Abstract
Objective: This case–control study was conducted in Lima, Peru, from June 1997 through January 1998 to assess whether alteration in maternal erythrocyte omega-3 (n-3) and omega-6 (n-6) fatty acids was associated with increased risk of preeclampsia. Methods: A total of 99 preeclampsia and 100 normotensive pregnant women were included. Maternal erythrocyte n-3 and n-6 fatty acids were determined using capillary gas chromatography/mass spectrometry and expressed as micromolar (mM) concentrations. We employed logistic regression procedures to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Result: n-3 fatty acids were consistently lower in preeclampsia cases than controls. After adjusting for confounders, the corresponding ORs for preeclampsia across decreasing quartiles of sum of long-chain n-3 fatty acids were 1.0, 3.3, 2.4, and 3.3, respectively (P=0.07 for trend). A similar pattern was observed for eicosapentenoic acid (20:5n-3, EPA) and docosahexenoic acid (22:6n-3, DHA). There was no clear evidence of an association between arachidonic acid (20:4n-6, AA) and preeclampsia risk, the ORs in successively lower quartiles were 1.0, 1.1, 1.0, and 1.5 (P=0.48 for trend). A similar pattern was seen for the sum of long-chain n-6 fatty acids. Conclusion: In Peruvian women, low erythrocyte n-3 fatty acids appeared to be associated with an increased risk of preeclampsia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia complicates approximately 6% of pregnancies in the United States and it is the third leading cause of maternal mortality, as well as an important cause of high perinatal mortality resulting from fetal growth restriction and preterm delivery [1]. Clinical symptom of preeclampsia is characterized by hypertension, proteinuria, and edema. The pathogenesis of preeclampsia remains a mystery, though several investigators speculate genetic, immunologic, and dietary factors that may be important etiological components [13, 22]. Endothelial dysfunction appears to be the common pathological features of the disorder [28]. Preeclampsia is also associated with altered synthesis of the eicosanoids thromboxane (TX) and prostacyclin (PG). Importantly, an elevated ratio of thromboxane A2 (TXA2) to prostacyclin I2 (PGI2) is evident in maternal plasma and placental tissue of patients with preeclampsia [2, 27].
Long-chain polyunsaturated fatty acids (PUFAs) are fatty acids with ≥20 carbon atoms and ≥3 double bonds. They are structural components of membrane phospholipids and are precursors of eicosanoids. Long-chain PUFAs are derived from the dietary essential fatty acids, linoleic acid (18:2n-6, LA), and α-linoleic acid (18:3n-3, ALA). The quantitatively most important long-chain PUFAs from 18:2n-6 is arachidonic acid (20:4n-6, AA), where 18:3n-3 is converted into eicosapentenoic acid (20:5n-3, EPA) and docosahexaenoic acid (22:6n-3, DHA). Where ALA, LA, and AA are essential fatty acids (yet they are not produced by the body cells) and must be acquired via consumption of foods such as flax seed, fish, vegetable oils, nuts seeds, and meats [24]. AA is the precursor of series-2 eicosanoids (TXA2 in platelets and PGI2 in vascular endothelium). EPA, on the other hand, is the precursor of series-3 eicosanoids (TXA3 in platelets and of PGI3 in vascular endothelium). TXA2, derived from the AA series 2-metabolic pathway, is a much more potent vasoconstrictor and platelet aggregator than the TXA3 that is derived from the EPA series 3-metabolic pathway [9]. PGI2 and PGI3 are equipotent vasodilators and inhibitors of platelet aggregation. Hence, a balance between the actions of TXA2 and prostacyclin (PGI2 or PGI3) maintains vascular function, platelet stability, and uteroplacental perfusion during pregnancy. Notably, dietary intake of n-3 and n-6 fatty acid determines, to a large extent, the ratio of AA to EPA; and can therefore, influence eicosanoid-mediated effects [11].
