Abstract
We studied the fatty acid (FA) content and composition of ten zoobenthic species of several taxonomic groups from different freshwater bodies. Special attention was paid to essential polyunsaturated fatty acids, eicosapentaenoic acid (EPA, 20:5n-3), docosahexaenoic acid (DHA, 22:6n-3), and arachidonic acid (ARA, 20:4n-6); and the n-3/n-6 and DHA/ARA ratios, which are important for consumers of higher trophic levels, i.e., fish. The content and ratios of these FA varied significantly in the studied zoobenthic species, consequently, the invertebrates were of different nutritional quality for fish. Eulimnogammarus viridis (Crustacea) and Dendrocoelopsis sp. (Turbellaria) had the highest nutrition value for fish concerning the content of EPA and DHA and n-3/n-6 and DHA/ARA ratios. Using canonical correspondence analysis we compared the FA profiles of species of the studied taxa taking into account their feeding strategies and habitats. We gained evidence that feeding strategy is of importance to determine fatty acid profiles of zoobenthic species. However, the phylogenetic position of the zoobenthic species is also responsible and may result in a similar fatty acid composition even if species or populations inhabit different water bodies or have different feeding strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The fatty acid composition and FA ratios of different taxonomic groups are widely used as biochemical markers of trophic interactions in aquatic ecosystems [1, 2]. FA markers have been used to trace the transfer of the organic matter through aquatic food webs and to evaluate diet patterns of the aquatic animals [3–5]. Some polyunsaturated fatty acids (PUFA), namely eicosapentaenoic and docosahexaenoic acids are essential components in the nutrition of aquatic invertebrates and fish [6, 7]. It should be noted that the essential PUFA are of large physiological importance for animals of different taxonomic levels, including humans [8–10]. Besides the PUFA content, the n-3/n-6 and DHA/ARA ratios are very important. These ratios are quite species-specific and their dietary levels must be optimal to sustain normal growth and development of organisms [11].
Benthic invertebrates (zoobenthos) are one of the most important components of riverine ecosystems and a major food source for fish. The most data on fatty acid content and the composition of benthic invertebrates come primarily from the aquaculture of marine economically important food species [12–16]. In contrast, data on the fatty acid composition of freshwater zoobenthos in rivers are scarce.
As it is known, the FA profiles of aquatic animals can be affected both by their food sources and by genetically predetermined metabolism [17]. The relative importance of these factors is continuously discussed, especially for zooplankton species. On the one hand, there is experimental evidence that some zooplankton species, e.g. Daphnia, have quite variable FA profiles that are strongly influenced by diet [18–21]. Variations in FA composition of natural zooplankton were also determined primarily by seasonal changes in the FA composition of their food, seston [22]. On the other hand, some zooplankton species are believed to selectively accumulate and metabolically alter dietary essential fatty acids [23–26].
In contrast to the comparatively well-studied zooplankton, there are sparse data on factors determining FA profiles of freshwater benthic invertebrates. Though there are several studies that demonstrate a capacity of zoobenthic species to synthesize long-chain PUFA from short-chain precursors. For instance, the free-living nematode Caenorhabditis elegans evidently has a unique pathway of PUFA synthesis from oleic acid to linoleic (LNA, 18:2n-6) and α-linolenic (ALA, 18:3n-3) acids and then to ARA and EPA, and, as a result, FA composition of C. elegans does not reflect the FA composition of its food [27]. Larvae of chironomids, Chironomus riparius are able to synthesize a large enough quantity of ARA and EPA from dietary LNA and ALA to support normal larval growth and development [28]. However, the above-mentioned zoobenthic species neither synthesized DHA nor accumulated this acid from their food [28]. We have previously demonstrated using fatty acids as trophic markers that benthic Chironomus plumosus selectively consumed food particles originating from green algae and cyanobacteria and contained little EPA and no DHA [4]. Riverine larvae of chironomids were shown to contain only traces of DHA [29, 30]. In contrast to the nematode and chironomids mentioned, larvae of caddisfly, Hydropsyche sp., have been shown to have a very limited ability to elongate and desaturate C18 PUFA to C20 PUFA [31]. There is evidence that the PUFA composition in gammarids depends on both diet and the inherent capacity of the animals to desaturate and elongate linoleic and linolenic acids to long-chain PUFA [32].
Kraffe and coauthors [33] have revealed a striking relationship between cardiolipin FA composition and phylogenetic groups of bivalve mollusks. They have suggested that cardiolipin FA profiles in bivalves are likely similar in species of the same phylogenetic group.
The majority of the above cited studies were focused on rather narrow phylogenetic groups of zoobenthos or did not consider the effect of phylogenetic factors. Therefore, a notion still occurs that trophic factors are key determinants of FA composition including essential PUFA in most aquatic invertebrates. We considered that studies covering a wider range of taxa and habitats were necessary to elucidate the true role of trophic and phylogenetic factors as determinants of FA profiles in zoobenthic invertebrates.
