The best prediction models for portable Cary 630 (MID FT-IR) equipment with 5-bounce ATR and temperature control were found for C14:0, EPA, DHA, C16:0 and C16:1 with RPDs of 5.71, 5.51, 4.75, 3.77 and 3.6, respectively (Table 5). Figure 5 shows the prediction versus measured for (A) EPA and (B) DHA PLS models. All models were at least classified for screening application, except for C14:0 and EPA which were defined as quality control application models (RPD >5). Although the correlation coefficient of prediction for FFA showed a goodness of fit with r val of 0.93, the low levels of FFA found in the dietary supplements resulted in a validation set without enough variability yielding poor prediction power (RPD <1.1).
The results of the prediction model confirm the capability to estimate the main FAs of fish oil supplements, an important quality parameter for Food and Drug Administration compliance. In addition, the regression model allows for good predictions of important FAs associated with health benefits of fish oil supplementation.
Conclusions
This study demonstrated a fast, simple, and reliable method for characterization of the primary FAs in dietary oil supplements, focusing on the two primary omega-3 fatty acids present. This method has the potential to support and validate labeling claims by using a portable mid-infrared system equipped with a temperature-controlled 5-bounce ATR accessory. The samples analyzed covered a wide range of EPA/DHA concentrations (0–60 %) and sources (fish body oils, cod liver oil, and flaxseed oil). Classification of samples was associated with EPA content, oil source, and factors associated with processing (alkyl ester or triglyceride). A discriminating factor was the carbonyl band (C=O), and regions associated with C=C and C–H bonds. The difference in the ester carbonyl band found in a comparison of the B and C groups is consistent with that found in FA methyl and ethyl esters versus triglyceride esters. Models were developed for rapidly determining the FA profile, particularly EPA and DHA contents expected for fish oils, by using MID FT-IR coupled with chemometrics. Models exhibited excellent performance in estimating DHA/EPA in omega-3 supplements (r val = 0.99, SEP = 1.1 % and RPD >4.8). The portable MID FT-IR technology provided precise FA composition information and allowed the determination of the state of these FAs, whether they are methyl/ethyl esters, FFAs, or triglycerides. We have shown the feasibility of using a portable unit that can provide in-field opportunities for testing in industry, replacing or complementing traditional techniques.
References
Sidhu KS (2003) Health benefits and potential risks related to consumption of fish or fish oil. Regul Toxicol Pharmacol 38:336–344
de Leiris J, de Lorgeril M, Boucher F (2009) Fish oil and heart health. J Cardiovasc Pharmacol 54:378–384
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Ackman RG, Ratnayake WMN, Olsson B (1988) The “basic” fatty acid composition of Atlantic fish oils: potential similarities useful for enrichment of polyunsaturated fatty acids by urea complexation. J Am Oil Chem Soc 65:136–138
Moffat CF, McGill AS (1993) Variability of the composition of fish oils—significance for the diet. Proc Nutr Soc 52:441–456
Schuchardt JP, Hahn A (2013) Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids 89:1–8
Dyerberg J, Madsen P, Moller JM, Aardestrup I, Schmidt EB (2010) Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot Essent Fatty Acids 83:137–141
Act DSHaE (1994) 103rd congress, 2nd session Publ No. 103–417
Mozaffarian D, Rimm EB (2006) Fish intake, contaminants, and human health: evaluating the risks and the benefits. J Am Med Assoc 296:1885–1899
Maria MS, Arianna S, Giuseppe OM (2004) Polychlorinated biphenyls, oil dietary supplements. J Food Prot 67:1787–1791
Zhang Y, Tan LH, Cao F, He Y (2013) Application of visible and near infrared spectroscopy for rapid and non-invasive quantification of adulterants in fish oil. Spectrosc Spectr Anal 33:1532–1536
Azizian H, Kramer JKG, Ehler S, Curtis JM (2010) Rapid quantitation of fish oil fatty acids and their ethyl esters by FT-NIR models. Eur J Lipid Sci Tech 112:452–462
Endo Y, Tagiri-Endo M, Kimura K (2005) Rapid determination of iodine value and saponification value of fish oils by near-infrared spectroscopy. J Food Sci 70:C127–C131
Cozzolino D, Murray I, Chree A, Scaife JR (2005) Multivariate determination of free fatty acids and moisture in fish oils by partial least-squares regression and near-infrared spectroscopy. LWT Food Sci Technol 38:821–828
Hernandez-Martinez M, Gallardo-Velazquez T, Osorio-Revilla G, Almaraz-Abarca N, Ponce-Mendoza A, Vasquez-Murrieta MS (2013) Prediction of total fat, fatty acid composition and nutritional parameters in fish fillets using MID-FTIR spectroscopy and chemometrics. LWT Food Sci Technol 52:12–20
