Abstract
The present prospective study examines proportions of maternal erythrocyte fatty acids across gestation and their association with cord erythrocyte fatty acids in normotensive control (NC) and preeclamptic pregnancies. We hypothesize that maternal fatty acid status in early pregnancy influences fetal fatty acid stores in preeclampsia. 137 NC women and 58 women with preeclampsia were included in this study. Maternal blood was collected at 3 time points during pregnancy (16–20th weeks, 26–30th weeks and at delivery). Cord blood was collected at delivery. Fatty acids were analyzed using gas chromatography. The proportions of maternal erythrocyte α-linolenic acid, docosahexaenoic acid, nervonic acid, and monounsaturated fatty acids (MUFA) (p < 0.05 for all) were lower while total n-6 fatty acids were higher (p < 0.05) at 16–20th weeks of gestation in preeclampsia as compared with NC. Cord 18:3n-3, 22:6n-3, 24:1n-9, MUFA, and total n-3 fatty acids (p < 0.05 for all) were also lower in preeclampsia as compared with NC. A positive association was observed between maternal erythrocyte 22:6n-3 and 24:1n-9 at 16–20th weeks with the same fatty acids in cord erythrocytes (p < 0.05 for both) in preeclampsia. Our study for the first time indicates alteration in maternal erythrocyte fatty acids at 16th weeks of gestation which is further reflected in cord erythrocytes at delivery in preeclampsia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia, one of the most common pregnancy complications is a leading cause of maternal and perinatal mortality worldwide [1]. It is characterized by increased maternal blood pressure, proteinuria and vascular dysfunction [2]. Preeclampsia is an independent cardiovascular risk factor for the mother, and recent studies suggest that the offspring also may have an increased cardiovascular and neurodevelopmental risk [3, 4]. Despite the severity of the problem the exact origins of preeclampsia are still unknown [5]. It has been suggested that poor maternal nutrition may play a key role in the pathogenesis of preeclampsia [6].
Maternal, fetal and neonatal essential fatty acids (EFA), linoleic acid (LNA, 18:2n-6), and α-linolenic acid (ALA, 18:3n-3) and their long chain polyunsaturated fatty acids (LCPUFA) derivatives, docosahexaenoic acid (DHA, 22:6n-3) and arachidonic acid (ARA, 20:4n-6) status are key determinants of health and disease in infancy and later in life [7]. During pregnancy there is a decrease in the maternal LCPUFA status as the developing fetus completely depends on the maternal essential fatty acids supply [8–10]. Studies have reported that higher intake of 22:6n-3 reduces the risk of preterm birth and increases the length of gestation and birth weight of the newborn [11, 12]. Other studies also support the positive association between infant plasma 20:4n-6 and birth weight [13, 14].
Lipids are the major constituents of brain tissue (about 50 %) and it thus becomes important that adequate amounts of high-quality fatty acids are available to the developing fetus [15]. 22:6n-3 is highly concentrated in the brain and confers a unique structural and functional property to cell membrane phospholipids (in the neuronal synapses of the brain) due to its longest side-chain and highest degree of unsaturation [16, 17]. 22:6n-3 is associated with cognitive functioning [18] and deficiency of 22:6n-3 in the brain is reported to lead to memory loss [19], and increase the risk for neurodegenerative diseases later in life [20].
20:4n-6, an LCPUFA is a major fatty acid in the brain [21] and is known to influence neurotransmission [22]. Nervonic acid (NA, 24:1n-9), a monounsaturated n-9 fatty acid plays an important role in the biosynthesis of myelin and is a major constituent of the myelin sheath. It is important for the development of the nervous system and early myelination [23]. In humans during the 24th–42nd weeks of gestation the growth rate of the brain is very high and involves processes like myelination and synapse formation [24]. Disturbed LCPUFA synthesis in preeclampsia is suggested to result in suboptimal neurodevelopment of the infants [25].
There are a number of studies which have examined the levels of LCPUFA in preeclampsia and reports are inconclusive with some showing lower [26–29], others no change [30] and still others high [31] 22:6n-3 levels. Further all these studies have been carried out during the third trimester of pregnancy or at the time of delivery. Our earlier cross-sectional studies have also shown lower proportions of maternal and placental 22:6n-3 in women with preeclampsia [32–35]. However, these fatty acid proportions were analyzed after the diagnosis of preeclampsia and may be confounded by the disease [27, 29, 31]. There is therefore a need to undertake prospective studies which will help resolve the above issues.
We hypothesize that maternal fatty acid status in early pregnancy influence fetal fatty acid stores in preeclampsia. Our recent longitudinal study reports placental, cord, and maternal plasma fatty acid levels at different points across gestation in women with preeclampsia [36]. Erythrocyte fatty acids are known to reflect long-term fatty acid intake because of less sensitivity to recent intake and a slower turnover rate [37]. Therefore, the present study analyzes maternal erythrocyte proportions of fatty acids at three different time points across gestation in women with preeclampsia and compares them with normotensive women. Further, the association of maternal fatty acids with cord fatty acids are also reported.