The aim of the present work was to compare the FA composition of freshwater benthic invertebrates of diverse taxonomic groups, potentially important for fish feeding, from different aquatic ecosystems. We addressed the following questions: (1) Whether phylogenetically close species inhabiting the same water body have different fatty acid spectra? (2) Can the populations of the same species inhabiting different water bodies with variable food sources, have a similar fatty acid composition? (3) Do phylogenetic factors are important determinants of essential EPA and DHA contents in benthic invertebrates?
Materials and Methods
Sampling and Fatty Acid Analysis
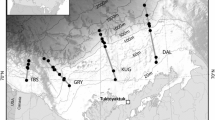
We included ten zoobenthic species in the study; most of them were taken from one water body, and only one species, C. plumosus, was sampled from two water bodies. Invertebrates were collected from the Yenisei River, Rybinskoe reservoir, Bugach reservoir and Chistoe lake (Table 1). The total number of samples was 100. Samples were collected in different periods of the year due to the seasonal occurrence and dominance of the species. The list of species, number of samples, and sampling periods are shown in Table 1. Each sample consisted of 2–20 individuals of various sizes, most of them were adults or premature larval stages. Whole bodies of the animals were taken for the analyses, except dreissenids, from which the closing muscle was removed and used for the following analysis.
Organisms from the river littoral were sampled using a Surber-type kick-bottom sampler by disturbing an area in a frame 40 × 35 cm upstream of an attached net (mouth 40 × 40 cm, mesh size 0.25 mm). Organisms from the reservoirs and the lake were sampled using Petersen-type bottom samplers. Immediately after sorting, the live animals were placed in beakers with tap water for 24 h to empty their guts. Then the animal’s body surfaces were gently wiped with filter paper and the animals were weighed and placed in chloroform:methanol mixture (2:1, v/v) and kept until further analysis at −20 °C.
Laboratory FA analyses and the comprehensive identification of fatty acids are described in detail elsewhere [29, 34]. Briefly, lipids from the samples were extracted with chloroform:methanol (2:1, v/v) 3 times simultaneously with mechanical homogenization of the tissues with glass beads. Before the extraction, a fixed volume of an internal standard solution (19:0) was added to the samples. The combined lipid extracts were filtered, dried by passing through anhydrous Na2SO4 layer and evaporated at 35 °C. The lipid extract was subjected to acidic methanolysis as described previously [5]. Fatty acid methyl esters (FAME) were analyzed on a gas chromatograph equipped with a mass spectrometer detector (GCD Plus, Hewlett-Packard, USA) and a 30-m long × 0.32-mm internal diameter capillary column HP-FFAP. The column temperature programming was as follows: from 100 to 190 °C at 3 °C/min, 5 min isothermally, to 230 °C at 10 °C/min, and 20 min isothermally. Other instrumental conditions were as described elsewhere [5]. Peaks of FAME were identified by their mass spectra compared to those in the database (Hewlett-Packard, USA) and to those of available authentic standards (Sigma, USA). Positions of double bonds in monoenoic acids were determined by GC–MS of FAME dimethyl disulfide adducts prepared as described elsewhere [35]. To determine double bond positions in polyenoic acids, GC–MS of dimethyloxazoline derivatives of FA was used [29].
Statistical Analysis
Means, standard errors (SE) and Fisher LSD tests were calculated conventionally [36]. To check differences among taxa and the seasonal effect, the data matrix was formed which included all samples of the animals. Mollusk samples were averaged for each date and used in the analysis. The data matrix included percentages of total FA of the most prominent FA and those of high marker significance, in the total eighteen FA. Canonical correspondence analysis of the data matrix was carried out using STATISTICA software (version 9; StatSoft Inc., Tulsa, OK, USA). To test differences among phylogenetic groups, such as classes Insecta, Oligochaeta, Crustacea, Turbellaria, Bivalvia, the data matrix was formed in similar manner as for the canonical correspondence analysis. Taxa groups comprised: Insecta—A. crymophila, E. setigera, P. olivacea, C. plumosus; Oligochaeta—L. variegates and T. tubifex; Crustacea—E. viridis; Turbellaria—Dendrocoelopsis sp.; Bivalvia—D. polymorpha and D. bugensis. The data matrix included percentages (mol% of the total) of essential FA, such as LNA, ALA, EPA, DPA, and DHA, and 16:0 and 18:1n-9 which is the most significant as energy sources. Tukey HSD test of the data matrix was carried out using STATISTICA software (version 9; StatSoft Inc., Tulsa, OK, USA).
Results
Fatty acid content and composition of zoobenthos from four phyla were studied. Taxonomy position and phylogenetic relationships between the studied species are shown in Fig. 1 [37].
The scheme of phylogenetic tree of benthic invertebrates, Dendrocoelopsis sp., D. polymorpha, D. bugensis, T. tubifex, L. variegatus, E. viridis, C. plumosus, P. olivacea, A. crymophila, and E. setigera [by 37]
More than fifty FA species were identified in samples (Tables 2, 3).
The levels of saturated fatty acids (SFA) in the studied species varied from 26.3 to 41.4% (mol% of the total) (Tables 2, 3). Levels of SFA were low in E. viridis and Dendrocoelopsis sp. and high in mollusks. Among SFA, 16:0, 18:0 and 14:0 dominated in most of the invertebrates, although their ratios significantly differed among the species (Tables 2, 3).
