Abstract
During early development, oviparous fish species must use finite lipid and fatty acid (FA) reserves for both catabolism and structural components. In cold environments, developing fish have the additional constraint of maintaining membrane fluidity for metabolic efficiency (homeoviscous adaptation), resulting in further demand on lower melting point FAs like n-3 polyunsaturated fatty acids (PUFAs). To examine whether marine fish embryos physiologically adapt to changing temperature environments, we incubated Pacific cod (Gadus macrocephalus) eggs at 5 temperatures (0, 2, 4, 6, and 8 °C) in the laboratory and sampled them repeatedly during development to measure changes in lipid/FA composition. Pacific cod embryos increased n-3 PUFA content during the egg stage in all temperature treatments, with the possible exception of 0 °C, where poor survival and hatch success limited our ability for continued sampling. At the beginning of the hatch cycle, free-swimming embryos shifted from lipogenesis to lipid catabolism. The rates of lipogenesis and catabolism were temperature dependent, and the distinct increase in unsaturated fatty acids at temperatures <8 °C was consistent with homeoviscous adaptation theory. However, with the possible exception of embryos at 0 °C, the relative amounts of essential fatty acids (e.g., EPA, DHA, AA) were conserved in a similar manner across incubation temperatures. Collectively, these data suggest Pacific cod are capable of homeoviscous adaptation but cannot tolerate temperatures approaching 0 °C despite their possible ability to biosynthesize PUFAs from other energetic sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature is a principle aspect of fish physiology and is often the most limiting environmental variable for survival of fish in the wild. Temperature effects in fish are generally quantified in terms of growth potential (e.g., Hurst et al. 2010), development rates (e.g., Laurel et al. 2008), thermal threshold limits on survival (Becker and Genoway 1979), feed conversion efficiency (Björnsson et al. 2001), and thermal preference (McCauley and Huggins 1979). Such metrics have been measured in multiple species and populations across a range of life stages in both marine and freshwater fish. Collectively, these studies have shown that the temperature response (sensu ‘thermal reaction norm’) in fish is remarkably variable between and within species, with some populations being more ‘cold-adapted’ than others (i.e., comparatively increased physiological performance at low temperatures) and others able to better exploit warm conditions by way of more rapid growth (e.g., Munch and Conover 2003).
The underlying mechanisms driving thermal adaptation in the early life history of fish are not well understood but are likely occurring at a cellular level by way of selective incorporation of lipids during embryogenesis. Lipids and more specifically fatty acids (FAs) are important to developing fish embryos, serving multiple purposes including energy, structural components for organogenesis (e.g., retinas, myotome, brain), and precursors for eicosanoids such as prostaglandins (Sargent et al. 1999). In oviparous species, these lipids and fatty acids are transferred from the mother during vitellogenesis prior to the release of eggs into the environment. Numerous studies have examined lipid/FA changes in developing fish eggs and larvae with the goal of understanding first-feeding nutrition (Copeman et al. 2002), maternal provisioning (Berkeley et al. 2004), buoyancy regulation (Laurel et al. 2010), and hatch success (Penney et al. 2006). The mechanisms and mediating factors that regulate lipid/FA catabolism are therefore important, especially in species of commercial or ecological significance where such effects can be measured at the population level.
Lipid stores generally decrease until the onset of first feeding (Fraser et al. 1988), but because fish are poikilotherms, these patterns are further mediated by their thermal environment. Temperature can impact developmental lipid/FA use by two mechanisms: (1) metabolism (efficiency and consumption rates) and (2) homeoviscous adaptation (Sinensky 1974), the idea that greater unsaturation of the fatty acyl chains decreases the melting point, resulting in more fluid structure in membranes. The latter mechanism has limited support (Cossins and Prosser 1978; Wiegand et al. 1991; Dey et al. 1993), but it is believed to be important for fish from cold regions where the maintenance of cellular fluidity is critical. Temperature effects on lipid/FA catabolism during embryogenesis have largely been ignored, but the increased requirements of polyunsaturated fatty acids (PUFAs) at low temperature have been hypothesized to negatively impact the development of fish embryos (Wang et al. 1987), as PUFA deficiency can lead to malpigmentation, visual impairment, and behavioral abnormalities in later larval stages (Copeman et al. 2002; Penney et al. 2006; Yanes-Roca et al. 2009). The most limiting PUFAs are the (n-3) and (n-6) PUFAs, especially 22:6n-3 (docosahexaenoic acid, DHA), 20:5n-3 (eicosapentaenoic acid, EPA), and 20:4n-6 (arachidonic acid, AA) (Wiegand 1996). During the early development of cold-water fish species, both DHA and EPA are important in the development of the nervous system and are also used for catabolism and other membrane structures. AA is involved with eicosanoid production that is considered to be important in the stress response of fish larvae (Sargent et al. 1999; Copeman et al. 2002). DHA, EPA, and AA are considered to be ‘essential fatty acids’ (EFAs), meaning they cannot be synthesized de novo from other carbon sources (e.g., protein) or shorter-chain FA precursors. Therefore, an increased demand of these finite PUFAs at low temperatures for membrane structure may be deleterious to the organism, especially at extreme cold temperatures when membrane fluidity cannot compensate for metabolic inefficiencies (Hurst 2007 and references therein).