It has been suggested that supplementing the diet of pregnant women with n-3 fatty acid-rich fish oil may lead to a reduced risk of preeclampsia [11, 20, 23]. Numerous studies have also documented the association between the alteration of dietary maternal blood n-6 fatty acids and the risk of preeclampsia [3, 21, 25]. However, not all studies reported similar results.
We use the data from a case–control study of preeclampsia risk factors conducted in Lima, Peru, from June 1997 through January 1998 to assess whether alteration in maternal erythrocyte omega-3 and omega-6 fatty acids is associated with increased risk of preeclampsia. We elected to test fatty acids in erythrocytes since erythrocyte turnover is approximately 120 days and erythrocyte fatty acid profiles might reflect relative patterns of dietary fat intake over a 2- or 3-month period.
Methods
Study population and data collection
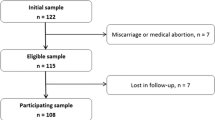
Potential study participants were recruited between June 1997 and January 1998 as part of a case–control study designed to examine the epidemiology of preeclampsia among Peruvian women. Details regarding data collection methods have been previously described [18]. Cases eligible for inclusion were those women with a diagnosis of preeclampsia. Using the then-current American College of Obstetricians and Gynecologists [1] diagnostic criteria, preeclampsia was defined as a persistent (i.e., lasting more than 6 h) 15 mm Hg diastolic rise or a 30 mm Hg rise in systolic blood pressure, or a persistent blood pressure of at least 140/90 mm Hg, and urine protein concentration ≥30 mg/dl (or 1+ on a urine dipstick). Potential preeclampsia cases were identified during the regular work hours of research nursing staff and recruited from Labor and Delivery wards at the Materno-Perinatal Institute and the Dos de Mayo Hospital in Lima, Peru. Of eligible cases were approached and asked to participate in the study, 97% elected to do so (193 of 199). Controls were selected among women with pregnancies uncomplicated by pregnancy-induced hypertension or proteinuria. Of the 204 controls approached, 196 (96%) agreed to participate in the study. We randomly selected 100 cases and 100 controls for the blood analysis. The power and sample size estimates indicated that a study of 100 preeclampsia cases and 100 controls gave sufficient power to test our study hypotheses. The Ethical Committee of the Dos De Mayo Hospital, the Materno-Perinatal Institute of Lima, and the Human Subjects Committee of the University of Washington Medical Center approved this investigation.
A standardized structured interview questionnaire was used to collect information regarding maternal sociodemographic, medical, reproductive, and lifestyle characteristics during in-person interviews. Maternal and infant records were reviewed after delivery to collect detailed information concerning antepartum, labor and delivery characteristics, and conditions of the newborn.
Laboratory analysis
Nonfasting, prelabor blood samples collected in EDTA tubes were immediately transported, in a cooler with ice, to the Blood Bank Laboratory of Dos de Mayo Hospital. Upon arrival at the laboratory, plasma was separated by centrifugation and erythrocytes were washed in a standard manner [16]. Washed erythrocytes and plasma were divided into 1.0-ml cryovials and were kept frozen at −70°C until analysis.
Maternal erythrocytes were shipped with frozen packs by overnight express to Metametrix Clinical Laboratory (Norcross, GA, USA). Fatty acids were measured by performing total transesterification reactions in anhydrous acidic methanol as described by Lepage [10]. Fatty acid methyl esters in the extract were analyzed by GC/MS (capillary gas chromatography/mass spectrophotometer, model 6890 GC with model 5972 MSD under Chemstation Software control from Hewlett-Packard Instruments, Palo Alto, CA, USA). The software converted peak areas into micromolar (mM) concentrations of individual fatty acids by comparison to known amounts of pure standards for calibration. The quantitative fatty acid assay avoids shortcomings of previous methods of reporting percentage that can obscure significant fatty acid relationships due to large variations in other major fatty acid components such as palmitic and oleic acids. Molar concentrations also allow for direct comparisons of amounts of fatty acid molecules present in the tissues, as opposed to quantization by weight. All analyses were performed without the knowledge of case or control status. There was insufficient blood for fatty acid determination for one preeclampsia case; hence, only 99 cases and 100 controls were available for statistical analyses.