Branched fatty acids (BFA) comprised mostly i15:0, ai15:0, i17:0 and ai17:0 and their levels varied from 1.0 to 9.2% of the total (Tables 2, 3). The distribution of 15:0 BFA differed from that of 17:0 BFA. The levels of i15:0 and ai15:0 were significantly higher in C. plumosus ‘C’ in comparison with other species (Tables 2, 3). The levels of i17:0 and ai17:0 were high in mollusks and oligochaetes, especially in L. variegatus.
Monoenoic acids (MUFA) varied from 17.8 to 36.5% of the total and were primarily represented by 16:1n-7, 18:1n-7, 18:1n-9, 20:1n-7, 20:1n-9 and 20:1n-11 (Tables 2, 3). However, MUFA profiles differed among the species studied. Dendrocoelopsis sp. and E. viridis showed significant levels of 18:1n-9, while mollusks had the highest level of 20:1n-9. It should be noted that 20:1n-11 was found in all studied species in traces, except mollusks and oligochaetes, which had this acid dominant among MUFA. Monoenoic C22 acids were found mainly in worms and mollusks (Table 2).
The levels of polyunsaturated fatty acids varied from 25.1 to 39.3% of the total. The lowest value of PUFA was found in larvae of Chironomidae and the highest value was in E. viridis and D. bugensis. Dominant PUFA patterns in the studied invertebrates were evidently species-specific (Tables 2, 3).
In samples of Dendrocoelopsis sp. 20:5n-3, 22:5n-3, ALA, LNA and 22:6n-3 dominated. Note that the studied planarian was characterized by a very high content of 22:5n-3 (Tables 2, 4).
In both mollusk species, 22:6n-3, 20:5n-3, 22:5n-3, 22:5n-6, 20:4n-6 dominated, while their levels of C16 and C18 PUFA were negligible. The levels of 22:6n-3 in mollusks were significantly higher in comparison with other species (Tables 2, 3).
In oligochaetes the following acids dominated: 20:5n-3, LNA, ALA, 20:4n-6, and 22:6n-3. The per cent levels of 20:5n-3 in both Oligochaeta species were high and comparable with those in some arthropod species, E. setigera, E. viridis, and P. olivacea (Tables 2, 3).
All chironomids had similar dominant PUFA: 20:5n-3, LNA, ALA, with their levels being markedly different. Both populations of C. plumosus, ‘B’ and ‘C’, contained significant levels of LNA (Table 3). In contrast, P. olivacea was rich in 20:5n-3. In addition, P. olivacea had the highest level of 16:2n-4; and C. plumosus ‘B’ had the highest level of 18:4n-3. Studied chironomids were lacking in DHA, moreover in C. plumosus ‘C’ DHA was not detected at all (Table 3).
Like in Chironomidae, the dominant PUFA of A. crymophila, E. setigera and E. viridis also comprised 20:5n-3, ALA and LNA. In addition, these invertebrates had significant percentage levels of 18:4n-3 and C16 PUFA, especially 16:2n-4, 16:3n-4, 16:4n-1. In contrast to most of the invertebrates, A. crymophila had the highest values of 16:4n-3 and 16:3n-3 (Table 3).
In the invertebrates studied the highest values of n-3/n-6 and DHA/ARA ratios were found in E. viridis and Dendrocoelopsis sp. (Table 4). A. crymophila and T. tubifex had comparatively high values of n-3/n-6 ratio and low values of DHA/ARA. High n-3/n-6 ratios were found in E. setigera and P. olivacea however, DHA/ARA ratios were very low in these species (Table 4). In both mollusks, n-3/n-6 ratios were similar to DHA/ARA ratios. In samples of C. plumosus ‘B’ and ‘C’ both ratios were very low (Table 4).
Using correspondence analysis, the zoobenthic invertebrates studied were represented in a two-dimensional space according to their levels (mol% of the total) of prominent fatty acids (Fig. 2). The first dimension explained 51.65% of inertia (of total Chi-square value) of the data set, and the second dimension reproduced 13.34%. Chi-square values for both dimensions and the total Chi-square were significant (p < 0.0001). The first dimension (Fig. 2) demonstrated large differences between mollusks on the one hand and larvae of insects on the other. E. viridis and worms had an intermediate position. These positions of invertebrates in the first dimension were provided mostly by difference in their levels of 22:5n-6 and 20:1n-11 on the one hand and 16:4n-3, 16:2n-4, LNA and ALA on the other. The second dimension, although comparatively less substantial was also significant, indicating that the largest differences between C. plumosus and A. crymophila with E. setigera is primary due to LNA and 16:4n-3. P. olivacea had an intermediate position. Besides, the second dimension demonstrated differences between T. tubifex, L. variegatus and E. viridis, Dendrocoelopsis sp. provided mostly by the difference in their levels of 15:0 and 18:1n-9 (Fig. 2). Note, none of each studied species showed evident seasonal tendencies in the two-dimensional space. For example, samples of C. plumosus collected in May, July and August were close to each other, while all samples of C. plumosus collected in August, were distant. The samples of A. crymophila collected in different seasons, such as February, August and November, were closer than the samples of winter months (Fig. 2).