Materials and Methods
Study Design
This prospective study which is a part of large ongoing departmental study recruits pregnant women in early pregnancy and was carried out at two hospitals, Department of Obstetrics and Gynaecology, Bharati Hospital and Gupte Hospital and Research Centre, Pune, India. The study protocol was approved by Bharati Vidyapeeth Medical College Institutional Ethical Committee (Ref No.: BVDU/MC/02).
Subjects
All women gave written consent for participating in the study and were recruited at 16–20th weeks of gestation which were followed across gestation. Preeclampsia was diagnosed as per the ACOG guidelines if there was presence of proteinuria (>1 + or 300 mg per 24 h) or high blood pressure (>140 and 90 mmHg) [38]. Proteinuria was measured on a dipstick test while blood pressure was measured with a mercury sphygmomanometer. These women were treated with antihypertensive drugs. As per the ACOG guidelines, it is recommended that diagnosis of hypertension requires two blood pressure determinations at least 4 h apart [38]. The women in our cohort had two blood pressure measurements in order to meet the criteria for diagnosis of preeclampsia. At delivery, they were classified as normotensive control (NC) delivering at term (total gestation ≥37 weeks and baby weight ≥2.5 kg) and those that develop preeclampsia during pregnancy. Thus, a total of 137 NC women and 58 women with preeclampsia were included in the present study. The present study includes women with preeclampsia delivering both at term and preterm.
Exclusion criteria were other pregnancy complications such as gestational diabetes, eclampsia, chronic hypertension, type I or type II diabetes mellitus, seizure disorder and renal or liver disease. All pregnancies were singleton and none of the women smoked or consumed alcohol during pregnancy. All women were routinely given iron (60 mg per tablet) and folic acid (500 μg per tablet) tablets during the first trimester of pregnancy as per the National Prophylaxis Programme. Gestational age was calculated from the last menstrual period and confirmed by routine ultrasonography.
Demographic characteristics were recorded at the time of recruitment i.e., 16–20th weeks of gestation, and subsequently at 26–30th weeks of gestation and at the time of delivery. Details of gestational age at delivery, mode of delivery, fetal sex, birth weight and placental weight were recorded. The current study includes the same group of pregnant women on whom plasma fatty acid levels were recently reported [36].
Dietary Assessments
Pregnant women were interviewed with a food frequency questionnaire (FFQ) during 16–20th weeks, 26–30th weeks and at delivery to estimate the frequency of intake of foods rich in 18:3n-3, 22:6n-3, and n-3 fatty acids. The FFQ was administered by a nutritionist. This FFQ has been validated for this population and has been used by us in a number of our departmental studies [32, 36, 39, 40].
The FFQ used in the present study was used to estimate the frequency of consumption of foods rich in 18:3n-3, 22:6n-3, and n-3 fatty acids consumed during the last 1 month for which scores were calculated. For example, an item consumed once a week has a score of 4 while that consumed daily has a score of 30.
Sample Collection and Processing
The first blood sample was obtained between 16 and 20 weeks of gestation, the second between 26 and 30 weeks of gestation, the third sample was taken just before going to the labor room. At each visit maternal blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA). At delivery, a section of the umbilical cord is clamped close to the baby and another clamp further down the umbilical cord. The umbilical cord was cut between the two clamps. Ten millilitres of cord blood remaining in the vein was then collected and also been reported by us earlier [41]. Plasma and erythrocytes were separated by centrifugation at 2000 rpm for 30 min. The erythrocyte fraction was washed 3 times with normal saline and was stored at −80 °C until further analysis.
Fatty Acid Analysis
The procedure for fatty acid analysis has been described in detail earlier [32–36, 39, 40, 42]. Briefly, transesterification of the total erythrocyte fatty acids was performed using hydrochloric acid–methanol. Methyl esters were separated using a PerkinElmer gas chromatograph (SP 2330, 30 m capillary Supelco column; PerkinElmer, Shelton, CT, USA). Peaks were identified by comparison with standard fatty acid methyl esters (Sigma-Aldrich). Fatty acids are expressed in mole percent (mol %).
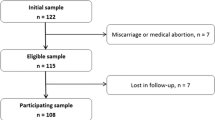
The number of maternal and cord erythrocyte samples analysed for fatty acid levels at various time points during pregnancy is shown in Fig. 1.
Statistical Methods
The data were analyzed using the SPSS/PC + package (Version 20, Chicago, IL, USA). Values are reported as mean ± SD (erythrocyte fatty acid proportions). Skewed variables were transformed to normality using the following transformations: log to the base 10. Pearson’s correlation was used to examine the associations between fatty acid levels and maternal age, body mass index (BMI), gestational age, parity, socioeconomic status (SES) and education as they have been shown to affect fatty acid levels [43–48]. Fatty acid levels were correlated with gestation and socioeconomic status and hence fatty acid levels were adjusted for these variables using multiple linear regression and were compared between NC and preeclampsia (p < 0.05) using independent t test. Mean values of fatty acid proportions from 16th week of gestation till delivery in NC and preeclampsia were compared using least significance difference estimated from one-way analysis of variance (p < 0.05). Correlation between variables was studied using Pearson’s correlation analysis after adjusting for gestation and socioeconomic status. Chi-square test was used for comparison of categorical variables. To compare two proportions, Z test of proportions was used. The variable sample number (n) in different measures was due to insufficient sample volume available. Although the data were incomplete, they are unlikely to have biased the results.
