Abstract
In several Russian northern lakes and rivers, Arctic cisco Coregonus autumnalis, least cisco C. sardinella, peled C. peled, tugun C. tugun, broad whitefish C. nasus, whitefish C. lavaretus and vendace C. albula were sampled in periods of officially permitted commercial fishery. Special attention was paid to contents (mg g−1 of wet weight) of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in muscle tissues (filets), which are essential for human nutrition. The highest values of EPA + DHA content in semi-anadromous fish and freshwater fish were recorded for C. autumnalis from the Yenisei River, 17.60 mg g−1 wet weight, and for C. lavaretus from the Sobachye Lake, 16.61 mg g−1 wet weight, respectively. Intra-genus variations of EPA + DHA contents of Coregonus species were from 1.87 to 17.60 mg g−1 wet weight. Since the congeneric species were genetically close to each other, the variations in EPA and DHA contents were thought to be caused primarily by ecological factors: migrational capability, type of feeding and trophic status of aquatic ecosystems. In general, the majority of studied species appeared to be of a high nutritive value for humans, although unfavorable environmental conditions could considerably diminish this value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, many extensive clinical and epidemiological studies have demonstrated a key importance of polyunsaturated fatty acids (PUFA) of the omega-3 (n-3) family, namely eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), for healthy functioning of human cardiovascular and neural systems [1,2,3,4]. To prevent many cardiovascular diseases and psychiatric disorders, a personal daily consumption of 0.5–1 g of EPA + DHA has been recommended by a number of national and international health organizations [5,6,7,8]. The main food source of EPA and DHA for most humans is fish [9,10,11,12]. However, various fish species differ in EPA and DHA contents in edible biomass by more than two orders of magnitude [10]. Some fish, especially freshwater species, have such low contents of EPA and DHA that it is impossible to obtain the recommended daily dose by eating these fish [e.g., 13, 14]. Thus, studies regarding PUFA contents of various fish species offer at least two benefits. First, continual improvement of databases on EPA and DHA contents in various fish species is necessary to provide individuals and public health officials with quantitative information on the attainability of desirable healthy PUFA intakes [5, 15]. Second, it is important to comprehend causes of the great variations of EPA and DHA in fish biomass.
In general, two groups of factors can control fatty acid (FA) composition and contents in aquatic animals: phylogenetic factors and ecological factors [14, 16, 17]. Relative contributions of these two groups of factors to fish FA contents, including that of EPA and DHA, are not completely known yet. Among ecological factors, feeding habits (planktivorous, benthivorous, piscivorous), habitat (marine vs. freshwater, pelagic vs. demersal and oligotrophic vs. eutrophic) and water temperature are regarded as controlling FA contents of fish. For instance, pelagic-feeding species are regarded as richer in lipids, including EPA and DHA, than demersal fish [12, 18]. Piscivorous fish are believed to have relatively higher EPA and DHA content [14, 19]. Marine fish seem to be richer in PUFA, including EPA and DHA, than freshwater species [20, 21]. Fish from oligotrophic water bodies appeared to have comparatively higher PUFA contents [22]. However, at least one phylogenetic factor, species identity, may outweigh the ecological factors with regard to EPA and DHA contents in fish [13, 23, 24]. Indeed, in spite of any ecological factors, the maximum value of EPA and DHA content in species from, for example, order Salmoniformes, are higher than that in order Cypriniformes [10]. Presumably, within each fish taxa (species, genus, …, order), there are genetically determined lower and upper limits of EPA and DHA contents, and only within these limits can variations of the PUFA contents be ascribed to ecological factors.
It is desirable to know the putative limits of EPA and DHA contents in fish taxa for many theoretical and applied purposes. For instance, we need to understand how global challenges, climate warming, anthropogenic pollution, eutrophication or biological invasions, which cause changes of natural fish species composition, will affect PUFA supplies for humans. The information about the taxon-specific limits also seems to be useful for fish aquaculture, especially for introducing new species potentially rich in EPA and DHA.
To determine the taxon-specific limits and to evaluate the contribution of ecological factors to EPA and DHA contents, it is necessary to quantify these contents as mass units, i.e., mg per g of fish biomass. Meanwhile, most published data are given in relative units, i.e. percent of total FA [25]. Nevertheless, to estimate the nutritive value of fish for humans, it is necessary to measure EPA and DHA contents in edible biomass (mg g−1), rather than then percent [18, 24, 26,27,28].
Thus, the aim of our study was to evaluate variations of fatty acid composition and contents of EPA and DHA within commercially important species of genus Coregonus in water bodies of the Russian Subarctic. To our knowledge, this was the first attempt to determine taxon (genus)-specific limits of EPA and DHA contents in wild fish. In addition, we aimed to test common ideas concerning differences in EPA and DHA contents between planktivorous and benthivorous fish using congeneric species. Last, we aimed to supplement existing data on EPA and DHA contents in fish with previously unexplored species.
Materials and Methods
Standards and Reagents
All organic solvents were of analytical grade and were purchased from Khimreactivsnab (Ufa, Russian Federation). Sodium of 99.8% grade was purchased from Acros Organic—Thermo Fisher Scientific (Geel, Belgium). We prepared 3 M sodium methoxide solution by cautiously dissolving sodium in methanol. The solution was stored at 4 °C no more than a week prior to usage. Standards of methyl esters of individual fatty acids (FAME) and their mixtures [29] were purchased from Sigma-Aldrich (USA). Solutions of the standard compounds were prepared in hexane at a concentration range of 0.5–5 mg mL−1 and analysed by GC–MS. Methyl ester of nonadecanoic acid (Sigma-Aldrich, USA) was used as an internal standard, a stock solution of which in chloroform at concentration of 1 mg mL−1 was prepared and stored at −20 °C.