The objectives of this study were to evaluate temperature effects on changes in the lipids and FAs of developing embryos of Pacific cod Gadus macrocephalus. Pacific cod is a highly fecund species (up to 5,000,000 eggs; McCain 2003) and is economically important in Alaskan waters. Eggs are released in spring when water temperatures can vary between 0 and 10 °C across their distributional range in the eastern Bering Sea and Gulf of Alaska. Unlike other commercially important gadids (e.g., walleye pollock Theragra chalcogramma and Atlantic cod Gadus morhua), Pacific cod embryos are capable of lipogenesis and possible n-3 PUFA synthesis in the egg stage (Laurel et al. 2010). Temperature effects on Pacific cod growth and mortality have been described (Laurel et al. 2008, 2011; Hurst et al. 2010) but, as with most fish species, the physiological mechanism by which temperature impacts these vital rates is not fully understood. In this study, we examined whether temperature mediates the allocation and synthesis of lipid/FAs during the egg and pre-feeding larval stage. Based on general thermal and homeoviscous adaptation theory, we tested the following hypotheses: (1) Pacific cod embryos proportionally increase PUFAs in their body in response to decreasing temperature (H 1), (2) metabolic inefficiencies at temperature extremes reduce absolute PUFA content at standardized developmental periods (H 2), and (3) the relative proportions of ‘essential’ FAs (e.g., arachidonic acid (AA), EPA, DHA) are conserved across temperature treatments (H 3).
Materials and methods
The Pacific cod eggs and unfed larvae used for the experiment were taken from the same series that examined the effects of temperature on hatch rates and post-hatch survival (see Laurel et al. 2008). The following is an abbreviated methodology for the collections and experimental design.
Egg collection and incubation
Adult Pacific cod were collected from a depth of 30 m by commercial jigging vessels in Chiniak Bay, Kodiak, AK, USA, during the spawning season in March 2006. Gametes were fertilized in the field from separate parents (1 female, 3 males) and held for 2 days at 4 °C before being shipped in insulated bottles to the Hatfield Marine Science Center (HMSC) in Newport, OR USA. A single female was used to avoid possible effects of differential maternal lipid/FA provisioning (sensu Chambers and Leggett 1996). Thermos bottles arrived 24 h later at the HMSC laboratory and eggs were equally divided across a series of 4-L plastic incubation trays (220 μm mesh sides and solid bottoms) distributed across 5 temperature treatments, that is, 0, 2, 4, 6, and 8 °C. Pacific cod eggs were scattered in a thin layer covering the bottom of each incubator tray at a density of 20 ml tray−1. Incubator trays were supplied with temperature controlled seawater (150 ml min−1) and light aeration to increase the flow of water over the eggs. Each egg incubator tray was nested in a separate seawater bath (1.0 × 1.0 × 0.5 m square tank), which was supplied with a higher rate of matched temperature controlled seawater at 2–3 L min−1. Three replicate seawater bath/egg incubators were constructed for each temperature treatment, that is, n = 15 total.
Sampling
Eggs and larvae were repeatedly sampled from incubation trays at different developmental stages. Sampling from replicate egg containers (n = 3) occurred the day eggs arrived at the HMSC (3 days post-fertilization, dpf) and every 3–10 days thereafter until the end of the hatch cycle. The sampling frequency varied to account for variable hatch timing and hatch cycle duration (days over which the first and last egg hatches in a batch of eggs) for Pacific cod embryos developing between 0 and 8 °C. Therefore, eggs were sampled at intervals approximating 0, 25, 50, and 75 % of the egg development cycle for each temperature treatment based on temperature-specific Pacific cod development times (~80 degree days to first hatch; Laurel et al. 2008). Larvae were sampled at ~40 % (early), 65 % (midway; 2 and 4 °C only), and 90 % (late) into the hatch cycle. All larvae sampled were technically 0 days post-hatch (DPH), but they varied in days post-fertilization (DPF) depending on the temperature and when they were sampled in the hatch cycle, that is, early, mid-, or late-hatch. Remaining larvae in the egg baskets were removed after each sampling period to ensure newly-hatched larvae were collected in subsequent sampling.