Results
Maternal and Neonatal Characteristics
The BMI of women with preeclampsia was higher as compared to those of NC women at 16–20th weeks, 26–30th weeks and at delivery (p < 0.01 for all). Systolic and diastolic blood pressure was higher in women with preeclampsia at all time points. Baby weight, baby length and baby head circumference were lower (p < 0.05 for all) in preeclampsia as compared with NC (Table 1).
Frequency of Intake of ALA, DHA and n-3 Fatty Acid Rich Foods
In our cohort, the 18:3n-3 rich foods consumed by the women include pulses (e.g., cowpea, rajma) and green leafy vegetables (e.g., fenugreek, spinach, colocasia leaves, amaranth, ambat chukka) while fish consumption was considered as a 22:6n-3 rich food. The frequency of consumption of 18:3n-3 rich foods was similar in both the normotensive control and preeclampsia groups at 16–20th weeks (p = 0.908), 26–30th weeks (p = 0.382) and at delivery (p = 0.537). Similarly, the frequency of consumption of 22:6n-3 and n-3 rich foods was similar in both the groups at 16–20th weeks (p = 0.779, p = 0.845, respectively), 26–30th weeks (p = 0.907, p = 0.542, respectively) and at delivery (p = 0.367, p = 0.273, respectively). The percent women consuming 18:3n-3, 22:6n-3 and n-3 fatty acid rich foods in both normotensive control and preeclampsia groups are shown in Table 2.
Proportions of Maternal Erythrocyte Fatty Acids Over Time During Pregnancy
Table 3 shows the maternal erythrocyte fatty acid proportions at three time points during pregnancy. 18:2n-6 proportions were higher (p < 0.05) in preeclampsia as compared with NC at 26–30th weeks. Dihomogamma linolenic acid (DGLA, 20:3n-6) and 20:4n-6 proportions were lower (p < 0.05) in preeclampsia as compared with NC at delivery. Total n-6 fatty acids were higher in preeclampsia at 16–20th weeks and 26–30th weeks (p < 0.05 for both) whereas lower (p < 0.05) at delivery as compared with NC. Proportions of 18:3n-3 were lower (p < 0.05) in preeclampsia as compared with NC at 16–20th weeks. 22:6n-3 proportions were lower in preeclampsia as compared with NC at 16–20th weeks, 26–30th weeks and at delivery (p < 0.05 for all). Total n-3 fatty acids were lower in preeclampsia at 26–30th weeks and at delivery (p < 0.05 for both). n-6:n-3 Fatty acid ratio was higher in preeclampsia at 26–30th weeks and at delivery (p < 0.05 for both). Saturated fatty acids (SFA) were higher (p < 0.05) in preeclampsia at delivery. Monounsaturated fatty acids (MUFA) were lower in preeclampsia at 16–20th weeks, 26–30th weeks and at delivery (p < 0.05 for all). 24:1n-9 Proportions were lower in preeclampsia at 16–20th weeks, 26–30th weeks and at delivery (p < 0.05 for all) in preeclampsia.
Proportions of Cord Erythrocyte Fatty Acids
Table 3 shows proportions of cord erythrocyte fatty acids. Cord erythrocyte 18:2n-6, 20:4n-6, and total n-6 fatty acid proportions were comparable between the two groups. Cord erythrocyte 18:3n-3 and 22:6n-3 proportions were lower (p < 0.05 for both) in preeclampsia as compared to NC. Total n-3 fatty acids were lower (p < 0.05) in preeclampsia as compared to NC. n-6:n-3 fatty acid ratio was higher in preeclampsia (p < 0.05). There was no change in the proportions of SFA between the two groups whereas proportions of MUFA were lower (p < 0.05) in preeclampsia as compared to NC. 24:1n-9 proportions were lower in preeclampsia (p < 0.05) as compared to NC.
Changes in Erythrocyte Fatty Acid Proportions From 16th Week of Gestation till Delivery in NC and Preeclampsia
Table 3 reports changes in fatty acid proportions from 16th week of gestation till delivery in NC and preeclampsia. The proportions of cord erythrocyte 18:3n-3 and 22:6n-3 were higher than the maternal values at all time points (p < 0.05 for all) in NC. The proportions of cord erythrocyte 22:6n-3 were higher than the maternal values at 26–30th weeks and at delivery (p < 0.05 for both) in preeclampsia.
The proportions of cord erythrocyte 18:2n-6 were lower whereas those of cord erythrocyte 20:4n-6 were higher than the maternal values at all time points (p < 0.05 for all) both in NC and preeclampsia. The cord erythrocyte n-6:n-3 fatty acid ratio were lower than the maternal values at all time points (p < 0.05 for all) both in NC and preeclampsia.