Aquatic Environments
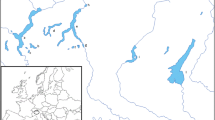
All sampled water bodies (Table 1) were oligotrophic (except nearly mesotrophic Lake Onega) and had low water temperature, 6.5–15 °C. Dominant phytoplankton taxa were Bacillariophyta [31, 38, 39]. A map of the sampled water bodies is given in Fig. 1.
Fish Sampling
Fish of commercial sizes were obtained from local authorized fishers just after catching.
Sampling was conducted in accordance with the BioEthics Protocol on Animal Care, approved by the Siberian Federal University. Species of genus Coregonus, collected in diverse water bodies, and numbers of samples are given in Table 2. Although feeding habits of these species were well known from literature, stomach contents of some specimens were taken for microscopic analyses to check their food items (Table 2).
Arctic cisco Coregonus autumnalis (Pallas, 1776) in the Yenisei River are semi-anadromous fish, which feed in the Yenisei Gulf (the Kara Sea) and migrate in the river for spawning [40]. The arctic cisco is a pelagic feeder which eats zooplankton, planktobenthic invertebrates and small fish [40] (Table 2).
Least cisco Coregonus sardinella Valenciennes, 1848 were caught in the Yenisei River and in the Sobachye Lake. Least cisco from the Yenisei River, like Arctic cisco, are semi-anadromous fish which feed in the Yenisei Gulf and migrate in the river for spawning. Least cisco from the Sobachye Lake are landlocked fish. The least cisco is primarily a zooplanktivore [40] (Table 2).
Peled Coregonus peled (Gmelin, 1789) in the Yenisei River is planktivore–benthivore [40] (Table 2).
Whitefish Coregonus lavaretus (Linnaeus, 1758) were caught in the Yenisei River, in the Sobachye Lake, in the Keret River and in Lake Onega. In the Keret River, C. lavaretus is semi-anadromous fish, which feed in the White Sea. C. lavaretus in all the water bodies were benthivorous [40,41,42,43] (Table 2).
Tugun Coregonus tugun (Pallas, 1814) were caught in the Yenisei River and in the Sobachye Lake. Tugun is a planktivorous–benthivorous species [40] (Table 2).
Broad whitefish Coregonus nasus (Pallas, 1776) were caught in the Yenisei River and in the Sobachye Lake. Broad whitefish is a benthivore [40] (Table 2).
Vendace Coregonus albula (Linnaeus, 1758) in the Bolshoie Krasnoie Lake is a planktivore [44, 45].
For biochemical analyses, samples of white muscle tissue of approximately 0.7–2 g, were taken 1–2 cm below the dorsal fin. When cutting the sample, we tried to avoid skin, red muscle and bones. The samples were subdivided into two subsamples, for moisture and fatty acid analyses, respectively. To measure moisture, subsamples of ca. 1–2 g of wet weight were weighed, dried at 75 °C until constant weight, and weighed dry. The other sub-samples of muscle tissue were immediately weighed, placed into chloroform/methanol mixture (2:1, by vol.) and kept until further analysis at −20 °C. The samples were transported frozen to the laboratory in 1–2 weeks. Lipid analyses were done within 2 months after sampling.
Fatty Acid Analysis
Lipids were extracted with chloroform/methanol (2:1, by vol.) three times, when tissues were simultaneously homogenized with glass beads in a mortar [11]. The extracts were dried with anhydrous Na2SO4 , and chloroform and methanol were roto evaporated under vacuum at 35 °C. The extracted lipid was dissolved in 1 mL of hexane, then 0.2 mL of 3 M methanolic sodium methoxide solution was added, and the mixture was shaken vigorously for 1 min. Subsequently, the mixture was kept quiet at ambient temperature for 5 min, and finally 2.5 mL of hexane and 5 mL of a saturated solution of NaCl were added. Contents were mixed for 1 min, transferred to a separatory funnel, and the lower aquatic layer was discarded. The hexane layer was washed one more time with an aliquot of the solution of NaCl and twice with 5 mL of distilled water. The hexane solution of FAME was dried with anhydrous Na2SO4, and hexane was removed by roto-evaporating at 30 °C. The FAME were redissolved in 150–300 μL of hexane prior to chromatographic analysis.
A gas chromatograph equipped with a mass spectrometer detector (model 6890/5975C; Agilent Technologies, USA) and with a 30-m long, 0.25-mm internal diameter capillary HP-FFAP column was used for FAME analysis. Detailed descriptions of the chromatographic and mass-spectrometric conditions are given elsewhere [46]. The FAME were quantified according to the peak area of the internal standard, 19:0-FAME, which we added to samples prior to lipid extraction.
Statistical Analysis
Kolmogorov–Smirnov one-sample test for normality D K–S, standard errors (SE), Student’s t tests, one-way ANOVA with post hoc Tukey HSD test, Kruskal–Wallis test (in the absence of normal distribution) and canonical correspondence analysis (CCA) [47] were calculated conventionally, using STATISTICA software, version 9.0 (StatSoft Inc., Tulsa, OK, USA).
Results
Moisture content of studied species had a small range of variation. C. lavaretus from the Sobachye Lake tended to have the lowest value of moisture, 66.1 ± 2.9%, while C. sardinella from the Sobachye Lake tended to have the highest value, 78.3 ± 0.5%. The difference between these values was statistically significant: P = 0.000129 after the Kruskal–Wallis test.
In all samples, 70 FA were identified. However, for the following analysis, only 25 quantitatively prominent FA (mean level > 0.5% at least in one fish species) were taken (Table 3). Thus, total sums of FA in Table 3 were lower than 100%.
The correspondence analysis demonstrated a marked partitioning of the same species from different water bodies, e.g., C. sardinella from the Yenisei River and the Sobachye Lake, C. tugun from the Yenisei River and the Sobachye Lake, and C. lavaretus from the Keret River and the Yenisei River (Fig. 2). Along Dimension 1, which represented the largest proportion of inertia, most overall differences in FA composition were found between C. lavaretus from the Keret River, on the one hand, and C. autumnalis and C. lavaretus from the Sobachye Lake, on the other hand (Fig. 2). These differences were mainly provided by contrast levels of 22:6n-3 and 16PUFA in the species (populations) (Fig. 2). Along Dimension 2, most differences were between C. autumnalis from the Yenisei River and C. tugun from the Sobachye Lake (Fig. 2). These differences primarily were due to the contrast between levels of Σ20:1 and 18:4n-3 in the species (Fig. 2).