Statistical analytical procedures
Frequency distributions of maternal sociodemographic characteristics, medical, and reproductive histories according to case and control statuses were examined. Comparisons of categorical variables were made between case and control subjects using Chi-square or Fisher’s exact tests. Nonpaired two-sample Student’s t test was used to compare the continuous variable in two groups if it was normally distributed.
To estimate the relative association between preeclampsia and concentrations of erythrocyte fatty acids, we categorized each subject according to quartiles determined by its distribution in normotensive control subjects [6]. We used the highest quartile category of each fatty acid as the reference group and calculated odds ratios (ORs) and 95% confidence intervals (CIs). Logistic regression procedures were used to calculate ORs and 95% CIs, adjusted for confounders [17]. In multivariable logistic regression models, significance for linear trend was assessed by treating the four quartiles as a continuous variable after assigning a score (i.e., 1, 2, 3, and 4) as its value. To assess confounding, we entered variables into a logistic regression model one at a time; we then compared the adjusted and unadjusted ORs. Final logistic regression models included covariates that altered unadjusted ORs by at least 10%, as well as those a priori.
All analyses were performed using Stata 7.0 statistical software (Stata, College Station, TX, USA). All reported P values are two-tailed, and all CIs were calculated at the 95% level. We considered that it was statistically significant if P<0.05.
Results
We present sociodemographic, medical, and reproductive characteristics of study subjects in Table 1. There were no statistically significant differences between preeclampsia cases and controls for covariates such as maternal age, race/ethnicity, or prepregnancy body mass index (BMI). Women with preeclampsia, however, were more likely to have delivered by cesarean section (63.6 vs. 37%; P<0.01) than normotensive control subjects. As expected, preeclampsia cases tended to deliver at earlier gestational ages, on average, as compared with controls (P<0.001).
We present unadjusted and adjusted ORs of preeclampsia for each quartile of n-3 fatty acids in Table 2. For most n-3 fatty acids, as well as for the aggregate variable (i.e., sum of long-chain n-3 fatty acids), we observed evidence of an inverse linear trend in the risk of preeclampsia with increasing concentrations of maternal erythrocyte fatty acids. After adjusting for possible confounding by parity, prepregnancy BMI, and gestational age at blood collection, we noted that preeclampsia risk increased for each successively lower quartile of ALA (18:3n-3) (ORs for successively lower quartile with the highest quartile as the referent group were as follows: 1.0, 1.5, 2.7, and 4.2, P value for trend=0.004). Hence, in this study population, women with erythrocyte ALA concentrations that were <0.96 mM had a 4.2-fold increased risk of preeclampsia (95% CI 1.5–12.1) as compared with those women who had concentrations that were ≥1.17 mM.
We also noted that the risk of preeclampsia was increased among women with the lowest concentrations of erythrocyte EPA concentrations (<3.13 mM, the lowest quartile) (OR=2.3; 95% CI 0.8–6.5) as compared to women who had concentrations that were ≥5.39 mM. The test statistic for a linear trend in preeclampsia risk in relation to declining concentrations of EPA did not reach statistical significance. A similar pattern of risk was noted for DHA (adjusted P value for trend=0.11). For the sum of long-chain n-3 fatty acids, women in the lowest quartile (<19.3 mM), as compared to those in the highest quartile (≥67.0 mM) had a 3.3-fold increased risk of preeclampsia (OR=3.3, 95% CI 1.2–9.5).
The findings regarding preeclampsia risk in relation to n-6 fatty acid concentrations in maternal erythrocytes are presented in Table 3. With the exception of AA, for each other individual fatty acids, we noted an inverse linear trend in the risk of preeclampsia with decreasing concentrations of n-6 fatty acids. For instance, women with the lowest concentrations of LA (<34.13 mM) had a 2.7-fold increased risk of preeclampsia (95% CI 1.0–7.1) as compared to women who had concentrations in the upper quartile (≥55.12 mM).