A total of 100 individual eggs or larvae was collected for each sampling period and pooled for each lipid/FA analysis. Eggs were sorted under a dissection scope to ensure viability prior to analysis. Viable eggs were transparent, and the embryo had advanced its development relative to embryos previously sampled. Sorted eggs and larvae were transferred to a 47 mm ashed glass fiber filter (Whatman GF/C) and rinsed with filtered seawater. Excess water was removed by gentle vacuum, and the filter containing eggs or larvae was then transferred to a test tube containing 2 ml of chloroform. Samples were flushed with nitrogen and placed into a freezer at −80 °C for later extraction and lipid/FA analysis.
Lipid and fatty acid analysis
Lipid classes were determined using thin layer chromatography with flame ionization detection (TLC/FID) with a MARK V Iatroscan (Iatron Laboratories, Tokyo, Japan) as described by Parrish (1987). Extracts were spotted on silica gel coated Chromarods, and a three-stage development system was used to separate lipid classes. The first separation consisted of 20-min developments in 99:1:0.05 hexane/diethyl ether/formic acid. The second separation consisted of a 40-min development in 80:20:1 hexane/diethyl ether/formic acid. The last separation consisted of a 15-min development in 100 % acetone followed by a 10-min development in 5:4:1 chloroform/methanol/water. After each separation, the rods were scanned and the 3 chromatograms were combined using T-data scan software (RSS Inc., Bemis, TN, USA). The signal detected in millivolts was quantified using lipid standards (Sigma, St. Louis, MO, USA). Lipid classes were expressed both in relative (mg g−1 wet weight) and absolute amounts (μg embryo−1).
The absolute amount of FA per larvae was determined using conversion factors described by Budge (1999) in which the glycerol, phosphate, and other functional groups are subtracted from the acyl lipid class mass in order to obtain the mass of fatty acids per lipid class. On the basis of an average fatty acid chain length in seafood, these conversion factors for the major lipid classes used here were ~0.47 steryl/wax esters (ST), ~0.95 triacyglycerols (TAG), 1.0 free fatty acids (FFA), ~0.90 diacylglycerols (DAG), ~0.37 acetone mobile polar lipids (AMPL), and ~0.72 for phospholipids (PL). While a separate study revealed higher variance in this method compared to using an internal standard [e.g., tricosanoic acid methyl ester (23:0)], mean amounts do not vary significantly between the two methods of calculation for Pacific cod eggs (see Laurel et al. 2010).
Total lipid extracts were transesterified using 14 % BF3/MeOH for 1.5 h at 85 °C. Iatroscan analyses showed this method derivatized 84–94 % of the acyl lipids. The FAMEs were analyzed on a HP 6890 GC FID equipped with a 7683 autosampler. The GC column was a ZB wax + (Phenomenex, USA). The column length was 30 m with an internal diameter of 0.25 μm and had a 1-m guard column on the front end. The column temperature began at 65 °C where it was held for 0.5 min. The temperature ramped to 195 °C at a rate of 40 °C min−1, held for 15 min, and then ramped to a final temperature of 220 °C at a rate of 2 °C min−1. This final temperature was held for 3.25 min. The carrier gas was hydrogen flowing at 2 ml min−1. The injector temperature started at 150 °C and ramped to a final temperature of 250 °C at a rate of 200 °C min−1. The detector temperature stayed constant at 260 °C. Peaks were identified using retention times from standards purchased from Supelco (37 component FAME, BAME, PUFA 1, PUFA 3). Chromatograms were integrated using the HP ChemStation Chromatograghy Software (Version B00.00).
Data analysis
We recognized a priori that temperature could possibly mediate lipid/FA composition on Pacific cod embryos by way of metabolic processes (rates and efficiencies) or by way of preferential retention (thermal adaptation). Selected lipid classes and FAs were therefore analyzed separately to address these two mechanisms. Metabolic effects of temperature were examined by fitting linear and piecewise regression models to lipid classes and FAs for each temperature treatment over the entire developmental period. Thermal adaptation was tested in two ways. The first analysis was tested for a negative relationship between temperature and polyunsaturated/saturated fatty acid ratios (PUFA/SFA). This was done both by linear regression and by best-fitting exponential decay and peak models to data to account for possible non-linearity. The second analysis used a series of 2-way ANOVAs to examine temperature effects at standardized developmental period, that is, egg stage (20–24 % into egg development) vs early larval stage (35–44 % cumulative hatch; see Laurel et al. 2010). These models were applied separately to absolute and relative data for lipid classes (ST, PL, and TAG) and highly represented FAs (e.g., 22:6n-3, 20:5n-3, 20:4n-6) and FA sums, for example, ∑PUFAs, ∑MUFAs. Examining normal probability plots and plots of residuals versus predicted values determined the suitability of all linear statistical models.