Canonical correspondence analysis of levels of fatty acids (% of total) in species of genus Coregonus: autY—C. autumnalis from the Yenisei River (red circles); sarY—C. sardinella from the Yenisei River (black circles); pelY—C. peled from the Yenisei River (blue circles); lavY—C. lavaretus from the Yenisei River (green circles); tugY—C. tugun from the Yenisei River (violet circles); nasY—C. nasus from the Yenisei River (light-blue circles); sarS—C. sardinella from the Sobachye Lake (black squares); tugS—C. tugun from the Sobachye Lake (violet squares); nasS—C. nasus from the Sobachye Lake (light-blue squares); lavS—C. lavaretus from the Sobachye Lake (green squares); lavK—C. lavaretus from the Keret River (green diamonds); lavO—C. lavaretus from Lake Onega (green triangles); albB—C. albula from the Bolshoie Krasnoie Lake (rose crosses). Dimension 1 and Dimension 2 represented 48.1 and 15.5% of inertia, respectively
C. autumnalis from the Yenisei River tended to have minimal mean levels of 17:0, 20:4n-6 and 22:5n-6, but also tended to have maximum levels of Σ20:1 and 24PUFA (Table 3). C. sardinella from the Yenisei River tended to have the highest levels of 20:2n-6 (Table 3). C. peled from the Yenisei River tended to have the highest levels of 15-17BFA and 18:3n-3 (Table 3). C. tugun from the Yenisei River tended to have the lowest levels of 20:5n-3, 22:5n-3 and 22:6n-3, but the highest level of 18:1n-9 (Table 3). C. sardinella from the Sobachye Lake tended to have the highest levels of 22:5n-6 (Table 3). C. tugun from the Sobachye Lake tended to have the highest levels of 18:2n-6 (Table 3). C. nasus from the Sobachye Lake tended to have the highest levels of 18:0 and 18:1n-7 (Table 3). C. lavaretus from the Sobachye Lake tended to have the lowest levels of 15:0, 16:0 and 18:0, but the highest levels of 16:1n-7 and 16PUFA (Table 3). C. lavaretus from the Keret River tended to have the lowest level of 14:0, 15-17BFA, 18:2n-6, 18:3n-3, 18:4n-3, 20:3n-3, 20:4n-3 and 24PUFA but the highest level of 16:0, 20:5n-3, 22:5n-3 and 22:6n-3 (Table 3). C. lavaretus from Lake Onega tended to have the lowest level of 18:1n-9 and Σ20:1, but the highest levels of 16:1n-9 and 20:4n-6 (Table 3). C. albula from the Bolshoie Krasnoie Lake tended to have the lowest level of 16:1n-7, 16PUFA and 18:1n-7, but the highest levels of 14:0 (Table 3). C. autumnalis from the Yenisei River tended to have the highest content of total FA while C. lavaretus from the Keret River tended to have the lowest content of total FA, (Table 3). C. lavaretus from two other habitats, Lake Onega and the Yenisei River, like this species from the Keret River, had comparatively low content of total FA, while C. lavaretus from the Sobachye Lake had very high content of total FA (Table 3).
Mean contents of EPA + DHA in the studied congeneric species varied from 1.87 ± 0.06 mg g−1 wet weight in C. lavaretus from Lake Onega to 17.60 ± 3.63 mg g−1 wet weight in C. autumnalis from the Yenisei River (Fig. 3). C. lavaretus from the Sobachye Lake also had very high content of EPA + DHA in biomass, 16.61 ± 2.80 mg g−1 wet weight (Fig. 3). Thus, variations of average EPA and DHA contents between the congeneric species were ~ 10-fold, from 0.7 mg g−1 of C. lavaretus from the Keret River to 9.9 mg g−1 of C. lavaretus from the Sobachye Lake and from 1.1 mg g−1 of C. lavaretus from Lake Onega to 9.0 mg g−1 of C. autumnalis from the Yenisei River, respectively (Fig. 3). Meanwhile, variations of average percentages of EPA and DHA were ~ 4-fold only, from 6.3% of C. tugun from the Yenisei River to 12.1% of C. lavaretus from the Keret River and from 5.9% of C. tugun from the Yenisei River to 26.5% of C. lavaretus from the Keret River, respectively (Table 3).
Mean content (mg g−1 wet weight) of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and their sum (EPA + DHA) in species of genus Coregonus: autY—C. autumnalis from the Yenisei River; sarY—C. sardinella from the Yenisei River; pelY—C. peled from the Yenisei River; lavY—C. lavaretus from the Yenisei River; tugY—C. tugun from the Yenisei River; nasY—C. nasus from the Yenisei River; sarS—C. sardinella from the Sobachye Lake; tugS—C. tugun from the Sobachye Lake; nasS—C. nasus from the Sobachye Lake; lavS—C. lavaretus from the Sobachye Lake; lavK—C. lavaretus from the Keret River; lavO—C. lavaretus from Lake Onega; albB—C. albula from the Bolshoie Krasnoie Lake. Bars represent standard error. The lowest and the highest means, which significantly (P < 0.05) differ from each other after the Kruskal–Wallis test, are labelled with letters a and b, respectively. Number of samples, n, for each species are given in Table 2
Discussion
Intra-genus variations of EPA + DHA contents of Coregonus species, revealed in this study, were from 1.87 to 17.60 mg g−1 wet weight. Values of the contents of another species of this genus, published in available literature, fell in the above range and varied from 3.1 mg g−1 wet weight in lake whitefish C. clupeaformis ([48], recalculated from dry weight using mean moisture content in Salmoniformes 72.5%) to 10.7 mg g−1 wet weight in European whitefish C. macrophtalmus ([14], recalculated from Table 5 of the source). Thus, in the present study we expanded the lower and upper limits of intra-genus variations of EPA + DHA contents in wild Coregonus species. Moreover, to our knowledge, the highest values of EPA + DHA content in anadromous and freshwater fish, published in available literature, were 11.06 mg g−1 wet weight in Chinook salmon (Oncorhynchus tshawytscha) [49] and 11.07 mg g−1 wet weight in lake trout (Salvelinus namaycush) [50], calculated from Table 5 of the source), respectively. In our study, the maximum value for semi-anadromous species, C. autumnalis, was 17.60 mg g−1 wet weight, and for the landlocked C. lavaretus from the Sobachye Lake this value was 16.61 mg g−1 wet weight. Hence, in the present work, we expanded considerably the upper limit of EPA + DHA contents for anadromous and freshwater fish.