The proportions of cord erythrocyte 24:1n-9 were lower than the maternal values at all time points (p < 0.05 for all) only in NC; however in preeclampsia the proportions of cord erythrocyte 24:1n-9 were lower at than the maternal values at all time points but were not statistically significant. The proportions of cord erythrocyte MUFA were lower than the maternal values at all time points (p < 0.05 for all) both in NC and preeclampsia.
Associations Between Cord Erythrocyte Fatty Acids and Maternal Erythrocyte Fatty Acids
There was a positive association between cord erythrocyte 22:6n-3, total n-3 fatty acids, MUFA and maternal erythrocyte 22:6n-3, total n-3 fatty acids and MUFA (r = 0.361, p < 0.001, n = 128; r = 0.283, p = 0.002, n = 128; r = 0.267, p = 0.004, n = 128 respectively) at 16–20th weeks in NC.
A positive association was seen between cord erythrocyte 24:1n-9, 22:6n-3, SFA and maternal erythrocyte 24:1n-9, 22:6n-3, SFA respectively (r = 0.276, p = 0.050, n = 56; r = 0.292, p = 0.040, n = 56; r = 0.280, p = 0.049, n = 56 respectively) at 16–20th weeks in preeclampsia.
There was a positive association of cord erythrocyte 24:1n-9, 22:6n-3, total n-3 fatty acids, 20:4n-6, total n-6 fatty acids, SFA, MUFA with respective maternal erythrocyte fatty acids respectively (r = 0.334, p = 0.001, n = 123; r = 0.581, p < 0.001, n = 123; r = 0.417, p < 0.001, n = 123; r = 0.352, p < 0.001, n = 123; r = 0.329, p = 0.001, n = 123; r = 0.336, p < 0.001, n = 123; r = 0.288, p = 0.003, n = 123 respectively) at 26–30th weeks in NC whereas a negative association was observed between cord erythrocyte 18:3n-3 and maternal erythrocyte 18:3n-3 (r = −0.200, p = 0.042, n = 123) at 26–30th weeks in NC.
Cord erythrocyte 22:6n-3, total n-3 fatty acids were positively associated with maternal erythrocyte 22:6n-3, total n-3 fatty acids respectively (r = 0.332, p = 0.042, n = 43; r = 0.382, p = 0.018, n = 43, respectively) in preeclampsia at 26–30th weeks.
There was a negative association between cord erythrocyte 18:2n-6 and maternal erythrocyte 18:2n-6 (r = −0.489, p < 0.001, n = 128) whereas a positive association was observed between cord erythrocyte 22:6n-3, total n-3 fatty acids and maternal erythrocyte 22:6n-3, total n-3 fatty acids (r = 0.331, p < 0.001, n = 128; r = 0.388, p < 0.001, n = 128, respectively) in NC at delivery.
Cord erythrocyte 18:2n-6 proportions were negatively associated with maternal erythrocyte 18:2n-6 proportions (r = −0.326, p = 0.037, n = 48) whereas a positive association was observed between cord erythrocyte 24:1n-9, 22:6n-3 and maternal erythrocyte 24:1n-9, 22:6n-3 (r = 0.340, p = 0.030, n = 48; r = 0.379, p = 0.015, n = 48, respectively) in preeclampsia at delivery.
Discussion
The current study reports erythrocyte fatty acid proportions in both NC women and women with preeclampsia since they are considered be a better measure reflecting intake over the past months as compared to fatty acids measured in plasma which reflect a short-term intake [37, 49]. Our recent studies on this cohort report an association of cord plasma fatty acids with maternal plasma fatty acids across gestation in a NC pregnancy [42] and alterations in plasma fatty acids across gestation in preeclampsia as compared with NC women [36]. However, since plasma fatty acid profile is influenced by the intake over the past weeks and is known to vary considerably [50, 51] it is necessary to examine changes in erythrocyte fatty acid proportions across gestation especially in pregnancy complications like preeclampsia. Reports indicate that cord erythrocyte fatty acids are a good surrogate measure for fetal fatty acid stores since they do not cross the placenta and would not be affected by changes in transport during parturition [52]. The current study therefore also reports the association of maternal erythrocyte fatty acids with cord erythrocyte fatty acids.
In the current study we observed lower proportions of maternal erythrocyte 18:3n-3, 22:6n-3 and total n-3 fatty acids at 16–20th weeks of gestation. We and few others have reported lower erythrocyte n-3 fatty acids in preeclampsia during the third trimester or at the end of pregnancy [27, 29, 32, 35]. There is only one prospective study that has examined maternal plasma phospholipid LCPUFA levels in pregnancy induced hypertension [8]. In contrast, to the above study our study suggests that erythrocyte n-3 fatty acid proportions are altered right from 16–20th weeks of gestation in preeclampsia. Our earlier study suggests that increased oxidative stress observed in women with preeclampsia is negatively associated with LCPUFA levels [35].
In the current study higher proportions of maternal erythrocyte total n-6 fatty acids were seen at 16–20th weeks, 26–30th weeks and at delivery in preeclampsia. Although 18:2n-6 and total n-6 fatty acid proportions were found higher in preeclampsia, proportions of 20:4n-6 were lower at delivery. These findings are in contrast with few other studies that have reported higher erythrocyte 20:4n-6 levels in preeclampsia in the third trimester [26, 31]. This reduction in 20:4n-6 levels could be due to the fact that it is further metabolized to thromboxane in preeclampsia [53].