Results of correspondence analysis of zoobenthic invertebrates and fatty acids (mol% of the total) represented in a two-dimensional space reproduced 65% of total inertia. Lv: L. variegatus, Tt: T. tubifex, DrP: D. polymorpha, DrB: D. bugensis, Ac: A. crymophila, Ev: E. viridis, Dsp: Dendrocoelopsis sp., Es: E. setigera, Po: P. olivacea, ChC: C. plumosus from Chistoe lake, ChB: C. plumosus from Bugach reservoir. The numbers indicate the months of sampling dates, e.g. 05 May, 08 August, 11 November. The FA which provided the major part of total inertia for axes D1 and D2 are circled
To find differences in percentages of the essential and quantitatively valuable FA between large phylogenetic groups, such as Classes, we performed Tukey HSD test (Table 5). The mean levels of all eight tested FA were different among the groups. Bivalvia were markedly high in 16:0, while Crustacea and Turbellaria showed the significantly high levels of 18:1n-9. The highest level of LNA and ALA was found in Insecta. Oligochaeta and Bivalvia were rich in ARA, while the former group and Crustacea were rich in EPA. C22 n-3 PUFA were highly variable, being the most prominent in Bivalvia, Turbellaria and Crustacea (Table 5).
Discussion
We found that the FA content and composition of the studied benthic invertebrates differed significantly. Levels of essential PUFA, EPA and DHA, in the species varied by more than an order of magnitude (Tables 2, 3). Some authors emphasized the importance of n-3/n-6 and DHA/ARA ratios in zoobenthos because higher aquatic consumers, fish, need food with optimal FA ratios to achieve high rates of growth and reproduction and to optimize immune functioning [11, 38]. Some data collected from the literature showed that n-3/n-6 and DHA/ARA ratios in the fish food should be at least 2–3 and >0, respectively, i.e. some ARA are also needed to give good growth and reproduction [11]. In addition, the ratio values depend on trophic positions of fish, e.g., n-3/n-6 and DHA/ARA ratios in the carnivorous-benthivorous freshwater fish were 3.8 and 4.8, respectively, while for carnivorous-piscivorous fish the ratios were 2.6 and 2.7, respectively [11]. In the Yenisei River, the habitat for seven of the studied invertebrates, one of the most abundant fish species is Siberian grayling Thymallus arcticus (Pallas) which is benthivorous during most of its life stages. The ratios of n-3/n-6 and DHA/ARA in Thymallus arcticus are 9.4 and 5.5, respectively [39]. These ratios in Thymallus arcticus are markedly higher in comparison with those in other carnivorous-benthivorous freshwater fish species described by Ahlgren and colleagues [11].
Considering the food quality for the dominant fish Thymallus arcticus, the ratios in E. viridis and Dendrocoelopsis sp. are the most optimal. The larvae of A. crymophila, E. setigera, P. olivacea and oligochaete T. tubifex are of good nutritional quality in respect to the n-3/n-6 ratio, but their DHA/ARA ratios are lower than required (Table 4). Thus, the zoobenthic species from the Yenisei River are of variable food quality in respect to the fatty acid ratios.
In the Bugach reservoir, the habitat for C. plumosus, the most abundant benthivorous fish is Carassius auratus gibelio (Bloch). The mean ratios in C. auratus are 3.3 and 3.9, respectively (Sushchik and Makhutova, unpublished data). In contrast to the fish, C. plumosus had very low ratios, as a result, it was of low food quality for the population of crucian carp. Note that crucian carp of the Bugach reservoir had comparatively lower FA ratios than found in other benthivorous species [11] probably due to the food being of low biochemical quality.
We compared n-3/n-6 and DHA/ARA ratios of the studied invertebrates with available data. On average, n-3/n-6 ratios of chironomids were reported to vary from 1 to 3 [29, 30, 40]. Three chironomids studied in the present work were from different water bodies and showed differences in n-3/n-6 ratio. Both populations of C. plumosus inhabited black silts and had a relatively low n-3/n-6 ratio, within 1–2, while the population of P. olivacea, inhabited a river site rich in epilithic biofilms, had significantly higher value, >3. Hence, C. plumosus showed an n-3/n-6 ratio close to the range reported in the literature, while riverine P. olivacea was enriched in n-3 PUFA.
The studied oligochaetes and larvae of Trichoptera and Ephemeroptera had ratios very similar to the literature data [29, 30, 40–42].
Many researchers reported n-3/n-6 and DHA/ARA ratios in Gammaridae as 1–3 and ≤1, respectively [29, 32, 34, 41, 43, 44]. These values were significantly lower than those for the gammarids in the present study (Table 4). It is noteworthy that the studied E. viridis had n-3/n-6 and DHA/ARA ratios similar to those of predatory Mysis relicta [45].