The new maximum values of EPA + DHA content in the semi-anadromous C. autumnalis and the freshwater C. lavaretus are still lower than the maximum value of EPA + DHA content in marine fish, published in available literature, 25.6 mg g−1 wet weight in Sardine (Sardinops sagax) [28]. Meanwhile, many freshwater fish species, especially in pristine cold oligotrophic Arctic lakes of Russia, which may have very high content of EPA and DHA, are as yet unexplored. In any case, regarding present findings, the common point of view on higher PUFA contents in marine fish [20, 21, 51] should be taken with caution. Indeed, EPA + DHA contents in C. autumnalis and in C. lavaretus were considerably higher than those in a majority of marine fish reviewed in [10]. The high nutritive value of freshwater fish for humans was revealed in this work. Thus, “more must be learned about the possible benefits of freshwater fish consumption in different areas of the world” [52, p. 1305].
Since congeneric and especially conspecific fish were believed to be genetically close to each other, the above variations in EPA and DHA contents were likely caused primarily by ecological factors. Among the ecological factors, water temperature was often regarded as a driver of the PUFA contents in fish. The effect of water temperature was explained by a hypothesis of “homeoviscous adaptation”, which predicted a decrease of a degree of saturation of phospholipid FA with an increase of temperature to maintain an optimal cell membrane fluidity [53]. For instance, Arts et al. [54] found that under a laboratory conditions an increase of water temperature from 12 to 19 °C caused a decrease of DHA content in juvenile Atlantic salmon (Salmo salar) from 4.6 to 3.3 mg g−1 wet weight (recalculated from dry weight using mean moisture content in Salmoniformes 72.5%). There are also some data on higher PUFA contents in wild fish in cold waters compared to those in warm waters [55, 56]. However, other authors did not find any significant effect of water temperature on the PUFA levels in fish in a laboratory or in natural waters [18, 57,58,59,60,61]. Moreover, in many works the putative peculiar role of EPA or DHA in the temperature adaptations of the cell membrane properties (fluidity, order, curvature and elastic stress) was not confirmed [53, 58, 62,63,64]. In any case, in our study water temperature in the subarctic water bodies was below 16 °C and hardly contributed considerably to the observed differences in EPA and DHA contents between the studied species. Indeed, in the Yenisei River, or in the Sobachye Lake, Coregonus species, which dwelt together under the same temperature, had significantly different contents of these PUFA.
Another important ecological factor, which affects FA composition and content in fish biomass, is nutrition. Fish food chains in inland waters are known to be based on autochthonous resources, microalgae and, to some extent, on allochthonous (terrestrial) organic matter. Autochthonous resources are regarded to be of a high biochemical quality for consumers, including fish, especially in oligotrophic water bodies, where diatom, cryptophyte and dinoflagellate algae, rich in EPA and DHA, are dominant species [22, 65]. In our study, all water bodies were oligotrophic, diatom-dominated rivers and lakes, except the mesotrophic Lake Onega. It is worth noting that C. lavaretus from Lake Onega had the lowest content of EPA and DHA in biomass. Hence, the above result seems to be in a good agreement with data of other authors on higher content of PUFA in fish from oligotrophic water bodies [22, 65]. Moreover, C. lavaretus from Lake Onega had the highest level of arachidonic acid 20:4n-6, which is regarded as marker of allochthonous (terrestrial) organic matter of comparatively low nutritive value [31]. Thus, the lowest content of EPA + DHA of C. lavaretus from Lake Onega among the studied fish was likely determined by the low quality of its food sources.
Planktivorous (pelagic-feeding) fish are considered to have higher EPA and DHA contents than benthivorous (demersal) species [12, 18]. According to the above point of view, in our study, in the Yenisei River planktivorous C. autumnalis and C. sardinella tended to have higher EPA and DHA contents than benthivorous C. lavaretus and C. nasus, while planktivorous–benthivorous C. peled and C. tugun had intermediate values. However, the high contents in C. autumnalis and C. sardinella may be explained by causes other than only pelagic feeding (see below). Moreover, in Sobachye Lake, the planktivorous C. sardinella had the lowest EPA + DHA content, while the highest content was characteristic of the benthivorous C. lavaretus. Thus, planktivorous species of Coregonus genus did not necessary have a higher EPA and DHA contents compared to benthivorous species.
As mentioned above, marine fish are commonly regarded to be richer in PUFA content compared with freshwater fish [20, 21, 51]. In our study, the highest EPA and DHA contents were characteristic of the semi-anadromous C. autumnalis, which fed in the Yenisei Gulf of the Kara Sea and then migrated in the Yenisei River for spawning. Indeed, C. autumnalis had the highest level of the sum of 20:1 fatty acids. These acids, namely 20:1n-9 and 20:1n-7, are known to be markers of marine copepods [66, 67]. Evidently, this species assimilated organic matter of marine origin, which seemed to be of very high nutritive value. For instance, marine planktonic copepods are extremely rich in lipids, which constitute up to 75% of their dry mass [68]. Moreover, C. autumnalis had the lowest proportion of the marker of low-quality terrestrial organic matter, 20:4n-6 [31]. Similarly, anadromous (marine) forms of another species of Salmoniformes, Oncorhynchus nerka, had considerable levels of Σ20:1 in their biomass, while in landlocked forms (kokanee) these FAs were nearly absent [24, 69]. In turn, levels of 20:4n-6 in the marine O. nerka were significantly lower than that in kokanee [24, 69]. Thus, the migrating C. autumnalis had explicit markers of food of marine origin, while the contribution of low-quality terrestrial organic matter was considerably lower, than that in the land-locked river and lake fish species.