We also observed a moderate inverse linear component of trend in increased risk of preeclampsia with decreasing concentrations of maternal erythrocyte docosatetranoic acid (22:4n6). The corresponding ORs for decreasing quartiles were 1.4, 1.7, and 2.6, respectively (P for trend=0.05). Dihomo-γ-linolenic acid (20:3n6, DGLA) was also inversely associated with preeclampsia risk in this population. The OR for extreme quartiles was 3.5 (95% CI 1.3–9.4). Eicosanoids derived from the DGLA series 1 metabolic pathway share biochemical properties and has physiological effects that are similar to those of eicosanoids derived from the EPA series 3 metabolic pathways [24].
However, no clear evidence of an association was observed between preeclampsia risk and variations in concentrations of AA, the most abundant n-6 fatty acid noted in maternal erythrocyte in this study population. The OR and 95% confidence for extreme quartiles of erythrocyte AA in this population was 1.5 (95% CI 0.6–3.7). Given the abundance of AA, we observed a similar pattern of risk when preeclampsia risk was evaluated in relation to the sum of long-chain n-6 fatty acids. The ORs for successive lower quartiles for the sum of long-chain n-6 fatty acids were 1.0, 1.0, 1.2, and 1.5, respectively, with the highest quartile as the referent group (P value for linear trend=0.35).
Discussion
Among Peruvian women, we noted that n-3 fatty acids were consistently lower in preeclampsia cases than controls. After adjusting for confounders, the corresponding ORs for preeclampsia across decreasing quartiles of sum of long-chain n-3 fatty acids were 1.0, 3.3, 2.4, and 3.3, respectively (P=0.07 for trend). A similar pattern was observed for EPA (20:5n-3) and DHA (22:6n-3).
Our results of association between maternal n-3 fatty acid concentrations (biochemical markers of habitual dietary intake of foods rich in n-3 fatty acids) and the risk of preeclampsia are generally consistent with other previous studies [12, 29, 30]. In a study including 22 preeclamptic cases and 20 controls, Williams et al. [30] reported that women in the lowest tertile of n-3 fatty acids in erythrocytes (median of the tertile=6.23%) were 7.6 times (95% CI: 1.4–40.6) more likely to have preeclampsia than those in the highest tertile (median of the tertile=8.50%). In another case–control study, Wang and colleagues [29] reported that plasma levels of n-3 fatty acids tended to be lower in women with preeclampsia as compared to controls. Importantly, the authors reported that plasma EPA concentrations were 90% lower in cases than controls. Plasma DHA concentrations were noted to be 14% lower in preeclampsia cases versus control subjects. However, results from previous studies were not consistent. Kesmodel et al. [7] observed no association between dietary n-3 fatty acid levels and risk of preeclampsia. In recent years controlled intervention studies have failed to show any protective effect of marine fatty acids on the risk of preeclampsia [26]. Although the precise reasons for conflicting are not known, some have speculated that differences in study population characteristics including base-line or prerandomization fatty acid levels may vary across studies. Others have speculated that the timing and dose of fatty acids offered in treatment arms were not uniform and may account for variations in results across studies.
Although there is increasing consensus that moderate intake of long-chain n-3 PUFAs are associated with reductions in the risk of cardiovascular diseases in men and nonpregnant women [5], there are indications that excessive intake of both n-3 and n-6 PUFAs may be harmful [3]. Clausen and his colleagues [3], in their prospective study of 3,133 subjects, reported that women with high (>7.5% of total energy intake) fatty acid intake (both n-3 and n-6 as measured by food frequency questionnaire) had a 2.6-fold increased preeclampsia risk (95% CI: 1.3–5.4) as compared to women with fatty acid intake that accounted for ≤5.2% their total energy intake.