There are many data on the FA composition of various taxa of mollusks, except for the dreissenids. The FA composition of mollusks is highly variable. Some representatives of mollusks, especially bivalves, have high levels of EPA and DHA [46–49], while others, especially gastropods, have high values of n-6 PUFA, 22:4n-6, 20:4n-6 and LNA [50, 51]. The mussels of D. polymorpha and D. bugensis in the present study were rich both in n-3 and n-6 C20-22 PUFA (Table 2). The bivalve mollusk Potamocorbula amurensis had the ratios similar to those in the studied dreissenids, n-3/n-6 ca. 2.5 and DHA/ARA ca. 2 [46].
The data on the FA composition of planarians are scarce. The only paper that describes the FA composition of phospholipids in the planarian Dugesia anceps is Ref.[52]. Both planarians, D. anceps studied by Politi and colleagues [52], and Dendrocoelopsis sp., studied in the present work, were characterized by unusually high levels of 22:5n-3. The level of this acid in the studied Dendrocoelopsis sp. was close to 20:5n-3, and it was 2–10 times higher than that of 22:6n-3 (Table 2). In contrast to the species studied in the present work, D. anceps had significant levels of n-6 PUFA, 20:4n-6, LNA and 22:5n-6 [52]. Ratios of n-3/n-6 and DHA/ARA in phospholipids of D. anceps were much lower than those in Dendrocoelopsis sp.
We used FA trophic markers to elucidate nutritional preferences of the studied invertebrates. We considered increased levels of C16 PUFA of n-1, n-4, n-7 family and 20:5n-3 in biomass of the animals as markers of consumption of diatoms. P. olivacea, A. crymophila, E. setigera, C. plumosus ‘B’ and E. viridis accumulated C16 PUFA of n-1, n-4, n-7 family and 20:5n-3, therefore, a significant part of their diet probably comprises diatoms. Diatoms were also present in diets of Dendrocoelopsis sp. and L. variegatus, but in comparatively lower proportions. High levels of C16 and C18 PUFA of n-3 and n-6 family in A. crymophila, both C. plumosus populations and E. setigera indicated that green algae and cyanobacteria were probably abundant in their diets. Hence, A. crymophila, E. setigera and C. plumosus ‘B’ probably had a mixed diet. High levels of bacterial FA and C18–22 SFA was found in deposit-feeding C. plumosus ‘C’, T. tubifex and L. variegatus, indicating that detritus composed a significant part of their diets. Content of DHA and ratios of 18:1n-9/18:1n-7 and PUFA/SFA were reported to be high in carnivorous species [53–55]. Thus, E. viridis, which contained the highest value of DHA and the highest 18:1n-9/18:1n-7 and PUFA/SFA ratios among the studied benthic invertebrates, seemed to be partly a carnivore. In spite of the above-mentioned ratios in Dendrocoelopsis sp. being moderate, planarians are known to be carnivores [56, 57].
We suppose that benthic organisms have variable requirements in dietary FA, essentially determined by genetic and metabolic factors. To meet such requirements, most zoobenthic species need to feed selectively and to have the capacity to selectively retain and (or) convert dietary FA, which is reflected in the FA composition of their bodies.
Some authors have discussed different feeding strategies of zoobenthic species, as detritivorous, omnivorous, carnivorous [e.g. 33, 54]. Using the canonical correspondence analysis we obtained evidence that feeding strategy can be of importance to predetermine the fatty acid profile of a zoobenthic species, however, the phylogenetic position of a species is a more powerful factor which results in close FA composition even if taxonomically close species or populations inhabit different places. We initially suggested that a seasonal variation within each invertebrate’s species composition is a very important factor. However, most species formed distinct groups in the two-dimensional space of the multivariate analysis. Note that these groups comprised samples of the same species taken in various seasonal periods (Table 1). One can easily see that phylogenetic differences were much larger than seasonal ones (Fig. 2). Thus, the seasonal variations in the FA composition of the zoobenthos were obviously a factor of secondary importance.
In correspondence analysis of the FA composition, we found large differences among mollusks, larvae of insects, and worms. However, samples of the crustacean E. viridis and the planarian Dendrocoelopsis sp. are very close to each other (Fig. 2). Although these species are very phylogenetically distant (Fig. 1), they both feed like carnivores, at least partly.
D. polymorpha and D. bugensis differ from other species by feeding strategy (filter feeders) and taxonomic position (particular phylum), and they were sampled from another water body. As a result, all samples of dreissenids formed the group that was the most distant from others (Fig. 2). A. crymophila and E. setigera, which formed a joint group, belonged to the same class Insecta (Figs. 1, 2). In addition, they inhabited the same site (Table 1). Note that samples of the two populations of the chironomid C. plumosus, although taken in waters from geographically very distant regions, formed a joint group, too. Obviously, populations of the same species have the close metabolic processes including FA biosynthesis and conversion capacity of dietary FA. Alternatively, C. plumosus has selective feeding [4] and thereby might have the same feeding spectrum even in different water bodies. Nevertheless, we consider the phylogenetic proximity as a factor that mostly determines similarity in FA profiles of the two different populations of C. plumosus. Another species of chironomids, P. olivacea, inhabitant of the river, formed a small separate group (Fig. 2).