Another semi-anadromous species from the Yenisei River, C. sardinella, also tended to have a higher level of Σ20:1 and a lower level of 20:4n-6 than land-locked C. sardinella from the Sobachye Lake. However, the migratory species from the Keret River, C. lavaretus, did not have an explicitly higher level of Σ20:1, and lower level of 20:4n-6 than land-locked species. In addition, it should be noted that some 20:1 acids are markers of mollusks [70]. Indeed, C. nasus from the Yenisei River, which consumed primarily mollusks, had a comparatively high level of Σ20:1.
Comparing anadromous (semi-anadromous) and land-locked conspecific fish, it should be taken in account that land-locked populations may have a higher capacity of biosynthesis (conversion) of long-chain PUFA, EPA and DHA from short-chain n-3 precursor FA compared to that of anadromous populations [71, 72]. For instance, this putative ability might partly explain the higher EPA + DHA content of land-locked C. lavaretus from the Sobachye Lake vs. the lower content of migratory C. lavaretus from the Keret River.
What range of variations of EPA and DHA content in fish muscle tissues can be provided by feeding conditions? Species of the order Salmoniformes, Atlantic salmon (Salmo salar), reared in aquaculture using food based on vegetable and fish oil, had EPA + DHA content 3.2 and 7.0 mg g−1, respectively [73]. Similarly, Oncorhynchus mykiss, reared in aquaculture using vegetable and fish oil, had EPA + DHA content 3.7 and 8.3 mg g−1, respectively [74]. The above inter-species ranges of variations, provided by the changing of food composition in aquaculture, are evidently narrower than the inter-genus ranges of variations of EPA + DHA content, revealed in our study. Thus, feeding conditions might not play the principal role in variations of EPA and DHA content in fish compared with the other ecological and phylogenetic factors. For instance, based on the putative importance of food, Ahlgren et al. [65] supposed that different fish species from the same ecosystem, with access to the same food items should have similar FA content. However, in our study, the congeneric benthivorous fish species from the Sobachye Lake, C. lavaretus and C. nasus, had significantly different EPA and DHA contents.
It is well known that contents of lipids (total fatty acids) in fish tissues are highly variable and depend on feeding and reproduction season [14, 19, 75]. In our study, content of total FA, which tightly correlated with total lipid content in fish [65], varied significantly. Since all species were sampled before spawning season, these variations were believed to be caused primarily by food availability in particular aquatic ecosystems. It is worth noting that all fish were obtained in the periods of officially permitted commercial fishery. The EPA and DHA content in fish is the indicator of their nutritive value for humans. Therefore, measuring of the nutritive value in the period of commercial fishery seemed to be reasonable.
In our study, a considerable discrepancy between levels (percentages) of PUFA and their content in mass units in fish biomass was found, as in many other studies [14, 24, 26,27,28]. Indeed, C. lavaretus from the Keret River had the highest EPA and DHA levels, 12.1 and 26.5%, respectively, while it had one of the lowest contents of EPA + DHA, 2.33 mg g−1 wet weight. This phenomenon might be explained by a difference between PUFA contents in polar lipids, phospholipids (PL) and neutral lipids, triacylglycerols (TAG). The functionally important EPA and DHA are known to be contained mostly in PL, which are structural lipids of cell membranes, and their constant proportions are essential for muscle tissue functioning [76]. Thus, a high proportion of EPA and DHA seems to be maintained in fish muscles even under unfavorable feeding conditions. Meanwhile, under favorable feeding conditions, fish accumulate storage lipids, TAG, which are relatively poor in PUFA and contain mainly saturated and monounsaturated FA [18, 77]. Therefore, fatty fish with high total lipid (total FA) contents have high EPA and DHA contents in mass units, but levels (percent of total FA) of these PUFA are ‘diluted’ by the other FAs in TAG. Hence, our study confirmed that the nutritive value of fish species for humans should be estimated based on mass units, mg per gram of consumed tissues, rather than on the basis of total FA percentage.
Total FA content, as the proxy of total lipid content [65], seemed to have a considerable effect on EPA + DHA content in fish species. Indeed, C. lavaretus from the Sobachye Lake had the highest total FA content and also the highest EPA + DHA content among the studied populations. In turn, C. lavaretus from Lake Onega, the Keret River and the Yenisei River had considerably lower total FA contents and accordingly lower EPA + DHA contents.
In the present work, the data on EPA and DHA contents in seven species of the genus Coregonus were obtained for the first time, except the only report for C. lavaretus [78]. A majority of these species in most of the studied water bodies appeared to be valuable food sources for humans regarding their high EPA and DHA content. However, environmental conditions of the species habitats should be taken into account in future works, since some ecological factors could diminish the species (genus)-specific contents of the essential PUFA in fish biomass.