Results
General patterns
The development time of eggs ranged between 14 days at 8 °C to 43 days at 0 °C. Similarly, hatch duration was temperature dependent, ranging from 6 days at 8 °C to 17 days at 0 °C. Hatch success was low in the 0 °C treatment and allowed for only a single analysis of larvae to be sampled early in the hatch cycle. However, egg survival was high and permitted full replicate sampling at all temperature treatments. The broad patterns of FA/lipid composition through development were similar among treatments (Fig. 1). In the first 25 % of development, there was a drop in total lipid content largely as the result of decreasing PL. Following this, lipid content increased in all the lipid classes up until the beginning of the hatch cycle. The increased lipid content at the beginning of the hatch cycle was substantially higher than in newly fertilized eggs in all temperature treatments for example, ~2 × greater total lipid, ~10 × greater TAG, ~2 × greater ST and ~1.6 × greater PL (Fig. 1).
Changes in the content (μg embryo−1) of a total lipid, b TAG, c sterol and d phospholipid in developing eggs and larvae of Pacific cod (Gadus macrocephalus) at 5 temperatures (0, 2, 4, 6, 8 °C). Percent development time is calculated as the day of sampling (days post-fertilization, DPF) divided by the days to the last hatching larva. Values are mean absolute amounts based on 2–3 replicate samples of pooled (n = 100) eggs or larvae (exception single replicate of newly-hatched larvae in 0 °C treatment). Dashed lines represent the onset of the hatch period
Lipids
The 2-way ANOVA indicated lipid content (μg embryo−1) significantly increased in all temperature treatments from egg to larval stages (Total Lipid, F 1,21 = 39.89, P < 0.001; TAG F 1,21 = 16.46, P < 0.001; PL F 1,21 = 45.86, P < 0.001; ST F 1,21 = 67.16, P < 0.001), but there was no effect of temperature as a single or interactive term in the model (P > 0.05 in all instances). Percent composition of these lipid classes also changed with development, with % PL decreasing and % TAG increasing from the egg stage to newly-hatched larvae (Table 1). These patterns were driven by temperature as indicated in the significant interaction between temperature and development stage (egg vs newly-hatched larvae) in the 2-way ANOVA (TAG F 4,17 = 8.80, P < 0.001; PL F 4,17 = 15.59, P < 0.001). Figure 2 shows the nature of the interaction; TAG increases were relatively higher from egg to hatch in warm treatments (6–8 °C), whereas PL was more highly conserved at cold temperatures (0–4 °C). No significant interaction between temperature and development was found for % ST composition of Pacific cod embryos, but ST content was significantly higher in the egg and newly-hatched larvae in the extreme cold temperature treatment (0 °C, ST F 4,17 = 11.04, P < 0.001). Newly-hatched larvae hatching later in the hatch cycle generally had lower lipid content, driven mostly by TAG and PL (Fig. 1), whereas ST content was more highly conserved.
Change in % composition of a PL and b TAG from the early egg stage (20–25 % development) to the newly-hatched larval stage of Pacific cod (Gadus macrocephalus) at 5 temperatures (0, 2, 4, 6, 8 °C). Values are mean relative amounts based on 2–3 replicate samples of pooled (n = 100) eggs or larvae (exception single replicate of newly-hatched larvae in 0 °C treatment)
Fatty acids
There were several notable changes in the FA composition of Pacific cod embryos from fertilization to first hatch. Fatty acid content (μg embryo−1) increased from egg to hatch in most instances, with the notable exception of 0 °C larvae where FA content actually decreased relative to the egg stage (Fig. 3). This pattern was statistically significant, as indicated in interaction term of the 2-way ANOVA for summed FA content (F 4,17 = 9.74, P < 0.001). Other single and grouped FA variables (18:1n-9, 20:1n-9, AA, EPA, DHA, ∑SFA, ∑MUFA, ∑PUFA, ∑n-3) trended the same way (P < 0.05; Fig. 3). However, no temperature effect was observed in 18:2n-6 despite a significant decrease from egg to early hatching larvae (F 4,17 = 5.55, P < 0.001; Fig. 3).
Content (μg embryo−1) of a select fatty acids and b summed fatty acid groups in newly-hatched Pacific cod (Gadus macrocephalus) larvae relative to newly fertilized eggs. Values are mean absolute amounts based on 2–3 replicate samples of pooled (n = 100) eggs or larvae (exception single replicate of newly-hatched larvae in 0 °C treatment)
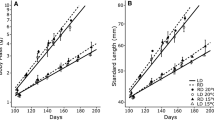
At the beginning of the hatch cycle, both % AA and DHA/EPA markedly increased across all temperature treatments as indicated by the significant piecewise regression fits (Fig 4a, b). The slopes of these relationships decreased with temperature (indicating a metabolic mechanism), but overall these developmental patterns in fatty acids were conserved across all temperatures. However, evidence of possible thermal adaptation was observed in the peak model fit to PUFA/SFA index [f = 2.303e (−0.5((T − 2.773)/12.432)^2)] with an r 2 of 0.97 (Fig. 5). Although the pattern is subtle, the model suggests a precipitous decline in the relative amount of unsaturated fatty acids in newly-hatched larvae that were incubated above 6 °C.