No association could be detected in these data between maternal erythrocyte n-6 fatty acids and preeclampsia. Our result is in disagreement with previous studies [3, 21]. Those investigators have noted that women with preeclampsia, as compared to normotensive pregnant women, had higher n-6 PUFAs. Other investigators, however, have reported there lower plasma AA levels in preeclampsia cases than in normotensive pregnant women [29].
Our study had several important strengths. The relative large sample size of our study allowed us to assess relative risk estimates with varying concentrations of fatty acids while adjusted for potential confounders. Nevertheless, we cannot exclude the possibility that residual confounding may still have affected the reported ORs. Differential misclassification of maternal erythrocyte fatty concentrations is unlikely, since all laboratory analyses were conducted without the knowledge of participants’ pregnancy outcome.
Potential limitations of our research, however, must be considered when interpreting our results. First, because of the cross-sectional design of our study, we cannot determine whether the observed case–control differences preceded preeclampsia, or whether the differences may be attributed to preeclampsia-related alterations in n-3 and n-6 fatty acid metabolism. Second, lack of information pertaining to maternal dietary habits limited our ability to assess maternal habitual dietary intake of these fatty acids and other nutrients and the risk of preeclampsia. Otto et al. [15], in an international comparative study, showed that the reduction in maternal essential fatty acid status during pregnancy is a general phenomenon, and is largely independent of differences in dietary habits and ethnic origin. Future studies that include collection of information about maternal dietary habits and which include assessment of measures of maternal erythrocyte fatty acids from blood samples collected in early pregnancy will overcome these important limitations.
Our cross-sectional studies of Peruvian women suggest that maternal dietary intake of n-3 fatty acids may be protective against preeclampsia. The biochemical roles that n-3 fatty acids play in reducing triglyceride [19] and decreasing platelet and leukocyte reactivity [4, 8], and their likely ability to reduce blood pressure [14] are consistent with these observations. An intervention trial of n-3 fatty acid supplementation in the preeclamptic group would also help clarify this cause or effect issue.
References
American College of Obstetricians, Gynecologists (1996) Hypertension in pregnancy. ACOG Tech Bull 219:1–8
Chavarria ME, Lara-Gonzalez L, Gonzalez-Gleason A, Garcia-Paleta Y, Vital-Reyes VS, Reyes A (2003) Prostacyclin/thromboxane early changes in pregnancies that are complicated by preeclampsia. Am J Obstet Gynecol 188:986–992
Clausen T, Slott M, Solvoll K, Drevon CA, Vollset SE, Henriksen T (2001) High intake of energy, sucrose, and polyunsaturated fatty acids is associated with increased risk of preeclampsia. Am J Obstet Gynecol 185:451–458
Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC (1989) The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med 320:265–271
Harris WS (2005) Extending the cardiovascular benefits of omega-3 fatty acids. Curr Atheroscler Rep 7:375–380
Hsieh C-C, Maisonneuve P, Boyle P, Macfarlane GJ, Robertson C (1991) Analysis of quantitative data by quantiles in epidemiologic studies: classification according to cases, noncases or all subjects. Epidemiology 2:137–140
Kesmodel U, Olsen SF, Salvig JD (1997) Marine n-3 fatty acid and calcium intake in relation to pregnancy induced hypertension, intrauterine growth retardation, and preterm delivery. A case-control study. Acta Obstet Gynecol Scand 76:38–44
Kramer HJ, Stevens J, Grimminger F, Seeger W (1996) Fish oil fatty acids and human platelets: dose-dependent decrease in dienoic and increase in trienoic thromboxane generation. Biochem Pharmacol 52:1211–1217