Abbreviations
- BFA:
-
Branched fatty acid(s)
- CCA:
-
Canonical correspondence analysis
- DHA:
-
Docosahexaenoic acid (22:6n-3)
- EPA:
-
Eicosapentaenoic acid (20:5n-3)
- FA:
-
Fatty acid(s)
- FAME:
-
Fatty acid methyl ester(s)
- GC–MS:
-
Gas chromatography–mass spectrometry
- PL:
-
Phospholipids
- PUFA:
-
Polyunsaturated fatty acid(s)
- TAG:
-
Triacylglycerol(s)
References
Hibbeln JR, Nieminen LRG, Blasbalg TL, Riggs JA, Lands WEM (2006) Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr 83:1483S–1493S
McNamara RK, Carlson SE (2006) Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 75:329–349
Adkins Y, Kelley DS (2010) Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem 21:781–792
De Caterina R (2011) n–3 Fatty acids in cardiovascular disease. N Engl J Med 364:2439–2450
Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J (2009) Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr 139:804S–819S
Kris-Etherton PM, Grieger JA, Etherton TD (2009) Dietary reference intakes for DHA and EPA. Prostaglandins Leukot Essent Fatty Acids 81:99–104
Casula M, Soranna D, Catapano AL, Corrao G (2013) Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: a meta-analysis of randomized, double blind, placebo controlled trials. Atheroscler Suppl 14:243–251
Nagasaka R, Gagnon C, Swist E, Rondeau I, Massarelli I, Cheung W, Ratnayake WMN (2014) EPA and DHA status of South Asian and White Canadians living in the National Capital Region of Canada. Lipids 49:1057–1069
Robert SS (2006) Production of eicosapentaenoic and docosahexaenoic acid-containing oils in transgenic land plants for human and aquaculture nutrition. Mar Biotechnol 8:103–109
Gladyshev MI, Sushchik NN, Makhutova ON (2013) Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins Other Lipid Mediat 107:117–126
Gladyshev MI, Makhutova ON, Gubanenko GA, Rechkina EA, Kalachova GS, Sushchik NN (2015) Livers of terrestrial production animals as a source of long-chain polyunsaturated fatty acids for humans: an alternative to fish? Eur J Lipid Sci Technol 117:17–1421
Tacon AGJ, Metian M (2013) Fish matters: importance of aquatic foods in human nutrition and global food supply. Rev Fish Sci 21:22–38
Kwetegyeka J, Mpango G, Grahl-Nielsen O (2008) Variation in fatty acid composition in muscle and heart tissues among species and populations of tropical fish in lakes Victoria and Kyoga. Lipids 43:1017–1029
Vasconi M, Caprino F, Bellagamba F, Busetto ML, Bernardi C, Puzzi C, Moretti VM (2015) Fatty acid composition of freshwater wild fish in subalpine lakes: a comparative study. Lipids 50:283–302
Chuang L-T, Bulbul U, Wen P-C, Glew RH, Ayaz FA (2012) Fatty acid composition of 12 fish species from the Black Sea. J Food Sci 77:C512–C518
Makhutova ON, Sushchik NN, Gladyshev MI, Ageev AV, Pryanichnikova EG, Kalachova GS (2011) Is the fatty acid composition of freshwater zoobenthic invertebrates controlled by phylogenetic or trophic factors? Lipids 46:709–721
Lau DCP, Vrede T, Pickova J, Goedkoop W (2012) Fatty acid composition of consumers in boreal lakes—variation across species, space and time. Freshw Biol 57:24–38
Litzow MA, Bailey KM, Prahl FG, Heintz R (2006) Climate regime shifts and reorganization of fish communities: the essential fatty acid limitation hypothesis. Mar Ecol Prog Ser 315:1–11
Sushchik NN, Gladyshev MI, Kalachova GS (2007) Seasonal dynamics of fatty acid content of a common food fish from the Yenisei River, Siberian grayling, Thymallus arcticus. Food Chem 104:1353–1358
Garg ML, Wood LG, Singh H, Moughan PJ (2006) Means of delivering recommended levels of long chain n-3 polyunsaturated fatty acids in human diets. J Food Sci 71:R66–R71
Rubio-Rodriguez N, Beltran S, Jaime I, de Diego SM, Sanz M, Carballido JR (2010) Production of omega-3 polyunsaturated fatty acid concentrates: a review. Innov Food Sci Emerg Technol 11:1–12
Taipale SJ, Vuorioc K, Strandberg U, Kahilainen KK, Jarvinen M, Hiltunen M, Peltomaa E, Kankaala P (2016) Lake eutrophication and brownification downgrade availability and transfer of essential fatty acids for human consumption. Environ Int 96:156–166
Ahlgren G, Vrede T, Goedkoop W (2009) Fatty acid ratios in freshwater fish, zooplankton and zoobenthos—are their specific optima? In: Arts MT, Kainz M, Brett MT (eds) Lipids in aquatic ecosystems. Springer, New York
Gladyshev MI, Lepskaya EV, Sushchik NN, Makhutova ON, Kalachova GS, Malyshevskaya KK, Markevich GN (2012) Comparison of polyunsaturated fatty acids content in filets of anadromous and landlocked sockeye salmon Oncorhynchus nerka. J Food Sci 77:C1306–C1310
Hixson SM, Sharma B, Kainz MJ, Wacker A, Arts MT (2015) Production, distribution, and abundance of long-chain omega-3 polyunsaturated fatty acids: a fundamental dichotomy between freshwater and terrestrial ecosystems. Environ Rev 23:414–424
Gladyshev MI, Sushchik NN, Gubanenko GA, Demirchieva SM, Kalachova GS (2007) Effect of boiling and frying on the content of essential polyunsaturated fatty acids in muscle tissue of four fish species. Food Chem 101:1694–1700
Gladyshev MI, Artamonova VS, Makhrov AA, Sushchik NN, Kalachova GS, Dgebuadze YY (2017) Triploidy does not decrease contents of eicosapentaenoic and docosahexaenoic acids in filets of pink salmon Oncorhynchus gorbuscha. Food Chem 216:66–69