Changes in the a % AA(20:4n-6) and b DHA/EPA in developing Pacific cod (Gadus macrocephalus) near or at the beginning of the hatch cycle at each incubation temperature (0, 2, 4, 6, 8 °C). Trend lines are based on breakpoint analysis. Rates of increase (i.e., slopes) to the right of the break were positively associated with temperature. Note, no significant break was detected in DHA/EPA in the 0 °C treatment (due to limited data), so trend lines were plotted before and after the onset of hatching. Values are based on pooled samples (n = 100) of eggs or larvae
Changes in the ratio of polyunsaturated to saturated fatty acids (PUFA/SFA) in newly-hatched Pacific cod (Gadus macrocephalus) larvae as a function of egg incubation temperature. The dotted trend line represents a linear regression (f = −0.018T + 2.323, r 2 = 0.58, P = 0.189, n = 12) to test the original hypothesis that PUFA/SFA is negatively related to temperature (T). However, the better-fitting polynomial (solid line, f = 2.303e(−0.5 ((T − 2.773)/12.432)^2), r 2 = 0.97, P < 0.001, n = 12) suggests this relationship may be curvilinear. Values are based on 2–3 replicate samples of pooled (n = 100) eggs or larvae (exception single replicate of newly-hatched larvae in 0 °C treatment)
Discussion
Our study is one of the first to examine lipid/FA acid changes in fish embryos across multiple temperature environments. We focused our attention on Pacific cod for two reasons. First, similar to other cold-water gadids, Pacific cod use polyunsaturated fatty acids (PUFAs) for both catabolism and maintaining membrane fluidity at low temperatures (Bell et al. 2006; Henderson and Tocher 1987). Second, Pacific cod are capable of lipogenesis and possible n-3 PUFA synthesis from other carbon sources for example, protein (Laurel et al. 2010). Given the dual role of these fatty acids, we originally hypothesized that Pacific cod embryos would increase total percent composition of PUFAs (essential and non-essential) in their body in response to lower temperature (H 1), but absolute PUFA content (and other lipids and fatty acids) would be reduced at the upper and lower temperature ranges given metabolic inefficiencies (H 2). Finally, we hypothesized that the percent egg/larval composition of essential fatty acids (AA, EPA, DHA) would be conserved in the same manner independent of incubation temperatures (H 3). Our results support H 1 and H 3, whereas H 2 has only partial support from the data. We discuss each of these hypotheses from both a physiological and ecological perspective. However, the biochemical mechanisms to these patterns will remain speculative until more detailed phospholipid class analyses (e.g., phosphatidylcholine and phosphatidylethanolamine) and follow-up molecular studies are conducted. The possibility that fluidity may be controlled by just a few compounds suggests that molecular species analyses by gas chromatography would provide additional valuable insight into the observed patterns.
Thermal adaptation (H 1)
Homeoviscous adaptation has been demonstrated across cold- and warm-adapted fish species and other taxa (Cossins and Prosser 1978, Dey et al. 1993), but within-species examples are scarce. A rare example is a study on goldfish (Carassius auratus L.), where newly-hatched larvae reared at 13 °C had higher proportions of PUFAs in their body than eggs/larvae incubated at 22 °C (Wiegand et al. 1991). We predicted that Pacific cod larval PUFA composition would also be negatively related to the temperature of their rearing environment, but beyond changes in catabolic rates, the effects of temperature on lipid and fatty acid composition were subtle. This may be due to a number of reasons. Firstly, eggs in our experiment were fertilized and transported to the lab at a common temperature, effectively reducing the time homeoviscous adaptation could be realized during the course of the experiment. Secondly, the physical properties of membranes depend more on specific pairings of FA in phospholipids (Fodor et al. 1995). Finfish membranes have high levels of sn-1 monoenoic, sn-2 polyenoic phospholipid molecular species (e.g., 18:1n-9/DHA) with cold adaptation, rendering the membranes less packed (Farkas et al. 2001). Nonetheless, PUFA/SFA did follow a negative pattern consistent with thermal (homeoviscous) adaptation, but this pattern was largely driven by the steep decrease in the 8 °C treatment. Hazel (1984) notes that the production of unsaturated FAs is stimulated by short exposures to cold temperatures, whereas long-term cold acclimatization can begin to have an opposite effect because they begin to impact desaturase activity. The relatively small change in PUFA/SFA observed in the colder treatments (0–6 °C) may therefore be a consequence of prolonged developmental times at these temperatures.