It is well known that n-3 fatty acids interact with fatty acids in the n-6 family in eicosanoid biosynthesis, and n-3 fatty acids are rapidly and reversibly incorporated into membranes of erythrocytes, largely at the expense of n-6 fatty acids [30]. n-6 PUFA derived eicosanoids are pro-inflammatory whereas the n-3 PUFA derived eicosanoids mostly promote anti-inflammatory activities and are known to be regulated by the n-6:n-3 ratio [54, 55]. The ratio of n-6 to n-3 fatty acids is considered to be an important determinant of health [56]. The increased n-6:n-3 ratio will trigger enhanced activation of the immune system and result in increased production of pro-inflammatory eicosanoids and cytokines [57]. In the present study we observed higher n-6:n-3 fatty acids at 26–30th weeks and at delivery in preeclampsia as compared with NC group. Higher levels of erythrocyte n-6 fatty acids and lower n-3 fatty acids are known to be associated with an increased risk of preeclampsia [29]. Further a recent report indicates that an increased n-6:n-3 ratio negatively affects fetal development [58].
Further proportions of MUFA were lower in maternal erythrocytes at all time points and were also lower in the cord erythrocyte in women with preeclampsia. 24:1n-9 is important for white matter development and its incorporation increases rapidly in the last trimester [59]. In preeclampsia, we observed lower proportions of 24:1n-9 across gestation and further proportions lowered in cord erythrocyte.
Cord erythrocyte proportions of 18:3n-3, 22:6n-3, and total n-3 fatty acids were lower in preeclampsia in the current study. However, there was no difference observed in the cord erythrocyte 18:2n-6, 20:4n-6 and total n-6 fatty acid proportions in both the groups. Thus, the lower levels of n-3 fatty acids observed in cord erythrocytes could be either due to a disturbed LCPUFA metabolism or decreased release from maternal stores [26].This study also examined an association between proportions of maternal erythrocyte fatty acids across gestation and proportions of cord erythrocyte fatty acids. There was a positive association between cord and maternal erythrocyte 22:6n-3 at 16–20th weeks, 26–30th weeks and at delivery both in NC and preeclampsia. These findings suggest that the maternal fatty acid stores at 16–20th weeks of gestation are reflective of the fetal stores. Therefore, an optimal n-3 fatty acid intake in early pregnancy may positively influence fetal fatty acid stores.
Further there was a negative association between cord and maternal erythrocyte 18:2n-6 at delivery in NC and preeclampsia. This is similar to our earlier results on plasma fatty acid proportions in normotensive women [42]. We also observed a positive association between cord erythrocyte and maternal erythrocyte 24:1n-9 at 16–20th weeks and at delivery in preeclampsia. Reports indicate lower levels of 24:1n-9 in plasma and erythrocytes of major depressive disorder patients [60]. Thus, lower levels of 22:6n-3 and 24:1n-9 may lead to poor fetal brain development in children born to mothers with preeclampsia.
When changes in the erythrocyte fatty acid proportions were analyzed from 16th week of gestation till delivery, it was observed that proportions of cord erythrocyte 22:6n-3 and 20:4n-6 were higher than the maternal values both in NC and preeclampsia. These results are in accordance with our previously published data on plasma fatty acid levels in NC and preeclampsia [36]. The higher proportions of cord erythrocyte 22:6n-3 and 20:4n-6 than the maternal values may be explained by ‘biomagnification’ of these fatty acids i.e., selective transfer of these LCPUFA by the placenta [61]. Further the lower levels of 18:2n-6 in cord erythrocytes as compared to maternal values both in NC and preeclampsia which may be due to the lower placental transfer of 18:2n-6 so as to prevent inhibition of ∆6 desaturase activity and enable higher rates of synthesis of 20:4n-6 and 22:6n-3 in fetal tissues [62].
In our earlier study, we observed lower levels of maternal plasma 20:4n-6 at 16–20th weeks of gestation in preeclampsia as compared with NC [36], however this reduction was observed at delivery for erythrocyte fatty acids. However, both plasma and erythrocyte 22:6n-3 levels were lower at 16–20th weeks of gestation in preeclampsia as compared with NC. This is in line with earlier reports indicating a strong correlation between plasma and erythrocyte 22:6n-3 content [63]. The changes in the levels of erythrocyte 22:6n-3 was consistent in preeclampsia across gestation. Cord levels of 22:6n-3 was lower both in plasma [36] and erythrocyte while 20:4n-6 levels were lower only in plasma in preeclampsia as compared with NC [36]. In case of 24:1n-9, no differences were seen in maternal and cord plasma in preeclampsia and NC [36], however a reduction in 24:1n-9 levels was seen in maternal erythrocytes from 16th week till delivery, and was further lower in cord erythrocytes in preeclampsia as compared with NC. The discrepancies observed between plasma and erythrocytes need further investigation.