Huynh MD, Kitts DD (2009) Evaluating nutritional quality of pacific fish species from fatty acid signatures. Food Chem 114:912–918
Popova ON, Haritonov AY, Sushchik NN, Makhutova ON, Kalachova GS, Kolmakova AA, Gladyshev MI (2017) Export of aquatic productivity, including highly unsaturated fatty acids, to terrestrial ecosystems via Odonata. Sci Total Environ 581–582:40–48
Gladyshev MI, Gribovskaya IV, Adamovich VV (1993) Disappearance of phenol in water samples taken from the Yenisei River and the Krasnoyarsk reservoir. Water Res 27:1063–1070. doi:10.1016/0043-1354(93)90071-O
Gladyshev MI, Kolmakova OV, Tolomeev AP, Anishchenko OV, Makhutova ON, Kolmakova AA, Kravchuk ES, Glushchenko LA, Kolmakov VI, Sushchik NN (2015) Differences in organic matter and bacterioplankton between sections of the largest Arctic river: mosaic or continuum? Limnol Oceanogr 60:1314–1331
Pichugin MY (2009) The development of an artificial hybrid and revealing elements of reproductive isolation between sympatric forms of Dryagin’s char and Salvelinus alpinus complex (Salmonidae) from Sobachye mountain lake (Taimyr). J Ichthyol 49:236–248
Leonov AV, Filatov NN, Zdorovennov RE, Zdorovennova GE (2006) Mathematical modeling of the ecosystem functioning conditions in the Chupa estuary of the White Sea: transformation of organogenic substances and bioproductivity of the marine environment. Water Resour 33:543–567
Ivanov VV, Brizgalo VA (2007) Hydrology and hydrochemistry of watershed. In: Filatov N, Terzhevik A (eds) The White Sea and its watershed under influences of climate and anthropogenic impacts. Karelian Research Center of the RAS, Petrozavodsk (in Russian)
Gritsevskaya GL, Kyabeleva GK, Nikolayeva LA, Semenov VN (1972) Hydrology and hydrochemistry of Solovetsky lakes. Proc SevNIORH 6:5–44 (in Russian)
Savina EA (1991) Evaluation of degree of water pollution of surface waterbodies of the Solovetsky archipelago. In: Boyarsky PV (ed) Unknown Solovkee. Russian Scientific Research Institute of Cultural and Natural Heritage, Moscow (in Russian)
Domanitsky AP, Dubrovina RG, Isaeva AI (1971) Rivers and lakes of Soviet Union. Gidrometeoizdat, Leningrad (in Russian)
Timakova TM (2010) General description of the lake. In: Filatov NN (ed) Lake Onega. Atlas. Karelian Research Centre of the RAS, Petrozavodsk (in Russian)
Glushchenko LA (2016) Hydrobiological study of Lake Sobachye. Annals of nature recorded by Federal Institution “Nature Reserves of Taimyr”, issue 3. Norilsk (in Russian)
Vyshegorodcev AA (2000) Fishes of the Yenisei. Nauka, Novosibirsk (in Russian)
Berger VY (1995) Biological resources and problems of their rational exploitation. In: Scarlato OA (ed) White Sea Part II. Zoological Institute of the RAS, St.-Petersburg (in Russian)
Reshetnikov YS, Lukin AA (2008) Coregonids. In: Kukharev VI, Lukin AA (eds) Bioresources of Lake Onego. Karelian Research Centre of the RAS, Petrozavodsk (in Russian)
Sterligova OP, Ilmast NV, Savosin DS (2016) Cyclostomata and fish of freshwater of Karelia. Karelian Scientific Center of RAS, Petrozavodsk (in Russian)
Rusakova SA (1972) Feeding of vendace of the Goreloye Lake and the Bolshoie Krasnoie Lake. Proc SevNIORH 6:85–89 (in Russian)
Borovikova EA, YaI Alekseeva, Schreider MJ, Artamonova VS, Makhrov AA (2013) Morphology and genetics of the ciscoes (Actinopterygii: Salmoniformes: Salmonidae: Coregoninae: Coregonus) from the Solovetsky Archipelago (White Sea) as a key to determination of the taxonomic position of ciscoes in Northeastern Europe. Acta Ichthyol Piscat 43:183–194
Gladyshev MI, Sushchik NN, Gubanenko GA, Makhutova ON, Kalachova GS, Rechkina EA, Malyshevskaya KK (2014) Effect of the way of cooking on contents of essential polyunsaturated fatty acids in filets of zander. Czech J Food Sci 32:226–231
Legendre P, Legendre L (1998) Numerical ecology. Elsevier Science, Amsterdam
Wagner T, Jones ML, Ebener MP, Arts MT, Brenden TO, Honeyfield DC, Wright GM, Faisal M (2010) Spatial and temporal dynamics of lake whitefish (Coregonus clupeaformis) health indicators: linking individual-based indicators to a management-relevant endpoint. J Great Lakes Res 36:121–134
Cladis DP, Kleiner AC, Freiser HH, Santerre CR (2014) Fatty acid profiles of commercially available finfish fillets in the United States. Lipids 49:1005–1101
Neff MR, Bhavsar SP, Braekevelt E, Arts MT (2014) Effects of different cooking methods on fatty acid profiles in four freshwater fishes from the Laurentian Great Lakes region. Food Chem 164:544–550
Guler GO, Aktumsek A, Cakmak YS, Zengin G, Citil OB (2011) Effect of season on fatty acid composition and n-3/n-6 ratios of zander and carp muscle lipids in Altinapa Dam Lake. J Food Sci 76:C594–C597
Philibert A, Vanier C, Abdelouahab N, Chan HM, Mergler D (2006) Fish intake and serum fatty acid profiles from freshwater fish. Am J Clin Nutr 84:1299–1307
Arts MT, Kohler CC (2009) Health and condition in fish: the influence of lipids on membrane competency and immune response? In: Arts MT, Kainz M, Brett MT (eds) Lipids in aquatic ecosystems. Springer, New York
Arts MT, Palmer ME, Skiftesvik AB, Jokinen IE, Browman HI (2012) UVB radiation variably affects n-3 fatty acids but elevated temperature reduces n-3 fatty acids in juvenile Atlantic salmon (Salmo salar). Lipids 47:1181–1192
Wall R, Ross RP, Fitzgerald GF, Stanton C (2010) Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 68:280–289