Lipid class composition (%TAG, PL, ST) of newly-hatched larvae also depended on the egg incubation temperature. However, given PL and TAG are both considered catabolic substrates in gadids (Fraser et al. 1988; Laurel et al. 2010), it is difficult to disentangle whether changes in these lipid components were the result of thermal adaptation or simply a consequence of metabolic inefficiencies at temperature extremes. The increased %ST content of embryos incubated at the coldest temperature treatment suggests thermal adaptation given the non-metabolic role of ST and their importance in membrane fluidity and structure.
Absolute changes in lipids/FAs (H 2 )
Lipid/FA biosynthesis in Pacific cod embryos appeared to be inefficient at the coldest temperature treatment, to the point where survival was severely impacted. Temperature had no effect on the relative lipid/FA composition in Pacific cod embryos, but absolute amounts of the major lipid classes and most prevalent FAs were 2- to 4-times lower in embryos reared at 0 °C. Hatching success was also poor to the point that there were insufficient larvae for continued sampling of later hatching larvae. Although speculative, the decreased hatch success at 0 °C may be linked to the embryo’s inability to sufficiently relocate PUFAs at these temperatures to maintain membrane fluidity and function. Larvae incubated at 0 °C were notably lower in 18:1n-9, AA, EPA, and DHA compared to larvae hatching in warmer temperature treatments. Increased requirements of PUFAs at cold temperatures have been hypothesized to negatively impact the development of fish embryos (Wang et al. 1987), and in post-feeding larvae, deficiencies in n-3 PUFAs can result in malpigmentation, visual impairment, and reduced swimming performance (Copeman et al. 2002). The embryos of closely related fish species also develop poorly and experience high mortality at temperatures around 0 °C (e.g., walleye Pollock (Nakatani and Maeda 1984); Atlantic cod (Pepin et al. 1997)), but the mechanisms have not been explored.
We observed increases in EFA content across most temperature treatments, consistent with observations of developing Pacific cod eggs at a single temperature (8 °C) in an earlier study (as discussed by Laurel et al. 2010). Although lipogenesis is relatively common and has been observed in the late embryonic stage of species with demersal or bathypelagic eggs (Cetta and Capuzzo 1982; Berg et al. 2001; Zhu et al. 2003), there is no known mechanism for de novo synthesis of these EFAs (e.g., DHA, EPA, AA) in vertebrates without access to shorter-chain fatty acid precursors. Interestingly, our study suggests extreme low temperatures may interfere with the process, evidenced both by the reduced amounts of lipids/FAs biosynthesized and the slow rates at which the process occurs.
Temperature-independent conservation of EFAs (H 3 )
The most distinct shift in lipid/FA composition in the embryos occurred at the beginning of the hatch cycle, most notably in AA and DHA/EPA. However, the patterns did not change with temperature other than the rate at which these FAs were being conserved, as evidenced in the decreasing slopes with increasing temperature. There was variation in the DHA/EPA among temperature treatments in late stage unfed larvae, notably in the 8 °C treatment, but we suspect these differences would disappear had there been an opportunity to sample a very late stage of hatch at 8 °C. Supporting this conjecture is the observation that DHA/EPA in early hatching larvae was nearly identical (1.5:1) among all temperature treatments.
Although Pacific cod may be capable of synthesizing EFAs, DHA/EPA levels are generally conserved in most marine fish larvae, even under changing food environments (Sargent et al. 1999). In developing eggs and larvae from cold-water marine species, increasing DHA/EPA is typical up to first feeding (Fraser et al. 1988), likely because EPA (20:5n-3) is more readily catabolized than DHA. However, North Pacific and North Atlantic differences in DHA/EPA in both the plankton (El-Sabaawi et al. 2009) and larval cod nutritional requirements (Copeman and Laurel 2010) suggest regional adaptations among related species. For example, DHA/EPA in Atlantic cod has been reported to be higher in the eggs (2:1, Finn et al. 1995a) and newly-hatched larvae (2.6:1, Finn et al. 1995b) than in Pacific cod. In our study, DHA/EPA in Pacific cod eggs started at 1.5:1 and increased to ~1.7:1 to 2.0:1 in late staged larvae across all the temperature treatments. Interestingly, Pacific cod larvae grow and survive best at lower DHA/EPA (0.8:1) (Copeman and Laurel 2010) compared to North Atlantic species (Sargent et al. 1999), possibly because North Pacific DHA/EPA is typically lower (~1:1) in common zooplankton species (El-Sabaawi et al. 2009). The maintenance of similar levels of DHA/EPA among newly-hatched cod larvae in our experiment, despite differences in the relative and absolute content of lipid classes among temperature treatments, argues that the ratio of DHA to EPA is highly important to the survival of the individual.