Thus, the present study carried out on erythrocytes showed more pronounced differences in the 22:6n-3 and 24:1n-9 levels from early pregnancy and continued across gestation till delivery in preeclampsia as compared with NC. However, as mentioned above, at this stage it is difficult to comment on the effect of increased erythropoeisis during pregnancy on LCPUFA levels in the erythrocytes as compared to our recently published results on fatty acid levels in plasma [36].
The role of maternal diet in the etiology of preeclampsia has recently received increased attention [64]. A number of studies have reported the involvement of certain nutrients in the pathogenesis of preeclampsia particularly n-3 fatty acids, which modulate endothelial function [65]. The dietary intake of NC women and women with preeclampsia were similar in the current study. We have recently reported a disturbed placental fatty acid metabolism in preeclampsia [36].The altered levels of erythrocyte LCPUFA levels observed in preeclampsia may be due to a disturbed fatty acid metabolism or release from maternal stores.
Our study for the first time reports lower proportions of maternal erythrocyte 18:3n-3, 22:6n-3, 24:1n-9, and MUFA in preeclampsia right from early pregnancy. Further these fatty acid proportions were also lower in the cord samples in preeclampsia. Our findings are of significance since 22:6n-3 and 24:1n-9 are involved in brain development and function and may influence risk for neurodevelopmental disorders in children born to mothers with preeclampsia. Our findings suggest that optimal LCPUFA stores in early pregnancy may be useful in improving fetal growth and development.
Abbreviations
- ALA, 18:3n-3:
-
α-Linolenic acid
- ARA, 20:4n-6:
-
Arachidonic acid
- BMI:
-
Body mass index
- DGLA, 20:3n-6:
-
Dihomogamma linolenic acid
- DHA, 22:6n-3:
-
Docosahexaenoic acid
- EFA:
-
Essential fatty acids
- EDTA:
-
Ethylenediaminetetraacetic acid
- LNA, 18:2n-6:
-
Linoleic acid
- LCPUFA:
-
Long chain polyunsaturated fatty acids
- MUFA:
-
Monounsaturated fatty acids
- NA, 24:1n-9:
-
Nervonic acid
- NC:
-
Normotensive control
- PUFA:
-
Polyunsaturated fatty acids
- SFA:
-
Saturated fatty acids
References
Roberts JM, Pearsons G, Cutler J, Lindheimer M (2003) Summary of the NHLBI Working Group on research on hypertension during pregnancy. Hypertension 41:437–445
George EM, Granger JP (2010) Recent insights into the pathophysiology of preeclampsia. Expert Rev Obstet Gynecol 5:557–566
Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P (2012) Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 129:e1552–e1561
Hakim J, Senterman MK, Hakim AM (2013) Preeclampsia is a biomarker for vascular disease in both mother and child: the need for a medical alert system. Int J Pediatr 2013:953150
Bell MJ (2010) A historical overview of preeclampsia-eclampsia. J Obstet Gynecol Neonatal Nurs 39:510–518
Wu G, Imhoff-Kunsch B, Girard AW (2012) Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol 26:4–26
Duttaroy AK (2000) Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am J Clin Nutr 71:315–322
Al MD, van Houwelingen AC, Badart-Smook A, Hasaart TH, Roumen FJ, Hornstra G (1995) The essential fatty acid status of mother and child in pregnancy-induced hypertension: a prospective longitudinal study. Am J Obstet Gynecol 172:1605–1614
Otto SJ, Houwelingen AC, Antal M, Manninen A, Godfrey K, Lopez-Jaramillo P, Hornstra G (1997) Maternal and neonatal essential fatty acid status in phospholipids: an international comparative study. Eur J Clin Nutr 51:232–242
Haggarty P (2004) Effect of placental function on fatty acid requirements during pregnancy. Eur J Clin Nutr 58:1559–1570
Haggarty P (2014) Meeting the fetal requirement for polyunsaturated fatty acids in pregnancy. Curr Opin Clin Nutr Metab Care 17:151–155
Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, Georgieff MK, Markley LA, Kerling EH, Shaddy DJ (2013) DHA supplementation and pregnancy outcomes. Am J Clin Nutr 97:808–815
Elias SL, Innis SM (2001) Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am J Clin Nutr 73:807–814
Koletzko B, Braun M (1991) Arachidonic acid and early human growth: is there a relation? Ann Nutr Metab 35:128–131
Simpson JL, Bailey LB, Pietrzik K, Shane B, Holzgreve W (2011) Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part II–vitamin D, vitamin A, iron, zinc, iodine, essential fatty acids. J Matern Fetal Neonatal Med 24:1–24
Wainwright PE (2002) Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc 61:61–69
Bradbury J (2011) Docosahexaenoic acid (DHA): an ancient nutrient for the modern human brain. Nutrients 3:529–554
Lund EK (2013) Health benefits of seafood; is it just the fatty acids? Food Chem 140:413–420