Oligochaetes, L. variegatus and T. tubifex, showed variable levels of most FA, therefore, they were the only phylogenetic group that did not show a clear trend in the multidimensional analysis (Fig. 2). This finding is probably explained by the feeding strategy of the Oligochaetes. Due to detritivorous feeding, their diet might change strongly during the studied period, and FA profiles of their bodies reflected this variation in the diet.
It is important to note that positions of C. plumosus, P. olivacea, and A. crymophila with E. setigera were rather close to each other in the first dimension, which explained the major part of the total inertia (Fig. 2). All of these species are of the same class, Insecta, but were sampled from different water bodies (the Yenisei River, the Chistoe lake and the Bugach reservoir). Moreover, their feeding strategies are variable. Therefore, the FA profiles of the studied species of Insecta were likely primarily dependent on the genetically predetermined FA metabolism rather than on the biochemical composition of their diet.
Besides the differences in FA profiles between the particular species studied, we revealed significant variation in the mean levels of essential PUFA for large taxa which joined several species (Table 5). It is interesting to remark that C22 n-3 PUFA are depleted in the phylogenetically advanced group, Insecta, compared to that in worms, mollusks and crustaceans. It is well known that DHA is crucial for nervous system functioning [9] and enriched in neural tissues of highly organized animals. It is surprising that the larvae of Insecta which have a rather well-developed nervous system and complicated behavior do not evidently possess a mechanism for accumulating C22 n-3 PUFA.
In general, the fatty acid content and composition of the studied zoobenthic species significantly differed, including the essential PUFA and the FA ratios which are important factors of biochemical food quality for fish. E. viridis (Crustacea, Gammaridae) and Dendrocoelopsis sp. (Turbellaria) were of the highest nutritional value for carnivorous-benthivorous fish. Answering the initially formulated questions we can conclude that: (1) most phylogenetically close species inhabiting the same water body have similar fatty acid spectra; (2) the populations of the same species inhabiting different water bodies, e.g., the populations of C. plumosus, may have similar fatty acid composition; (3) just as trophic strategies, phylogenetic factors seem to be the important determinants of fatty acid profiles in benthic invertebrates.
Abbreviations
- ALA:
-
α-Linolenic acid, 18:3n-3
- ARA:
-
Arachidonic acid, 20:4n-6
- BFA:
-
Branched fatty acids
- DHA:
-
Docosahexaenoic acid, 22:6n-3
- DPA:
-
Docosapentaenoic acid, 22:5n-3
- EPA:
-
Eicosapentaenoic acid, 20:5n-3
- FA:
-
Fatty acid(s)
- FAME:
-
Fatty acid methyl ester(s)
- LNA:
-
Linoleic acid, 18:2n-6
- MUFA:
-
Monounsaturated fatty acid(s)
- NMI:
-
Nonmethylene interrupted
- PUFA:
-
Polyunsaturated fatty acid(s)
- SFA:
-
Saturated fatty acid(s)
References
Desvilettes Ch, Bourdier G, Amblard C, Barth B (1997) Use of fatty acids for the assessment of zooplankton grazing on bacteria, protozoans and microalgae. Freshw Biol 38:629–637
Leveille JC, Amblard C, Bourdier G (1997) Fatty acids as specific algal markers in a natural lacustrian phytoplankton. J Plankton Res 19:469–490
Ederington M, McManus GB, Harvey R (1995) Trophic transfer of fatty acids, sterols, and a triterpenoid alcohol between bacteria, a ciliate, and the copepod Acartia tonsa. Limnol Oceanogr 40:860–867
Gladyshev MI, Sushchik NN, Skoptsova GN, Parfentsova LS, Kalachova GS (1999) Use of biochemical markers provides evidence of selective feeding in zoobenthic omnivorous organisms of a fish-rearing pond. Doklady Biol Sci 364:67–69
Gladyshev MI, Emelianova AY, Kalachova GS, Zotina TA, Gaevsky NA, Zhilenkov MD (2000) Gut content analysis of Gammarus lacustris from Siberian lake using biochemical and biophysical methods. Hydrobiologia 431:155–163
Brett MT, Muller-Navarra DC (1997) The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw Biol 38:483–499
Muller-Navarra DC, Brett MT, Liston AM, Goldman CR (2000) A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403:74–77
Arts MT, Ackman RG, Holub BJ (2001) ‘Essential fatty acids’ in aquatic ecosystems: a crucial link between diet and human health and evolution. Can J Fish Aquat Sci 58:122–137
Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF (2001) The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res 40:1–94
Broadhurst CL, Wang Y, Crawford MA, Cunnane SC, Parkington JE, Schmidt WF (2002) Brain-specific lipids from marine, lacustrine, or terrestrial food resources: potential impact on early African Homo sapiens. Comp Biochem Physiol B 131:653–673