Conclusion
Despite changing metabolic rates, Pacific cod embryos largely conserved their lipid/FA composition irrespective of their incubation temperature. A range of temperatures used in this study are not as extreme as used in other studies, but they are near the temperature extremes this species faces throughout their range during spring spawning. Within this range, we still observed evidence of thermal adaptation in temperature-specific PUFA/SFA levels and percent composition of TAG, ST, and PL. However, contrary to the predictions of homeoviscous adaptation, the absolute amounts of PUFAs were low in the coldest temperature treatment (0 °C) as was overall hatch success. The limited sample size at the coldest temperature makes it impossible to reach definitive conclusions; however, these results suggest that it would be worth exploring whether temperature-specific patterns in PUFA content exist in other species, especially in relation to hatch success and post-hatch larval survival. A further manipulation of temperature exposure times within the development period, both pre- and post-fertilization, would also be an interesting follow-up study. Together, these results indicate the need for further biochemical investigations of marine fish species during embryogenesis.
References
Becker CD, Genoway RG (1979) Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ Biol Fish 4:245–256
Bell JG, Strachan F, Good JE, Tocher DR (2006) Effect of dietary echium oil on growth, fatty acid composition and metabolism, gill prostaglandin production and macrophage activity in Atlantic cod (Gadus morhua L.). Aquaculture 37:606–617
Berg OK, Hendry AP, Svendsen B, Bech C, Arnekleiv JV, Lohrmann A (2001) Maternal provisioning of offspring and the use of those resources during ontogeny: variation within and between Atlantic salmon families. Funct Ecol 15:13–23
Berkeley SA, Chapman C, Sogard SM (2004) Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology 85:1258–1264
Björnsson B, Stinarsson A, Oddgeirsson M (2001) Optimal temperature for growth and feed conversion of immature cod (Gadus morhua L.). ICES J Mar Sci 58:29–38
Budge SM (1999) Fatty acid biomarkers in a cold water marine environment. PhD Dissertation Memorial University of Newfoundland, St. John’s, NF, Canada
Cetta CM, Capuzzo JM (1982) Physiological and biochemical aspects of embryonic and larval development of the winter flounder Pseudopleuronectes americanus. Mar Biol 71:327–337
Chambers RC, Leggett WC (1996) Maternal influences on variation in egg sizes in temperate marine fishes. Am Zool 36:180–196
Copeman LA, Laurel BJ (2010) Experimental evidence of fatty acid limited growth and survival in Pacific cod (Gadus macrocephalus) larvae. Mar Ecol Prog Ser 412:259–272
Copeman LA, Parrish CC, Harel M, Brown JA (2002) Effects of docosahexaenoic, eicosapentaenoic, and arachidonic acids on the early growth, survival, lipid composition and pigmentation of yellowtail flounder (Limanda ferruginea): a live food enrichment experiment. Aquaculture 210:285–304
Cossins AR, Prosser CL (1978) Evolutionary adaptation of membranes to temperature. Proc Natl Acad Sci 75:2040–2043
Dey I, Buda C, Wiik T, Halver JE, Farkas T (1993) Molecular and structural composition of phospholipid membranes in livers of marine and freshwater fish in relation to temperature. Proc Natl Acad Sci 90:7498–7502
El-Sabaawi R, Dower JF, Kainz M, Mazumder A (2009) Interannual variability in fatty acid composition of the copepod Neocalanus plumchrusin the Strait of Georgia, British Columbia. Mar Ecol Prog Ser 382:151–161
Farkas T, Fodor E, Kitajka K, Halver JE (2001) Response of fish membranes to environmental temperature. Aquac Res 32:645–655
Finn RN, Fyhn HJ, Evjen MS (1995a) Physiological energetics of developing embryos and yolk- sac larvae of Atlantic cod (Gadus morhua). I. Respiration and nitrogen metabolism. Mar Biol 124:355–369
Finn RN, Henderson RJ, Fyhn HJ, Evjen MS (1995b) Physiological energetics of developing embryos and yolk-sac larvae of Atlantic cod (Gadus morhua). II. Lipid metabolism and enthalpy balance. Mar Biol 124:371–379
Fodor E, Jones RH, Buba C, Kitajka K, Dey I, Farkas T (1995) Molecular architecture and biophysical properties of phospholipids during thermal adaptation in fish: an experimental and model study. Lipids 30:1119–1126
Fraser AJ, Gamble JC, Sargent JR (1988) Changes in lipid content, lipid class composition of developing eggs and unfed larvae of cod (Gadus morhua L.). Mar Biol 99:307–314