Jump DB (2002) The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem 277:8755–8758
Heaton AE, Meldrum SJ, Foster JK, Prescott SL, Simmer K (2013) Does docosahexaenoic acid supplementation in term infants enhance neurocognitive functioning in infancy? Front Hum Neurosci 7:774
SanGiovanni JP, Chew EY (2005) The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res 24:87–138
Wauben IPM, Wainwright PE (1999) The influence of neonatal nutrition on behavioural development: a critical appraisal. Nutr Rev 57:35–44
Rutherford MA, Supramaniam V, Ederies A, Chew A, Bassi L, Groppo M, Anjari M, Counsell S, Ramenghi LA (2010) Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 52:505–521
Georgieff MK (2007) Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 85:614S–620S
Cetin I, Koletzko B (2008) Long-chain omega-3 fatty acid supply in pregnancy and lactation. Curr Opin Clin Nutr Metab Care 11:297–302
Mackay VA, Huda SS, Stewart FM, Tham K, McKenna LA, Martin I, Jordan F, Brown EA, Hodson L, Greer IA, Meyer BJ, Freeman DJ (2012) Preeclampsia is associated with compromised maternal synthesis of long-chain polyunsaturated fatty acids, leading to offspring deficiency. Hypertension 60:1078–1085
Qiu C, Sanchez SE, Larrabure G, David R, Bralley JA, Williams MA (2006) Erythrocyte omega-3 and omega-6 polyunsaturated fatty acids and preeclampsia risk in Peruvian women. Arch Gynecol Obstet 274:97–103
Wang Y, Walsh SW, Kay HH (2005) Placental tissue levels of nonesterified polyunsaturated fatty acids in normal and preeclamptic pregnancies. Hypertens Pregnancy 24:235–245
Williams MA, Zingheim RW, King IB, Zebelman AM (1995) Omega-3 fatty acids in maternal erythrocytes and risk of preeclampsia. Epidemiology 6:232–237
Mahomed K, Williams MA, King IB, Mudzamiri S, Woelk GB (2007) Erythrocyte omega-3, omega-6 and trans fatty acids in relation to risk of preeclampsia among women delivering at Harare Maternity Hospital, Zimbabwe. Physiol Res 56:37–50
Bakheit KH, Ghebremeskel K, Pol K, Elbashir MI, Adam I (2010) Erythrocyte omega-3 and omega-6 fatty acids profile in Sudanese women with pre-eclampsia. J Obstet Gynaecol 30:151–154
Kulkarni A, Mehendale S, Pisal H, Kilari A, Dangat K, Salunkhe S, Taralekar V, Joshi S (2011) Association of omega-3 fatty acids and homocysteine concentrations in pre-eclampsia. Clin Nutr 30:60–64
Kulkarni AV, Mehendale SS, Yadav HR, Joshi SR (2011) Reduced placental docosahexaenoic acid levels associated with increased levels of sFlt-1 in preeclampsia. Prostaglandins Leukot Essent Fatty Acids 84:51–55
Dangat KD, Mehendale SS, Yadav HR, Kilari AS, Kulkarni AV, Taralekar VS, Joshi SR (2010) Long-chain polyunsaturated fatty acid composition of breast milk in pre-eclamptic mothers. Neonatology 97:190–194
Mehendale S, Kilari A, Dangat K, Taralekar V, Mahadik S, Joshi S (2008) Fatty acids, antioxidants, and oxidative stress in pre-eclampsia. Int J Gynaecol Obstet 100:234–238
Wadhwani N, Patil V, Pisal H, Joshi A, Mehendale S, Gupte S, Wagh G, Joshi S (2014) Altered maternal proportions of long chain polyunsaturated fatty acids and their transport leads to disturbed fetal stores in preeclampsia. Prostaglandins Leukot Essent Fatty Acids 91:21–30
Sun Q, Ma J, Campos H, Hankinson SE, Hu FB (2007) Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 86:74–81
American College of Obstetricians and Gynecologists (ACOG) (2013) Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol 122:1122–1131
Kilari AS, Mehendale SS, Dangat KD, Yadav HR, Gupta A, Taralekar VS, Joshi SR (2010) Long chain polyunsaturated fatty acids in mothers of preterm babies. J Perinat Med 38:659–664
Kilari AS, Mehendale SS, Dangat KD, Yadav HR, Kulakarni AV, Dhobale MV, Taralekar VS, Joshi SR (2009) Long chain polyunsaturated fatty acids in mothers and term babies. J Perinat Med 37:513–518
Dhobale M, Mehendale S, Pisal H, D’Souza V, Joshi S (2012) Association of brain-derived neurotrophic factor and tyrosine kinase B receptor in pregnancy. Neuroscience 216:31–37
Wadhwani NS, Pisal HR, Mehendale SS, Joshi SR (2015) A prospective study of maternal fatty acids, micronutrients and homocysteine and their association with birth outcome. Matern Child Nutr 11:559–573
van Gool CJ, Thijs C, Dagnelie PC, Henquet CJ, van Houwelingen AC, Schrander J, Menheere PP, van den Brandt PA (2004) Determinants of neonatal IgE level: parity, maternal age, birth season and perinatal essential fatty acid status in infants of atopic mothers. Allergy 59:961–968
Baack ML, Puumala SE, Messier SE, Pritchett DK, Harris WS (2015) What is the relationship between gestational age and docosahexaenoic acid (DHA) and arachidonic acid (ARA) levels?. Prostaglandins Leukot Essent Fatty Acids. 100:5–11. doi:10.1016/j.plefa.2015.05.003 (In press)