Ahlgren G, Vrede T, Goedkoop W (2009) Fatty acid ratios in freshwater fish, zooplankton and zoobenthos—are there specific optima? In: Arts MT, Brett MT, Kainz MJ (ed) Lipids in aquatic ecosystems, Springer, Dordrecht
Lim C, Ako H, Brown CL, Hahn K (1997) Growth response and fatty acid composition of juvenile Penaeus uannamei fed different sources of dietary lipid. Aquaculture 151:143–153
Deerign MJ, Fielder DR, Hewitt DR (1997) Growth and fatty acid composition of juvenile leader prawns, Penaeus monodon, fed different lipids. Aquaculture 151:131–141
Milke LM, Bricelj VM, Parrish CC (2004) Growth of postlarval sea scallops, Placopecten magellanicus, on microalgal diets, with emphasis on the nutritional role of lipids and fatty acids. Aquaculture 234:293–317
Özyurt G, Duysak Ö, Akamca E, Tureli C (2006) Seasonal changes of fatty acids of cuttlefish Sepia officinalis L. (Mollusca: Cephalopoda) in the north eastern Mediterranean sea. Food Chem 95:382–385
Sinanoglou VJ, Meimaroglou D, Miniadis-Meimaroglou S (2008) Triacylglycerols and their fatty acid composition in edible Mediterranean molluscs and crustacean. Food Chem 110:406–413
Napolitano GE (1998) Fatty acids as trophic and chemical markers in freshwater ecosystems. In: Arts MT, Wainman BC (eds) Lipids in freshwater ecosystems. Springer, New York
Brett MT, Muller-Navarra DC, Ballantyne AP, Ravet JL, Goldman CR (2006) Daphnia fatty acid composition reflects that of their diet. Limnol Oceanogr 51:2428–2437
Muller-Navarra DC (2006) The nutritional importance of polyunsaturated fatty acids and their use as trophic markers for herbivorous zooplankton: does it contradict? Arch Hydrobiol 164:501–513
Weers PMM, Siewertsen K, Gulati RD (1997) Is the fatty acid composition of Daphnia galeata determined by the fatty acid composition of the ingested diet? Freshw Biol 48:731–738
Von Elert E (2002) Determination of limiting polyunsaturated fatty acids in Daphnia galeata using a new method to enrich food algae with single fatty acids. Limnol Oceanogr 47:1764–1773
Gladyshev MI, Sushchik NN, Makhutova ON, Dubovskaya OP, Kravchuk ES, Kalachova GS, Khromechek EB (2010) Correlations between fatty acid composition of seston and zooplankton and effects of environmental parameters in a eutrophic Siberian reservoir. Limnologica 40:343–357
Graeve M, Albers C, Kattner G (2005) Assimilation and biosynthesis of lipids in arctic Calanus species based on feeding experiments with a 13C labelled diatom. J Exp Mar Biol Ecol 317:109–125
Veloza AJ, Chu F-LE, Tang KW (2006) Trophic modification of essential fatty acids by heterotrophic protists and its effects on the fatty acid composition of the copepod Acartia tonsa. Mar Biol 148:779–788
Smyntek PM, Teece MA, Schulz KL, Storch AJ (2008) Taxonomic differences in the essential fatty acid composition of groups of freshwater zooplankton relate to reproductive demands and generation time. Freshw Biol 53:1768–1782
Ravet JL, Brett MT, Arhonditsis GB (2010) The effects of seston lipids on zooplankton fatty acid composition in Lake Washington, Washington, USA. Ecology 91:180–190
Wallis JG, Watts JL, Browse J (2002) Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem Sci 27:467–473
Goedkoop W, Demandt M, Ahlgren G (2007) Interactions between food quantity and quality (long-chain polyunsaturated fatty acids concentrations) effects on growth and development of Chironomus riparius. Can J Fish Aquat Sci 64:425–436
Sushchik NN, Gladyshev MI, Moskvicheva AV, Makhutova ON, Kalachova GS (2003) Comparison of fatty acid composition in major lipid classes of the dominant benthic invertebrates of the Yenisei river. Comp Biochem Physiol B 134:111–122
Bell JG, Ghioni C, Sargent JR (1994) Fatty acid composition of 10 freshwater invertebrates which are natural food organisms of Atlantic salmon parr (Salmo salar); a comparison with commercial diet. Aquaculture 128:301–313
Torres-Ruiz M, Wehr JD, Perrone AA (2010) Are net-spinning caddisflies what they eat? An investigation using controlled diets and fatty acids. J N Am Benthol Soc 29:803–813
Maazouzi C, Masson G, Izquierdo MS, Pihan J-C (2007) Fatty acid composition of the amphipod Dikerogammarus villosus: Feeding strategies and trophic links. Comp Biochem Physiol A 147:868–875
Kraffe E, Grall J, Le Duff M, Soudant P, Marty Y (2008) A striking parallel between cardiolipin fatty acid composition and phylogenetic belonging in marine bivalves: a possible adaptative evolution? Lipids 43:961–970
Makhutova ON, Sushchik NN, Kalachova GS, Ageev AV (2009) Fatty acid content and composition of freshwater planaria Dendrocoelopsis sp. (Planariidae, Turbellaria, Platyhelminthes) from the Yenisei River. J Sib Fed Univ Biol 2:135–144