Sanchez-Alonso I, Carmona P, Careche M (2012) Vibrational spectroscopic analysis of hake (Merluccius merluccius L.) lipids during frozen storage. Food Chem 132:160–167
Bosque-Sendra JM, Cuadros-Rodriguez L, Ruiz-Samblas C, de la Mata AP (2012) Combining chromatography and chemometrics for the characterization and authentication of fats and oils from triacylglycerol compositional data—a review. Anal Chim Acta 724:1–11
Aursand M, Standal IB, Axelson DE (2007) High-resolution (13)C nuclear magnetic resonance spectroscopy pattern recognition of fish oil capsules. J Agric Food Chem 55:38–47
Guillen MD, Carton I, Goicoechea E, Uriarte PS (2008) Characterization of cod liver oil by spectroscopic techniques. New approaches for the determination of compositional parameters, acyl groups, and cholesterol from H-1 nuclear magnetic resonance and Fourier transform infrared spectral data. J Agric Food Chem 56:9072–9079
Ozen BF, Weiss I, Mauer LJ (2003) Dietary supplement oil classification and detection of adulteration using fourier transform infrared spectroscopy. J Agric Food Chem 51:5871–5876
Bellorini S, Strathmann S, Baeten V, Fumiere O, Berben G, Tirendi S, von Holst C (2005) Discriminating animal fats and their origins: assessing the potentials of Fourier transform infrared spectroscopy, gas chromatography, immunoassay and polymerase chain reaction techniques. Anal Bioanal Chem 382:1073–1083
Zhang H-Z, Lee T-C (1997) Rapid near-infrared spectroscopic method for the determination of free fatty acid in fish and its application in fish quality assessment. J Agric Food Chem 45:3515–3521
Vongsvivut J, Heraud P, Zhang W, Kralovec JA, McNaughton D, Barrow CJ (2012) Quantitative determination of fatty acid compositions in micro-encapsulated fish-oil supplements using Fourier transform infrared (FTIR) spectroscopy. Food Chem 135:603–609
Ritter JCS, Budge SM, Jovica F (2013) Quality analysis of commercial fish oil preparations. J Sci Food Agric 93:1935–1939
Hamilton K, Brooks P, Holmes M, Cunningham J, Russell FD (2010) Evaluation of the composition of omega-3 fatty acids in dietary oil supplements. Nutr Diet 67:182–189
Tatarczyk T, Engl J, Ciardi C, Laimer M, Kaser S, Salzmann K, Lenners R, Patsch JR, Ebenbichler CF (2007) Analysis of long-chain omega-3 fatty acid content in fish-oil supplements. Wien Klin Wochen 119:417–422
Rodriguez-Saona LE, Barrett DM, Selivonchick DP (1995) Peroxidase and lipoxygenase influence on stability of polyunsaturated fatty-acids in sweet corn (Zea-Mays L) during frozen storage. J Food Sci 60:1041–1044
Blanco M, Castillo M, Peinado A, Beneyto R (2006) Application of multivariate curve resolution to chemical process control of an esterification reaction monitored by near-infrared spectroscopy. Appl Spectrosc 60:641–647
American Oil Chemists Society (2009) AOCS Method Ca 5a-40: Official methods and recommended practices of the AOCS: Sampling and analysis of commercial fats and oils. Urbana Champaign, IL
Savitzky A, Golay MJE (1964) Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 36:1627–1639
Opperman M, de Marais W, Spinnler Benade AJ (2011) Analysis of omega-3 fatty acid content of South African fish oil supplements. Cardiovasc J Afr 22:324–329
Kolanowski W (2010) Omega-3 LC PUFA contents and oxidative stability of encapsulated Fish oil dietary supplements. Int J Food Prop 13:498–511
Fierens C, Corthout J (2007) Omega-3 fatty acid preparations—a comparative study. J Pharm Belg 62:115–119
Fantoni CM, Cuccio AP, Barrera-Arellano D (1996) Brazilian encapsulated fish oils: oxidative stability and fatty acid composition. J Am Oil Chem Soc 73:251–253
Guillen MD, Cabo N (1997) Characterization of edible oils and lard by Fourier transform infrared spectroscopy. Relationships between composition and frequency of concrete bands in the fingerprint region. J Am Oil Chem Soc 74:1281–1286
Zimmerman D, Feng P, Vick B (1978) Characterization and occurrence of cyclic fatty-acids produced by plant extracts. Abstr Pap Am Chem S 175:67
Guillen MD, Cabo N (1997) Infrared spectroscopy in the study of edible oils and fats. J Sci Food Agric 75:1–11
Shenk JS, Westerhaus MO (1996) Calibration the ISI way. In: Davies AMC, Williams P (eds) Near infrared spectroscopy: the future waves. NIR Publications, Chichester
Williams P, Norris KH (2001) Near-infrared technology in the agricultural and food industries. American Association of Cereal Chemists, St. Paul
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Plans, M., Wenstrup, M.J. & Rodriguez-Saona, L.E. Application of Infrared Spectroscopy for Characterization of Dietary Omega-3 Oil Supplements. J Am Oil Chem Soc 92, 957–966 (2015). https://doi.org/10.1007/s11746-015-2666-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2666-8