Yeh LL, Kuller LH, Bunker CH, Ukoli FA, Huston SL, Terrell DF (1996) The role of socioeconomic status and serum fatty acids in the relationship between intake of animal foods and cardiovascular risk factors. Ann Epidemiol 6(4):290–298
Cohen BE, Garg SK, Ali S, Harris WS, Whooley MA (2008) Red blood cell docosahexaenoic acid and eicosapentaenoic acid concentrations are positively associated with socioeconomic status in patients with established coronary artery disease: data from the Heart and Soul Study. J Nutr 138:1135–1140
Sands SA, Reid KJ, Windsor SL, Harris WS (2005) The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids 40:343–347
Hamosh M, Goldman AS (eds) (2012) Human lactation 2: maternal and environmental factors. Springer Science & Business Media, Berlin
Fuhrman BJ, Barba M, Krogh V, Micheli A, Pala V, Lauria R, Chajes V, Riboli E, Sieri S, Berrino F, Muti P (2006) Erythrocyte-membrane phospholipid composition as a biomarker of dietary fat. Ann Nutr Metab 50:95–102
Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M (1997) Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 38:2012–2022
Hon GM, Abel S, Smuts CM, van Jaarsveld P, Hassan MS, van Rensburg SJ, Erasmus RT, Matsha T (2012) Gas chromatography results interpretation: absolute amounts versus relative percentages. In: Bekir Salih, Ömür Çelikbıçak (eds) Gas Chromatography–Biochemicals, Narcotics Essential Oils, In Tech
Weiler H, Fitzpatrick-Wong S, Schellenberg J, McCloy U, Veitch R, Kovacs H, Kohut J, Kin Yuen C (2005) Maternal and cord blood long-chain polyunsaturated fatty acids are predictive of bone mass at birth in healthy term-born infants. Pediatr Res 58:1254–1258
Wetzka B, Nüsing R, Charnock-Jones DS, Schäfer W, Zahradnik HP, Smith SK (1997) Cyclooxygenase-1 and -2 in human placenta and placental bed after normal and preeclamptic pregnancies. Hum Reprod 12:2313–2320
Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C (2012) Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab 2012:539426
Uauy R, Mena P, Rojas C (2000) Essential fatty acids in early life: structural and functional role. Proc Nutr Soc 59:3–15
Harris WS (2006) The omega-6/omega-3 ratio and cardiovascular disease risk: uses and abuses. Curr Atheroscler Rep 8:453–459
Simopoulos AP (2002) Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 21:495–505
Bascuñán KA, Valenzuela R, Chamorro R, Valencia A, Barrera C, Puigrredon C, Sandoval J, Valenzuela A (2014) Polyunsaturated fatty acid composition of maternal diet and erythrocyte phospholipid status in Chilean pregnant women. Nutrients 6:4918–4934
Ntoumani E, Strandvik B, Sabel KG (2013) Nervonic acid is much lower in donor milk than in milk from mothers delivering premature infants–of neglected importance? Prostaglandins Leukot Essent Fatty Acids 89:241–244
Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I, Abeling NG, Duran M, Schene AH (2010) Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One 5:e10635
Luxwolda MF, Kuipers RS, Sango WS, Kwesigabo G, Dijck-Brouwer DA, Muskiet FA (2012) A maternal erythrocyte DHA content of approximately 6 g % is the DHA status at which intrauterine DHA biomagnifications turns into bioattenuation and postnatal infant DHA equilibrium is reached. Eur J Nutr 51:665–675
Novak EM, King DJ, Innis SM (2012) Low linoleic acid may facilitate Δ6 desaturase activity and docosahexaenoic acid accretion in human fetal development. Prostaglandins Leukot Essent Fatty Acids 86:93–98
Arterburn LM, Hall EB, Oken H (2006) Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr 83:1467S–1476S
Xu H, Shatenstein B, Luo ZC, Wei S, Fraser W (2009) Role of nutrition in the risk of preeclampsia. Nutr Rev 67:639–657
Roberts JM, Balk JL, Bodnar LM, Belizan JM, Bergel E, Martinez A (2003) Nutrient involvement in preeclampsia. J Nutr 133:1684S–16892S
Acknowledgments
The authors acknowledge all the subjects who volunteered in this study and nurses of Bharati Hospital and Gupte Hospital who helped in collecting the samples. The authors also acknowledge the Department of Biotechnology (DBT), Ministry of Science and Technology, India for partially funding this study (No BT/PR-10593/MED/12/396/2008). We also thank Council of Scientific and Industrial Research (CSIR), New Delhi, India for granting the Senior Research Fellowship (SRF) to Nisha S. Wadhwani.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest to disclose.
About this article
Cite this article
Wadhwani, N.S., Narang, A.S., Mehendale, S.S. et al. Reduced Maternal Erythrocyte Long Chain Polyunsaturated Fatty Acids Exist in Early Pregnancy in Preeclampsia. Lipids 51, 85–94 (2016). https://doi.org/10.1007/s11745-015-4098-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4098-5