Christie WW (1989) Gas Chromatography and Lipids. A Practical Guide. The Oily Press, England
Campbell RC (1967) Statistics for Biologists, University Press, Cambridge
Halanych KM (2004) The new view of animal phylogeny. Annu Rev Ecol Evol Syst 35:229–256
Arts MT, Kohler CC (2009) Health and condition in fish: the influence of lipids on membrane competency and immune response. In: Arts MT, Brett MT, Kainz MJ (eds) Lipids in aquatic ecosystems, Springer, Dordrecht
Sushchik NN, Gladyshev MI, Kalachova GS, Makhutova ON, Ageev AV (2006) Comparison of seasonal dynamics of the essential PUFA contents in benthic invertebrates and grayling Thymallus arcticus in the Yenisei river. Comp Biochem Physiol B 145:278–287
Goedkoop W, Sonesten L, Ahlgren G, Boberg M (2000) Fatty acids in profundal benthic invertebrates and their major food resources in Lake Erken, Sweden: seasonal variation and trophic indications. Can J Fish Aquat Sci 57:2267–2279
Sushchik NN, Gladyshev MI, Kravchuk ES, Ivanova EA, Ageev AV, Kalachova GS (2007) Seasonal dynamics of long-chain polyunsaturated fatty acids in littoral benthos in the upper Yenisei River. Aquat Ecol 41:349–365
Torres-Ruiz M, Wehr JD, Perrone AA (2007) Trophic relations in a stream food web: importance of fatty acids for macroinvertebrate consumers. J N Am Benthol Soc 26:509–522
Biandolino F, Prato E (2006) A preliminary investigation of the lipids and fatty acids composition of Gammarus aequicauda (Crustacea: Amphipoda) and its main food source. J Mar Biol Ass 86:345–348
Kolanowski W, Stolyhwo A, Grabowski M (2007) Fatty acid composition of selected fresh water gammarids (Amphipoda, Crustacea): a potentially innovative source of omega-3 LC PUFA. J Am Oil Chem Soc 84:827–833
Schlechtriem Ch, Arts MT, Johannsson OE (2008) Effect of long-term fasting on the use of fatty acids as trophic markers in the opossum shrimp Mysis relicta—a laboratory study. J Great Lakes Res 34:143–152
Canuel EA, Cloern JE, Ringelberg DB, Guckert JB, Rau GH (1995) Molecular and isotopic tracers used to examine sources of organic matter and its incorporation into the food webs of San Francisco Bay. Limnol Oceanogr 40:67–81
Murphy KJ, Mooney BD, Mann NJ, Nichols PD, Sinclair AJ (2002) Lipid, FA, and sterol composition of New Zealand green lipped mussel (Perna canaliculus) and Tasmanian blue mussel (Mytilus edulis). Lipids 37:587–595
Berge J-P, Barnathan G (2005) Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv Biochem Eng Biotechnol 96:49–125
Pernet F, Tremblay R, Comeau L, Guderley H (2007) Temperature adaptation in two bivalve species from different thermal habitats: energetics and remodelling of membrane lipids. J Exp Biol 210:2999–3014
Fried B, Rao KS, Sherma J, Huffman JE (1993) Fatty acid composition of Goniobasis virginica, Physa sp. and Viviparus malleatus (Mollusca: Gastropoda) from lake Musconetcong, New Jersey. Biochem Syst Ecol 21:809–812
Zhukova N (2007) Lipid classes and fatty acid composition of the tropical Nudibranch mollusks Chromodoris sp. and Phyllidia coelestis. Lipids 42:1169–1175
Politi LE, De Santos SV, De Linares LV (1992) Phospholipids and fatty acids in intact and regenerating Dugesia anceps, a fresh water planaria. Zool Sci 9:671–674
Cripps GC, Atkinson A (2000) Fatty acid composition as an indicator of carnivory in Antarctic krill, Euphausia superba. Can J Fish Aquat Sci 57:31–37
Falk-Petersen S, Hagen W, Kattner G, Clarke A, Sargent J (2000) Lipids, trophic relationships, and biodiversity in Arctic and Antarctic krill. Can J Fish Aquat Sci 57:178–191
Stevens CJ, Deibel D, Parrish CC (2004) Species-specific differences in lipid composition and omnivory indices in Arctic copepods collected in deep water during autumn (North Water Polynya). Mar Biol 144:905–915
Cazzaniga NJ, Tamburi N, Carrizo M, Ponce GF (2002) Feeding Girardia anceps (Platyhelminthes: Tricladida) in the laboratory. J Freshw Ecol 17:93–98
Tranchida MC, Macia A, Brusa F, Micieli MV, Garcia JJ (2009) Predation potential of three flatworm species (Platyhelminthes: Turbellaria) on mosquitoes (Diptera: Culicidae). Biol Control 49:270–276
Acknowledgments
The work was supported by grants from the Russian Foundation for Basic Research (RFBR) No. 09-04-01085 and No. 08-05-00095, by “Thematic plan programs” from the Ministry of Education and Sciences of Russian Federation (Theme B-4 of Siberian Federal University).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Makhutova, O.N., Sushchik, N.N., Gladyshev, M.I. et al. Is the Fatty Acid Composition of Freshwater Zoobenthic Invertebrates Controlled by Phylogenetic or Trophic Factors?. Lipids 46, 709–721 (2011). https://doi.org/10.1007/s11745-011-3566-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-011-3566-9