Hazel JR (1984) Effects of temperature on the structure and metabolism of cell membranes in fish. Am J Physiol Regul Integr Comp Phys 246:460–470
Henderson RJ, Tocher DR (1987) The lipid composition and biochemistry of freshwater fish. Prog Lipid Res 26:281–347
Hurst TP (2007) Causes and consequences of winter mortality in fish. J Fish Biol 71:315–345
Hurst TP, Laurel BJ, Ciannelli L (2010) Thermal dependence of growth rates of early life stages of Pacific cod (Gadus macrocephalus). Fish Bull 108:382–392
Laurel BJ, Copeman LA, Hurst TP, Davis MW (2008) The role of temperature on the growth and survival of early and late hatching Pacific cod larvae (Gadus macrocephalus). J Plankton Res 30:1051–1060
Laurel BJ, Copeman LA, Parrish C, Hurst TP (2010) The ecological significance of lipid/fatty acid synthesis in developing eggs and unfed larvae of Pacific cod (Gadus macrocephalus). Mar Biol 157:1713–1724
Laurel BJ, Hurst TP, Ciannelli L (2011) An experimental examination of temperature interactions in the ‘match–mismatch’ hypothesis for Pacific cod larvae. Can J Fish Aquat Sci 68:51–61
McCain B (2003) Essential fish habitat west coast groundfish draft revised appendix. National Marine Fisheries Service, Northwest Fisheries Science Center, Seattle, WA
McCauley RW, Huggins NW (1979) Ontogenetic and non-thermal seasonal effects on thermal preferenda of fish. Am Zool 19:267–271
Munch SB, Conover DO (2003) Rapid growth results in increased susceptibility to predation in Menidia menidia. Evolution 57:2119–2127
Nakatani T, Maeda T (1984) Thermal effect on the development of walleye pollock eggs and their upward speed to the surface. Bull Jpn Soc Sci Fish 50:937–942
Parrish CC (1987) Separation of aquatic lipid classes by Chromarod thin-layer chromatography with measurement by Iatroscan flame ionization detection. Can J Fish Aquat Sci 44:722–731
Penney RW, Lush PL, Wade J, Brown JA, Parrish CC, Burton MPM (2006) Comparative utility of egg blastomere morphology and lipid biochemistry for prediction of hatching success in Atlantic cod, Gadus morhua L. Aquac Res 37:272–283
Pepin P, Orr SC, Armstrong JT (1997) Time to hatch and larval size in relation to temperature and egg size in Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 54(Suppl 1):2–10
Sargent JR, McEvoy LA, Estevez A, Bell JG, Bell MV, Henderson RJ, Tocher DR (1999) Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture 179:217–229
Sinensky M (1974) Homeoviscous adaptation—a homeostatic process that regulates viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci 71:522–525
Wang YL, Buddington RK, Dorosho SI (1987) Influence of temperature on yolk utilization by the white sturgeon, Acipenser transmontanus. J Fish Biol 30:263–271
Wiegand MD (1996) Composition, accumulation and utilization of yolk lipids in teleost fish. Rev Fish Biol Fish 6:259–286
Wiegand MD, Kitchen CL, Hataley JM (1991) Incorporation of yolk fatty acids into body lipids of goldfish (Carassius auratus L.) larvae raised at two different temperatures. Fish Phys Biochem 9:199–213
Yanes-Roca C, Rhody N, Nystrom M, Main KL (2009) Effects of fatty acid composition and spawning season patterns on egg quality and larval survival in common snook (Centropomus undecimalis). Aquaculture 287:335–340
Zhu P, Parrish CC, Brown JA (2003) Lipid and amino acid metabolism during early development of Atlantic halibut (Hippoglossus hippoglossus). Aquac Int 11:43–52
Acknowledgments
This project was supported in part with funding from the North Pacific Research Board (NPRB) grant #R0605. We thank Allan Stoner and Michael Davis for reviewing earlier drafts of this manuscript. Thanks also to Scott Haines, Tom Hurst, Paul Iseri, and Michele Ottmar for providing assistance in the laboratory. Brian Knoth and Alisa Abookire assisted with egg collections in the field. Boat charters were kindly provided by Tim Tripp aboard the F/V Miss O. Thanks finally to J. Wells for the patient assistance and laboratory analysis of lipid classes and fatty acids. This manuscript is NPRB publication # is 352.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. A. Peck.
Rights and permissions
About this article
Cite this article
Laurel, B.J., Copeman, L.A. & Parrish, C.C. Role of temperature on lipid/fatty acid composition in Pacific cod (Gadus macrocephalus) eggs and unfed larvae. Mar Biol 159, 2025–2034 (2012). https://doi.org/10.1007/s00227-012-1989-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-1989-3