Leaf A, Weber PC (1988) Cardiovascular effects of n-3 fatty acids. N Engl J Med 318:549–557
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Mann N, Sinclair A, Pille M, Johnson L, Warrick G, Reder E, Lorenz R (1997) The effect of short-term diets rich in fish, red meat, or white meat on thromboxane and prostacyclin synthesis in humans. Lipids 32:635–644
Mills JL, DerSimonian R, Raymond E, Morrow JD, Roberts LJ 2nd, Clemens JD, Hauth JC, Catalano P, Sibai B, Curet LB, Levine RJ (1999) Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: a multicenter prospective study. JAMA 282:356–362
Mittendorf R, Lain KY, Williams MA, Walker C (1996) Preeclampsia: a nested case-control study of risk factors and their interaction. J Reprod Med 41:491–496
Morris MC, Sacks F, Rosner B (1993) Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 88:523–533
Otto SJ, Houwelingen AC, Antal M, Manninen A, Godfrey K, Lopez-Jaramillo P, Hornstra G (1997) Maternal and neonatal essential fatty acid status in phospholipids: an international comparative study. Eur J Clin Nutr 51:232–242
Rose HG, Oklander M (1965) Improved procedure for the extraction of lipoids from human erythrocytes. J. Lipid Res 6:428–431
Rothman KJ, Greenland S (1998): Modern epidemiology. 2nd edn. Lippincott-Raven Publishers, Philadelphia
Sanchez SE, Zhang C, Williams MA, Ware-Jauregui S, Larrabure G, Bazul V, Farrand A (2000) Tumor necrosis factor-α soluble receptor p55 (sTNFp55) and risk of preeclampsia in Peruvian women. J Reprod Immunol 47:49–63
Saynor R, Verel D, Gillott T (1984) The long-term effect of dietary supplementation with fish lipid concentrate on serum lipids, bleeding time, platelets and angina. Atherosclerosis 50:3–10
Schiff E, Ben-Baruch G, Barkai G, Peleg E, Rosenthal T, Mashiach S (1993) Reduction of thromboxane A2 synthesis in pregnancy by polyunsaturated fatty acid supplements. Am J Obstet Gynecol 168:122–124
Shouk TA, Omar MN, Fayed ST (1999) Essential fatty acids profile and lipid peroxides in severe pre-eclampsia. Ann Clin Biochem 36:62–65
Sibai BM, Gordon T, Thoms E, Caritis SN, Klebanoff M, McNellis D, Paul RH (1995) Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol 172:642–648
Sorensen JD, Olsen SF, Pedersen AK, Boris J, Secher NJ, FitzGerald GA (1993) Effects of fish oil supplementation in the third trimester of pregnancy on prostacyclin and thromboxane production. Am J Obstet Gynecol 168:915–922
Sprecher H (1981) Biochemistry of essential fatty acids. Prog Lipid Res 20:13–22
Velzing-Aarts FV, van der Klis FR, van der Dijs FP, Muskiet FA (1999) Umbilical vessels of preeclamptic women have low contents of both n-3 and n-6 long-chain polyunsaturated fatty acids. Am J Clin Nutr 69:293–298
Villar J, Merialdi M, Gülmezoglu AM, Abalos E, Carroli G, Kulier R, de Onis M (2003) Characteristics of randomized controlled trials included in systematic reviews of nutritional interventions reporting maternal morbidity, mortality, preterm delivery, intra-uterine growth restriction & small for gestational age and birth weight outcomes. J Nutr 133:1632S–1639S
Walsh SW (1985) Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol 152:335–340
Walsh SW (1998) Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Sem Reprod Endocrinol 16:93–104
Wang YP, Kay HH, Killam AP (1991) Decreased levels of polyunsaturated fatty acids in preeclampsia. Am J Obstet Gynecol 164:812–818
Williams MA, Zingheim RW, King IB, Zebelman AM (1995) Omega-3 fatty acids in maternal erythrocytes and risk of preeclampsia. Epidemiology 6:232–237
Acknowledgement
This research was supported by awards from the National Institutes of Health (T37-TW00049; RO3-TW01159; and R01-32562).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, C., Sanchez, S.E., Larrabure, G. et al. Erythrocyte omega-3 and omega-6 polyunsaturated fatty acids and preeclampsia risk in Peruvian women. Arch Gynecol Obstet 274, 97–103 (2006). https://doi.org/10.1007/s00404-006-0140-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-006-0140-4