Pethybridge HR, Parrish CC, Morrongiello J, Young JW, Farley JH, Gunasekera RM, Nichols PD (2015) Spatial patterns and temperature predictions of tuna fatty acids: tracing essential nutrients and changes in primary producers. PLoS One 10(7):e0131598
Gokce MA, Tasbozan O, Celik M, Tabakoglu SS (2004) Seasonal variations in proximate and fatty acid compositions of female common sole (Solea solea). Food Chem 88:419–423
Laurel BJ, Copeman LA, Parrish CC (2012) Role of temperature on lipid/fatty acid composition in Pacific cod (Gadus macrocephalus) eggs and unfed larvae. Mar Biol 159:2025–2034
Murzina SA, Nefedova ZA, Falk-Petersen S, Ripatti PO, Ruokolainen TR, Pekkoeva SN, Nemova NN (2013) Lipid status of the two high latitude fish species, Leptoclinus maculatus and Lumpenus fabricii. Int J Mol Sci 14:7048–7060
Wijekoon MPA, Parrish CC, Mansour A (2014) Effect of dietary substitution of fish oil with flaxseed or sunflower oil on muscle fatty acid composition in juvenile steelhead trout (Oncorhynchus mykiss) reared at varying temperatures. Aquaculture 433:74–81
Gribble MO, Karimi R, Feingold BJ, Nyland JF, O’Hara TM, Gladyshev MI, Chen CY (2016) Mercury, selenium and fish oils in marine food webs and implications for human health. J Mar Biol Assoc UK 96:43–59
Stillwell W, Wassall SR (2003) Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids 126:1–27
Dymond MK (2015) Mammalian phospholipid homeostasis: homeoviscous adaptation deconstructed by lipidomic data driven modelling. Chem Phys Lipids 191:136–146
Dymond MK (2016) Mammalian phospholipid homeostasis: evidence that membrane curvature elastic stress drives homeoviscous adaptation in vivo. J R Soc Interface 13:20160228
Ahlgren G, Sonesten L, Boberg M, Gustafsson I-B (1996) Fatty acid content of some freshwater fish in lakes of different trophic levels—a bottom-up effect? Ecol Freshw Fish 5:15–27
Graeve M, Albers C, Kattner G (2005) Assimilation and biosynthesis of lipids in Arctic Calanus species based on feeding experiments with a 13C labelled diatom. J Exp Mar Biol Ecol 317:109–125
Kattner G, Hagen W (2009) Lipids in marine copepods: latitudinal characteristics and perspectives to global warming. In: Arts MT, Kainz M, Brett MT (eds) Lipids in aquatic ecosystems. Springer, New York
Lee RF, Hagen W, Kattner G (2006) Lipid storage in marine zooplankton. Mar Ecol Prog Ser 307:273–306
Ozawa A, Satake M, Fujita T (1993) Comparison of muscle lipid composition between marine and landlocked forms of sockeye salmon (Oncorhynchus nerka). Comp Biochem Physiol B 106:513–516
Makhutova ON, Protasov AA, Gladyshev MI, Sylaieva AA, Sushchik NN, Morozovskaya IA, Kalachova GS (2013) Feeding spectra of bivalve mollusks Unio and Dreissena from Kanevskoe Reservoir, Ukraine: are they food competitors or not? Zool Stud 52:56
Rollin X, Peng J, Pham D, Ackman RG, Yvan Larondelle (2003) The effects of dietary lipid and strain difference on polyunsaturated fatty acid composition and conversion in anadromous and landlocked salmon (Salmo salar L.) parr. Comp Biochem Physiol B 134:349–366
Betancor MB, Olsen RE, Solstorm D, Skulstad OF, Tocher DR (2016) Assessment of a land-locked Atlantic salmon (Salmo salar L.) population as a potential genetic resource with a focus on long-chain polyunsaturated fatty acid biosynthesis. Biochim Biophys Acta 1861:227–238
Torstensen BE, Froyland L, Ornsrud R, Lie O (2004) Tailoring of a cardioprotective muscle fatty acid composition of Atlantic salmon (Salmo salar) fed vegetable oils. Food Chem 87:567–580
Stone DAJ, Oliveira ACM, Plante S, Smiley S, Bechtel P, Hardy RW (2011) Enhancing highly unsaturated omega-3 fatty acids in phase-fed rainbow trout (Oncorhynchus mykiss) using Alaskan fish oils. Aquacult Nutr 17:E501–E510
Sushchik NN, Rudchenko AE, Gladyshev MI (2017) Effect of season and trophic level on fatty acid composition and content of four commercial fish species from Krasnoyarsk Reservoir (Siberia, Russia). Fish Res 187:178–187
Mairesse G, Thomas M, Gardeur J-N, Brun-Bellut J (2006) Effects of geographic source, rearing system, and season on the nutritional quality of wild and farmed Perca fluviatilis. Lipids 41:221–229
Benedito-Palos L, Calduch-Giner JA, Ballester-Lozano GF, Perez-Sanchez J (2013) Effect of ration size on fillet fatty acid composition, phospholipid allostasis and mRNA expression patterns of lipid regulatory genes in gilthead seabream (Sparus aurata). Br J Nutr 109:1175–1187
Suomela J-P, Lundén S, Kaimainen M, Mattila S, Kallio H, Airaksinen S (2016) Effects of origin and season on the lipids and sensory quality of European whitefish (Coregonus lavaretus). Food Chem 197:1031–1037
Acknowledgements
The work was supported by Grant of Russian Science Foundation No. 16-14-10001. We are grateful to Ya. I. Alekseeva, V. S. Artamonova, I. L. Schurov, V. A. Shirokov for their kind help in sample collecting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
About this article
Cite this article
Gladyshev, M.I., Sushchik, N.N., Makhutova, O.N. et al. Fatty Acid Composition and Contents of Seven Commercial Fish Species of Genus Coregonus from Russian Subarctic Water Bodies. Lipids 52, 1033–1044 (2017). https://doi.org/10.1007/s11745-017-4304-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-017-4304-8